Abstract
Since endoscopic submucosal dissection (ESD) has been accepted as the treatment of choice for early gastric cancer (EGC) without risk of lymph node metastasis, synchronous gastric epithelial neoplasia is no longer rare in the clinical practice. Knowledge about the characteristics associated with synchronous gastric epithelial neoplasia is of great importance to prevent delayed diagnosis.
Between November 2008 and December 2014, a retrospective study was conducted in a single tertiary referral hospital. Consecutive patients who underwent ESD due to EGC or high-grade dysplasia were analyzed to evaluate the incidence of synchronous gastric epithelial neoplasia and the factors associated with synchronous and overlooked synchronous lesions.
A total of 488 patients were analyzed in this study. Synchronous lesions were found in 59 patients (12.1%) during the mean 37.7 months of follow-up. Among 77 synchronous lesions, 25 lesions (32.4%) were overlooked at the time of initial ESD. Age of ≥ 65 years, moderate to severe endoscopic atrophic gastritis, and elevated morphology of primary lesions were associated with synchronous gastric epithelial neoplasia. An important factor associated with overlooked lesions is the non-elevated morphology of lesions.
Careful endoscopic examination of the whole stomach is necessary in patients who are older and who have moderate to severe atrophic gastritis and elevated morphology of lesions to prevent delayed diagnosis of synchronous gastric epithelial neoplasia, especially non-elevated lesions.
Keywords: atrophic gastritis, early gastric cancer, endoscopic submucosal dissection, neoplasia, synchronous
1. Introduction
Gastric adenocarcinoma is a common gastrointestinal malignancy worldwide. The prognosis of gastric cancer is dependent on the lymph nodes or distant metastasis. The prognosis of patients with early gastric cancer (EGC) who underwent surgical gastrectomy was good (stage IA over 95.1% and stage IB 88.4%).[1] According to the National Cancer Screening program of the Republic of Korea, adults over 40 years can have biennial gastric cancer screening free of charge. The screening endoscopic rate for gastric cancer was reported as 70.8% in the Republic of Korea,[2] and the proportion of EGC had reached 57.6% of patients who underwent gastrectomy because of gastric cancer.[3]
Endoscopic submucosal dissection (ESD) has been accepted as a treatment modality for EGC without the risk of lymph node metastasis [4,5] because the survival outcomes following ESD have been found to be comparable to surgical resection.[6] The 5-year survival rates of endoscopic resection for EGCs that met the absolute and expanded indication were over 95%.[7,8] In contrast to surgical gastrectomy, ESD is capable of organ preservation and provides a better quality of life than gastrectomy. Gastric cancer is highly associated with Helicobacter pylori infection, and the prevalence of H pylori infection in Korea was reported to be 59.6% in 2005.[9] The prevalence of atrophic gastritis and intestinal metaplasia in Korea was 20.1% to 40.7% and 21.2% to 32.7%, respectively.[10] The prevalence of atrophic gastritis and intestinal metaplasia increases with age.[11] After organ-preserving treatment of EGC such as endoscopic resection, the premalignant gastric lesions (atrophic gastritis or intestinal metaplasia) remain. Therefore, multiple gastric epithelial neoplasia may be found before or after ESD. The reported rates of synchronous lesions after endoscopic resection and surgical gastrectomy for gastric cancer were 2% to 19.2% and 4.8% to 14.6%, respectively.[12]
The early detection of multiple gastric epithelial neoplasia is important to preserve the stomach after ESD. In the present study, the clinical characteristics of synchronous gastric epithelial neoplasia before or after ESD for EGCs or high-grade dysplasia were evaluated. Moreover, the factors associated with overlooked synchronous lesions were analyzed.
2. Patients and methods
2.1. Patients
The medical records of patients with EGC or high-grade dysplasia who were treated using ESD were retrospectively reviewed between November 2008 and December 2014 at the Pusan National University Yangsan Hospital in the Republic of Korea. During the study period, 596 consecutive patients were treated using ESD. The indications of the ESD for EGC are absolute or expanded indications.[4,5] Absolute indication was differentiated mucosal cancer of <20 mm in size. Expanded indications were as follows:
-
(1)
differentiated mucosal cancer without ulcerations,
-
(2)
differentiated mucosal cancer with ulceration <30 mm in size,
-
(3)
differentiated submucosal cancer <30 mm (invasion depth <500 μm), and
-
(4)
undifferentiated mucosal cancer without ulceration <20 mm in size.
The exclusion criteria were as follows: beyond indications of endoscopic resection (n = 49), piecemeal resection or resection margin positive (n = 30), lesions in the remnant stomach after surgical resection (n = 2), and absence of follow-up endoscopic examination after ESD (n = 27). After exclusions, a total of 488 patients with high-grade dysplasia or EGCs were enrolled and analyzed (Fig. 1). Written informed consents were obtained from all patients before performing ESD. The study was approved by the Ethics Committee that belongs to our Institutional Review Board.
Figure 1.
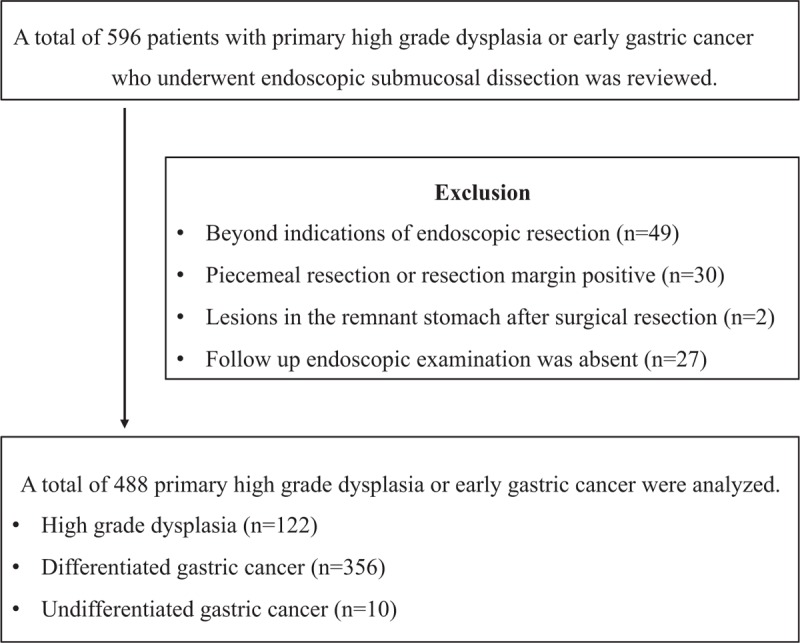
Study flow.
2.2. Clinicopathological characteristics and definitions
All endoscopic photographs and data were retrospectively reviewed by an endoscopist (CW Choi MD, PhD). The procedure time was defined as the time from marking around the lesion to complete removal of the lesion, including the time required for hemostasis. The macroscopic appearances of lesions were defined by the Paris classification, based on the characteristics of the endoscopic findings (elevated, flat, and depressed).[13] The location of the lesions was described as the lower third, mid-third, or upper third of the stomach using the Japanese classification of gastric cancer.[14] The lesion size was measured using the pathological examination (maximal diameter). Surface redness and whitish discoloration were defined as discoloration on the mucosal surface of the lesion compared to the surrounding mucosa. Surface nodularity was defined as the presence of irregularly raised or nodular mucosa. A lesion with an ulceration or scar secondary to previous ulceration was regarded as an ulcer. If visible fibrosis was identified during ESD, the presence of submucosal fibrosis was recorded. The extent of atrophic gastritis was classified according to the Kimura and Takemoto classification:[15] mild (normal to closed type 2), moderate (closed type 3 to open type 1), and severe (open type 2 to open type 3).
Synchronous lesions were defined as gastric epithelial neoplastic lesions (adenomatous lesions and EGCs) that have already been detected before the initial ESD or found endoscopically and confirmed pathologically with endoscopic forceps biopsy within 1 year of ESD. If multifocal lesions were found during endoscopic examinations, the larger or advanced pathologic lesions were selected as the primary lesions, and the others were termed as synchronous lesions. For each synchronous lesion, endoscopic findings were recorded.
2.3. Statistical analysis
Univariate analysis using chi-square test or Fisher exact test for categorical variables and Student t test for continuous variables were performed. Multivariate analysis with multiple logistic regression models was performed with associated factors for synchronous or overlooked synchronous lesions. P < .05 was considered statistically significant. Statistical calculations were performed using the Statistical Package for the Social Sciences (SPSS) version 21.0 for Windows (IBM Corp., Armonk, NY).
3. Results
3.1. Baseline characteristics of patients who underwent ESD for EGC or high-grade dysplasia
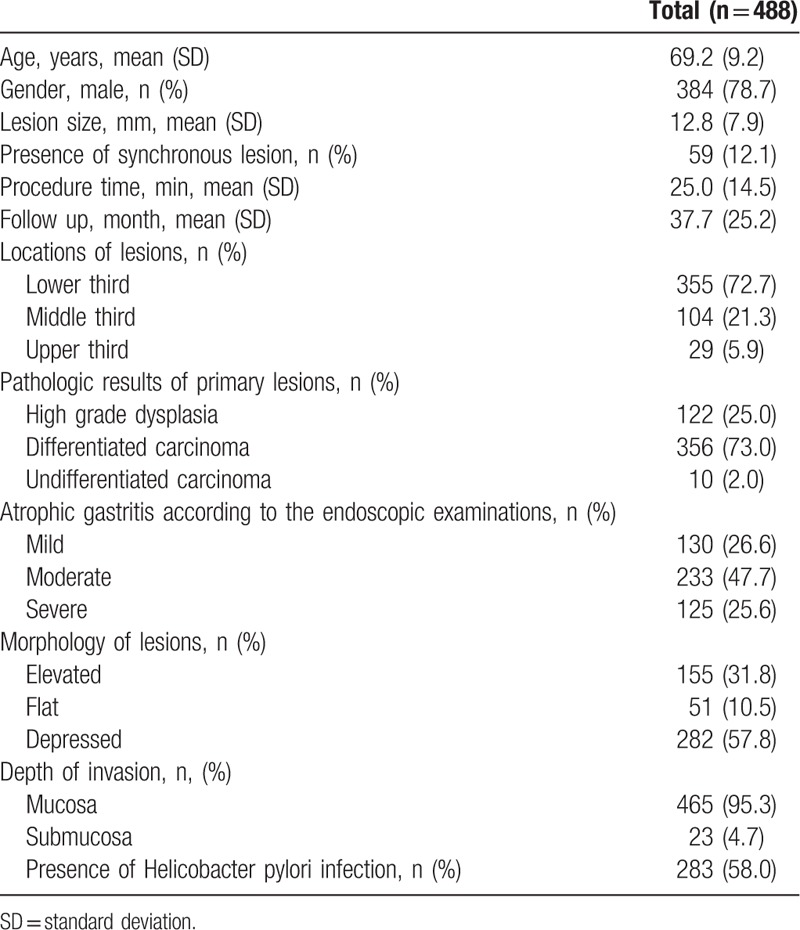
In this study, 488 patients were enrolled (122, 356, and 10 patients with high-grade dysplasia, differentiated cancer, and undifferentiated cancer, respectively). The patient population was predominantly male (78.7%, 384/488), with a mean age of 69.2 ± 9.2 years. The predominant location of the primary lesions was the lower third of the stomach (72.7%, 355/488), and the mean lesion size was 12.8 ± 7.9 mm. The most common gross type was depressed type (57.8%, 282/488) (Table 1). H pylori was detected in 58% of patients (283/488), and moderate extent of atrophic gastritis was common (47.7%, 233/488) (Table 1).
Table 1.
Baseline clinical characteristics of patients with primary high grade dysplasia or early gastric cancers.
3.2. Characteristics of synchronous lesions
The synchronous lesions were termed as “overlooked” if
-
(1)
endoscopic photos of the lesions were absent before or during ESD;
-
(2)
endoscopic photos showed lesions before or during ESD, but not endoscopic biopsy; and
-
(3)
pathological analysis following endoscopic forceps biopsy did not reveal neoplastic lesions.
Data were analyzed for patients with and without synchronous lesions (Table 2) and then analyzed for synchronous lesions themselves (Table 4).
Table 2.
Clinicopathologic differences between patients with synchronous and without synchronous lesions.
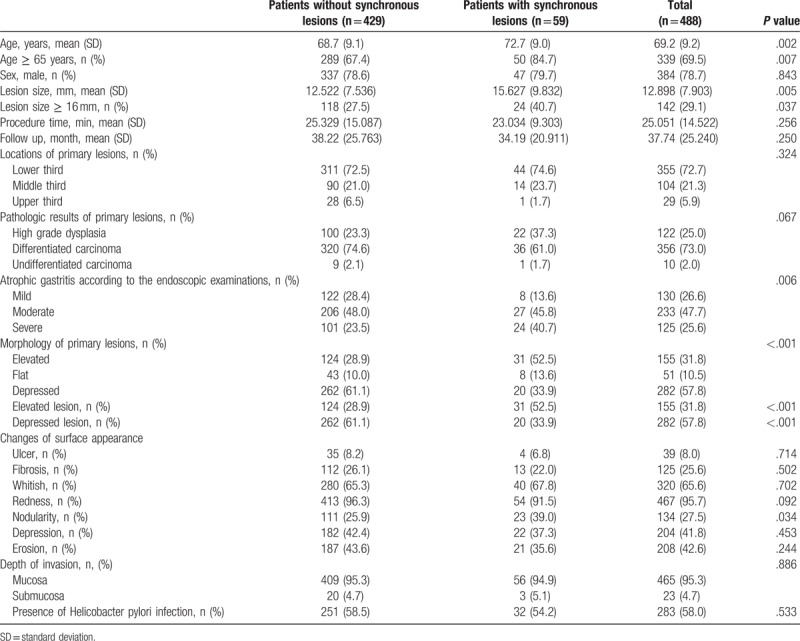
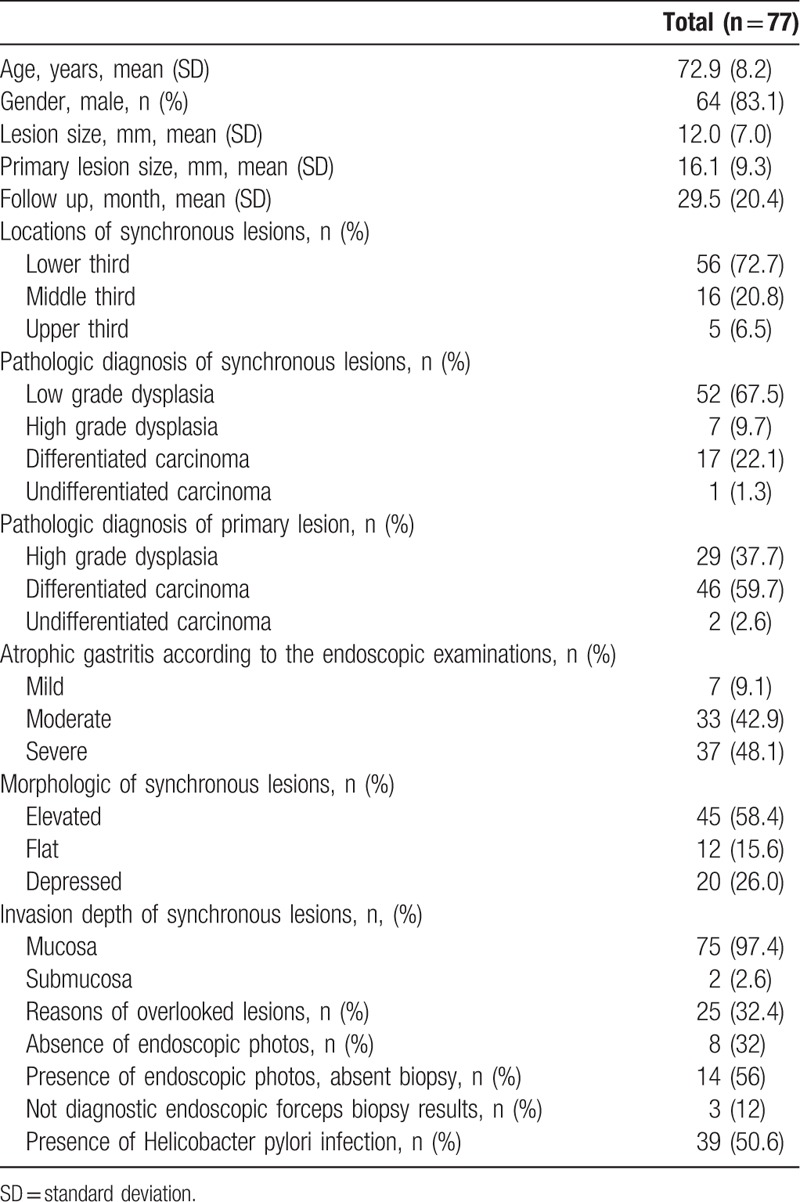
Table 4.
Primary characteristics of synchronous lesions.
3.3. Comparison between patients with and without synchronous lesions
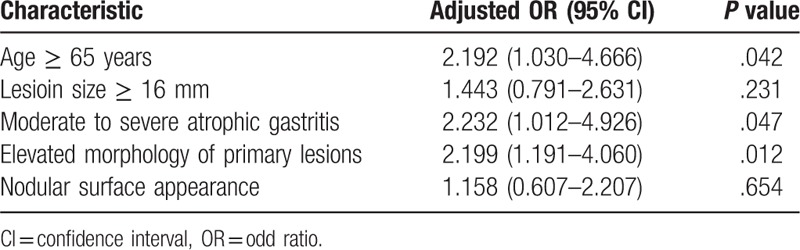
Synchronous lesions were detected in 12.1% (59/488) of patients (Table 2). From univariate analysis, older age, lesion size of ≥16 mm, extent of atrophic gastritis, morphology of primary lesions, and nodular surface appearance were significant risk factors (Table 2). After multivariate analysis, age of ≥65 years (odds ratio [OR] 2.192, 95% confidence interval [CI] 1.030–4.666, P = .042), moderate to severe atrophic gastritis (OR 2.232, 95% CI 1.012–4.926, P = .047), and elevated morphology of primary lesions were significant (OR 2.199, 95% CI 1.191–4.060, P = .012) (Table 3).
Table 3.
Risk factor analysis associated with synchronous lesions: multivariate analysis.
3.4. Characteristics of the synchronous lesions and risk factor analysis associated with overlooked synchronous lesions
A total of 77 synchronous lesions were found. The patient population was predominantly male (83.1%, 64/77), with a mean age of 72.9 ± 8.2 years. The predominant location of the primary lesions was the lower third of the stomach (72.7%, 56/77), and the mean lesion size was 12.0 ± 7.0 mm. The pathologic diagnoses of synchronous lesions were low-grade dysplasia, differentiated carcinoma, high-grade dysplasia, and undifferentiated carcinoma in 52, 17, 7, and 1 of the cases, respectively. The most common gross type was elevated type (58.4%, 45/77). A total of 25 lesions (32.4%) were diagnosed after initial ESD (overlooked lesions). The reasons for overlooked lesions were as follows: absence of endoscopic photos (32%, 8/25), presence of endoscopic photos without biopsy (56%, 14/25), and nondiagnostic endoscopic forceps biopsy results (12%, 3/25) (Table 4).
The characteristics associated with overlooked synchronous lesions were analyzed. From the univariate analysis, the morphology of synchronous lesions, whitish discoloration, redness, and erosive changes were significant (Table 5). After multivariate analysis, flat or depressed morphology was the only significant risk factor (OR 4.329, 95% CI 1.219–15.384, P = .023) (Table 6). Among the synchronous lesions, most of the lesions could be resected with ESD (85.7%, 66/77). Surgical resection was performed in 3 patients (2 differentiated EGC and 1 undifferentiated EGC). Because of patients’ refusal of treatment, 8 lesions resectable with ESD was not resected (6 low-grade dysplasia and 2 differentiated EGC) (Fig. 2). During follow-up examinations, gastric cancer-related mortality was absent.
Table 5.
Comparisons between detected and overlooked synchronous lesions: univariate analysis.
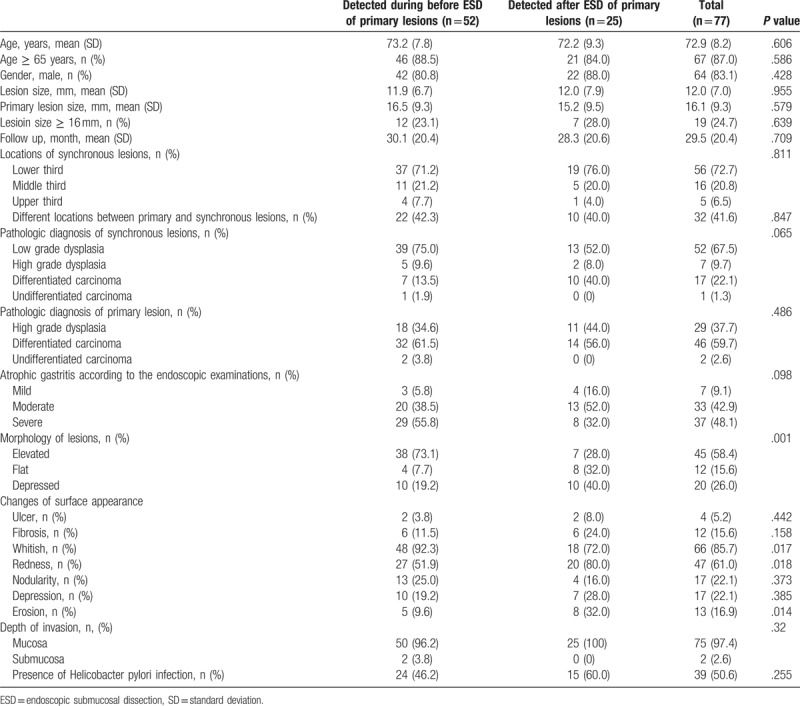
Table 6.
Risk factor analysis associated with overlooked synchronous lesions: multivariate analysis.
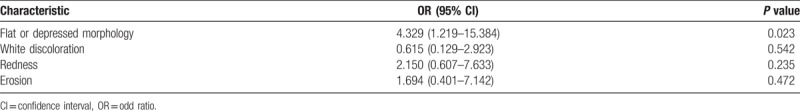
Figure 2.
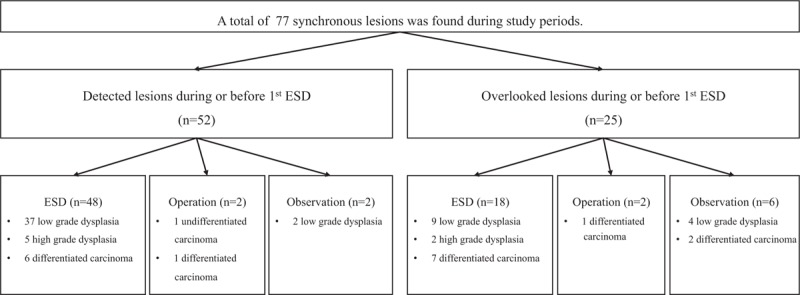
Clinical outcomes of synchronous lesions. ESD = endoscopic submucosal dissection.
4. Discussion
In the present study, synchronous gastric epithelial neoplasia was detected in 12.1% of patients. Other studies reported about approx 6.8% to 8.1% incidence of synchronous gastric cancers after endoscopic resection of EGC.[12,16] Because we included the adenomatous lesions as synchronous lesions, the incidence of synchronous lesions may be higher than previous reported studies. After exclusion of low-grade dysplasia as synchronous lesions (n = 52), the incidence of synchronous lesions was 5.1%. The benefit of ESD compared with surgical gastrectomy is the preservation of the stomach and the significant improvement in the quality of life. If synchronous lesions are found within 12 months after initial ESD, the lesions might be overlooked or missed before or during initial ESD. If the synchronous lesions have high risk of lymph node metastasis, additive surgical resection should be considered. In the present study, additive surgical gastrectomies were performed in 3 patients after diagnosis of deep submucosal cancer after ESD. During the follow-up period, gastric cancer-related mortality was absent.
The associated clinical factors with the occurrence of multiple synchronous gastric epithelial neoplasia were age of ≥65 years, moderate to severe atrophic gastritis, and elevated morphology of primary lesions. Gastric cancer is a common gastrointestinal malignancy in the Republic of Korea. The prevalence of H pylori infection in adults was reported as 59.6% in 2005.[9] There was no statistically significant difference according to H pylori infection in this study. H pylori eradication may induce regression in pre-existing adenomatous lesions or delay the occurrence of metachronous lesions rather than curing synchronous lesions. The effect of the eradication is likely to inhibit the progression or remain stable rather than histological recovery of synchronous lesions.[17–20] The mean age of the present study was 69.2 ± 9.2 years. The reported prevalence of atrophic gastritis over 60 years in Korea was 66.9% to 69.5% in the antrum and 43.7% to 44.5% in the body.[11] Previous studies reported that the cumulative incidence of gastric cancer was highly associated with the extent of atrophic gastritis.[21] Gastric cancer may occur in 10% of severe atrophic gastritis (open type 2–3 according to the Kimura–Takemoto classification).[15,21] The reason of elevated morphologic lesions as associated occurrence of synchronous lesions remains unclear.
Among the synchronous lesions, 32.4% of the lesions were not detected before or during initial ESD. Among the 25 overlooked or missed lesions, 22 lesions had no endoscopic photo or absent endoscopic forceps biopsy. Even if the synchronous lesions were captured on images, endoscopists were not recognized as a lesion and inappropriate targeting biopsy or poor biopsy tissue also could increase the incidence of synchronous lesions. Endoscopists require much effort to increase the diagnostic rate of EGC already present. High-definition white light endoscopy may detect minute EGC. Narrow band imaging (NBI) may be helpful in finding the lesion after the white light endoscopic evaluation in characterizing and distinguishing EGC and margins. However NBI is not used to evaluate the whole stomach as to replace conventional endoscopy. Standardized endoscopic examinations, such as adequate air insufflation to separate the gastric folds, irrigation to remove mucous, and mapping the stomach with photo documentation, are important.[22] Nevertheless, evaluating these parameters may be difficult and no useful index has been developed. Key endoscopic characteristics of EGC such as changes in the surface (elevated or depressed lesions), changes in color (whitish or reddish), and spontaneous bleeding should be paid more attention during endoscopic examinations.[23] The most important endoscopic factor associated with overlooked synchronous lesions was a flat or depressed morphology. To decrease the miss rate of these lesions, adequate time of endoscopic examination may be important. Previous studies reported that examination time is a possible indicator of quality of upper gastrointestinal endoscopy for asymptomatic patients.[24,25] Endoscopists who do not use adequate time may overlook neoplastic lesions. Endoscopists’ experience might be a possible important factor. A previous study reported that the miss rate was associated with the endoscopist's inexperience (<500 endoscopy cases).[26] To increase the rate of finding the lesion, endoscopist with more than a year of experience have carried out diagnostic exams and confirming the lesions by experienced endoscopist before finishing the exams in our study.
Among the overlooked lesions, nondiagnostic endoscopic forceps biopsy results were found in 3 lesions. Although endoscopic forceps biopsy was the most important diagnostic tool before resection, diagnostic discrepant results between endoscopic forceps biopsy and resected specimen may be present. We previously reported the follow-up results of nondiagnostic endoscopic forceps biopsy. Among gastric indefinite neoplasia from endoscopic forceps biopsy, 21.8% of the lesions were diagnosed as EGC during follow-up.[27] Therefore, follow-up endoscopic examinations and repeated endoscopic forceps biopsy are necessary for suspicious neoplastic lesions. Regular surveillance examination is important to detect overlooked synchronous lesions before progressing to invasive cancers. In the present study, the scheduled endoscopic examinations were performed 3 months after the initial ESD, and regular endoscopic examinations were recommended every 6 months for 2 years and annually thereafter. The cancer-related mortality was absent. Another previous study reported advanced gastric cancer detected more than 1 year after ESD in 0.12% cases. Based on these findings, follow-up endoscopic examinations within 1 year after initial ESD are strongly recommended.
The present study has several limitations. First, it was conducted in a single center, and the sample size might be small to generalize the results. Also, there were potential information biases due to the retrospective design of this study. However, the results of the present and other studies, so far, are informative and recommend the use of caution during endoscopic treatment before or after of ESD. Second, although intestinal metaplasia is an important premalignant factor, the severity of intestinal metaplasia could not be examined because biopsy was not routinely performed in Korea to diagnose atrophic gastritis or intestinal metaplasia. Secondary change due to intestinal metaplasia would mask the pre-existing lesions. The procedure time which is 1 of the important factors of diagnosing the lesion also could not be measured because of the retrospective study design. Third, there was heterogeneity in the experience of the endoscopist performing the procedures. Diagnostic rate would be increased in case of an expert, however as this cannot be generalized and this could be 1 of limitations.
In summary, synchronous gastric epithelial neoplasia was found in 12.1% of patients, and 32.4% of synchronous lesions were not diagnosed adequately before or during initial ESD. Patients’ age of ≥65 years, moderate to severe atrophic gastritis, and elevated morphology of primary lesions were found to be high-risk factors associated with the presence of synchronous lesions. A flat or depressed morphology of lesions might be overlooked during evaluation. Regular follow-up endoscopic examination within 1 year of ESD should be considered to detect overlooked lesions before they progress to invasive cancer.
Author contributions
Conceptualization: Cheol Woong Choi.
Formal analysis: Cheol Woong Choi.
Investigation: Hyung Wook Kim, Cheol Woong Choi, Dae Hwan Kang, Su Bum Park, Su Jin Kim, Hyeong Seok Nam.
Supervision: Cheol Woong Choi.
Validation: Jung Sik Choi.
Writing – original draft: Hyeong Seok Nam, Hyung Wook Kim, Cheol Woong Choi.
Writing – review & editing: Cheol Woong Choi, Hyeong Seok Nam.
Cheol Woong Choi orcid: 0000-0001-8867-3039
Footnotes
Abbreviations: CI = confidence interval, EGC = early gastric cancer, ESD = endoscopic submucosal dissection, OR = odds ratio.
HS Nam and HW Kim share first authorship.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh american joint committee on cancer/international union against cancer classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010;116:5592–8. [DOI] [PubMed] [Google Scholar]
- [2].Suh M, Choi KS, Lee YY, et al. Cancer screening in Korea, 2012: results from the Korean National Cancer Screening Survey. Asian Pac J Cancer Prev 2013;14:6459–63. [DOI] [PubMed] [Google Scholar]
- [3].Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 2011;11:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim YI, Kim YW, Choi IJ, et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 2015;47:293–301. [DOI] [PubMed] [Google Scholar]
- [7].Min BH, Kim ER, Kim KM, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015;47:784–93. [DOI] [PubMed] [Google Scholar]
- [8].Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485–93. [DOI] [PubMed] [Google Scholar]
- [9].Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev 2015;20:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eshmuratov A, Nah JC, Kim N, et al. The correlation of endoscopic and histological diagnosis of gastric atrophy. Dig Dis Sci 2010;55:1364–75. [DOI] [PubMed] [Google Scholar]
- [12].Nishida T, Tsujii M, Kato M, et al. Endoscopic surveillance strategy after endoscopic resection for early gastric cancer. World J Gastrointest Pathophysiol 2014;5:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].The paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003; 58:S3–43. [DOI] [PubMed] [Google Scholar]
- [14].Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- [15].Mihara M, Haruma K, Kamada T, et al. The role of endoscopic findings for the diagnosis of Helicobacter pylori infection: evaluation in a country with high prevalence of atrophic gastritis. Helicobacter 1999;4:40–8. [DOI] [PubMed] [Google Scholar]
- [16].Lee HJ, Lee YJ, Lee JY, et al. Characteristics of synchronous and metachronous multiple gastric tumors after endoscopic submucosal dissection of early gastric neoplasm. Clin Endosc 2018;51:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang YJ, Kim N, Lee HS, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication—a prospective study for up to 10 years. Aliment Pharmacol Ther 2018;47:380–90. [DOI] [PubMed] [Google Scholar]
- [18].Nam SY, Park BJ, Ryu KH, et al. Effect of Helicobacter pylori infection and its eradication on the fate of gastric polyps. Eur J Gastroenterol Hepatol 2016;28:449–54. [DOI] [PubMed] [Google Scholar]
- [19].Suzuki S, Gotoda T, Suzuki H, et al. Morphologic and histologic changes in gastric adenomas after helicobacter pylori eradication: a long-term prospective analysis. Helicobacter 2015;20:431–7. [DOI] [PubMed] [Google Scholar]
- [20].Mera RM, Bravo LE, Camargo MC, et al. Dynamics of Helicobacter pylori infection as determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018;67:1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shichijo S, Hirata Y, Niikura R, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc 2016;84:618–24. [DOI] [PubMed] [Google Scholar]
- [22].Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11–22. [PMC free article] [PubMed] [Google Scholar]
- [23].Uedo N, Yao K, Ishihara R. Screening and treating intermediate lesions to prevent gastric cancer. Gastroenterol Clin North Am 2013;42:317–35. [DOI] [PubMed] [Google Scholar]
- [24].Kawamura T, Wada H, Sakiyama N, et al. Examination time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig Endosc 2017;29:569–75. [DOI] [PubMed] [Google Scholar]
- [25].Park JM, Huo SM, Lee HH, et al. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology 2017;153:460–9 e461. [DOI] [PubMed] [Google Scholar]
- [26].Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013;62:1425–32. [DOI] [PubMed] [Google Scholar]
- [27].Goo JJ, Choi CW, Kang DH, et al. Risk factors associated with diagnostic discrepancy of gastric indefinite neoplasia: who need en bloc resection. Surg Endosc 2015;29:3761–7. [DOI] [PubMed] [Google Scholar]


