Abstract
Background:
Carbon monoxide (CO) poisoning may result in acute neurological sequelae, cognitive sequelae, and delay neurological sequelae. The administration of hyperbaric oxygen (HBO) to prevent the development of delayed neurological sequelae in CO poisoning have extensively investigated but conflicting results have been reported. We performed a systematic literature review and meta-analysis of randomized controlled trials (RCTs) evaluating HBO treatment and its effect on neuropsychometric dysfunction after CO poisoning.
Methods:
We searched Medline, Embase, Pubmed, and the Cochrane Register of Controlled Trials from inception to December 2017. Eligible studies compared HBO therapy with normobaric oxygen (NBO) in patients with CO poisoning.
Results:
Six studies compared HBO with NBO in CO poisoning patients. Compared with patients treated with NBO, a lower percentage of patients treated with HBO reported headache (16.2% vs 16.5%, relative risk [RR] = 0.83, 95% CI = 0.38–1.80), memory impairment (18.2% vs 23.8%, RR = 0.80, 95% CI = 0.43–1.49), difficulty concentrating (15.0% vs 18.4%, RR = 0.86, 95% CI = 0.55–1.34), and disturbed sleep (14.7% vs 16.2%, RR = 0.91, 95% CI = 0.59–1.39). Two sessions of HBO treatment exhibited no advantage over one session.
Conclusions:
The meta-analysis indicated that compared with CO poisoning patients treated with NBO, HBO treated patients have a lower incidence of neuropsychological sequelae, including headache, memory impairment, difficulty concentrating, disturbed sleep, and delayed neurological sequelae. Taking into consideration the cost-effectiveness of one session of HBO, one session of HBO treatment could be an economical option for patients with CO poisoning with high severity.
Keywords: carbon monoxide poisoning, hyperbaric oxygen, neuropsychometric dysfunction
1. Introduction
Carbon monoxide (CO) is a toxic gas that is difficult to detect because it is colorless, odorless, tasteless, and initially nonirritating. The most common sources of accidental CO poisoning are faulty or inadequately ventilated gas heating appliances, fires, mining accidents, and automobile exhaust fumes. Moreover, CO generated from charcoal smoke is sometimes utilized for committing suicide.[1] Symptoms of mild acute CO poisoning include lightheadedness, confusion, headache, vertigo, and flu-like effects; exposure to CO for long periods can cause severe toxicity in the central nervous and cardiovascular systems. In addition to acute neurological sequelae (loss of consciousness, coma, and death), neurological and cognitive sequelae, including poor concentration, memory problems, personality changes, psychosis, and Parkinsonism, may be observed in people recovering from CO poisoning. Delayed neurological sequelae may also develop after a period of apparent normality.[2] Approximately 30% of patients exhibited chronic neurological symptoms caused by late encephalopathy up to 1 year after CO poisoning.[3]
Recommended treatments for CO poisoning include removal from the site of exposure, administration of supplemental oxygen, and general supportive care. The elimination half-life of carboxyhemoglobin (approximately 320 minutes in room air) is shortened by approximately 5-fold after the administration of 100% oxygen at atmospheric pressure (normobaric oxygen [NBO]). The administration of hyperbaric oxygen (HBO) hastens the elimination of carboxyhemoglobin.[4]
Studies have extensively investigated the use of HBO to prevent the development of delayed neurological sequelae in CO poisoning; however, conflicting results have been reported. HBO, which is available at only a few hospitals, is more expensive than NBO. The possible complications of HBO treatment include barotrauma, claustrophobia, sinus damage, pneumothorax, and gas emboli.[5,6] Establishing the benefit/risk ratio of HBO treatment and its superiority over NBO treatment in CO poisoning is difficult.
In the present study, we performed a systematic literature review and meta-analysis of randomized controlled trials (RCTs) evaluating HBO treatment and its effect on neuropsychometric dysfunction after CO poisoning.
2. Methods
2.1. Protocol and guidelines
We conducted and reported this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, explanation and elaborate documentation, and checklist. Ethical approval and patient consent were not required because our study was retrieved from previous published studies.
2.2. Data sources
Literature searches were performed using 4 electronic databases (Medline, Embase, PubMed, and Cochrane databases) by 2 reviewers (CHL and WHS). The searches were performed from the inception of each electronic database (1950 for Medline, 1947 for Embase, 1951 for PubMed, and 1996 for Cochrane databases) and until December 2017. For the search strategy, we combined the terms “carbon monoxide poisoning” and “hyperbaric oxygen” or “HBO” or “hyperbaric oxygen therapy” or “HBOT.” The search was limited to RCTs without restriction of the date of publication, country, or language. Citations that included the search terms in the title, abstract, or article or medical subject heading terms were selected. In addition, we checked the references of potential RCTs. We identified other studies by hand searching the reference sections of these papers and by contacting known experts in the field. Finally, unpublished trials were retrieved from the ClinicalTrials.gov registry (http://clinicaltrials.gov/).
2.3. Study selection
We included only full-text RCTs that reported on the efficacy and safety of HBO treatment in comparison with those of NBO treatment for CO poisoning in adults. Only studies that reported neurological outcomes or mortality as trial endpoints were included. We excluded observational, uncontrolled, or nonrandomized interventional studies. CHL and WHS independently screened the titles and abstracts of retrieved reports for potential eligibility, and then checked reference lists for potentially relevant studies. Disagreements were resolved through discussion with a third reviewer (YCC). Results were set as the presence of signs or symptoms of neurologic injury after randomization and verse controlled groups with NBO alone. Studies that did not meet the inclusion criteria were excluded. We also excluded studies that enrolled patients who had undergone other intervention or treatment, those whose abstracts were presented in medical conferences, and those based on expert opinions. Duplicate subject publications within separate unique studies were not reported twice. The effect of 2 sessions versus one session of HBO treatment was further analyzed.
2.4. Outcome measures
The primary outcome was the percentage of patients having adverse neurological effects, including headache, memory impairment, difficulty concentrating, and disturbed sleep; full recovery; moderate sequelae; delayed neurological sequelae; and asthenia, all of which are plausibly related to CO poisoning.
2.5. Data extraction and quality assessment
CHL and WHS used a standardized electronic form to independently extract the study characteristics and outcome data from the included RCTs. Discrepancies were resolved through discussion, and any disagreement was resolved by YCC. When possible, we used data from intention-to-treat (ITT) analyses. The authors of the studies were contacted for additional information when necessary. CHL and WHS independently assessed the risk of bias in the included studies by using the Cochrane Collaboration's risk of bias tool. This risk was assessed according to individual domains, with the following aspects reported: allocation generation, allocation concealment, blinding, the length of follow-up, the percentage of loss to follow-up, and whether ITT analysis was conducted.
2.6. Data synthesis and analysis
We performed a random-effects meta-analysis (RevMan software, version 5.3, the Cochrane Collaboration, Copenhagen, Denmark) weighted by the Mantel–Haenszel method to estimate pooled risk ratios and 95% confidence intervals (CIs). The Q statistics and I2 test were used to assess heterogeneity. All data were combined with the Mantel–Haenszel random-effects model, which provides a more appropriate estimation of the average treatment effect when trials are statistically heterogeneous and usually yields wider CIs, resulting in a more conservative statistical claim. For outcome measures, statistical significance was set at P < .05.
3. Results
3.1. Characteristics of the trials
The review process is outlined in Figure 1. The initial search strategy yielded 288 citations, of which 211 were deemed ineligible based on the screening of titles and abstracts. Of the remaining 77 full-text articles, 71 were excluded, because 27 did not meet the eligibility criteria, 23 were not randomized trials, 12 were review articles, 2 were not human studies, 6 were not journal articles or did not have a relevant study design, and 1 was a questionnaire. Finally, 6 studies and 8 eligible trials were included.[7–12]
Figure 1.
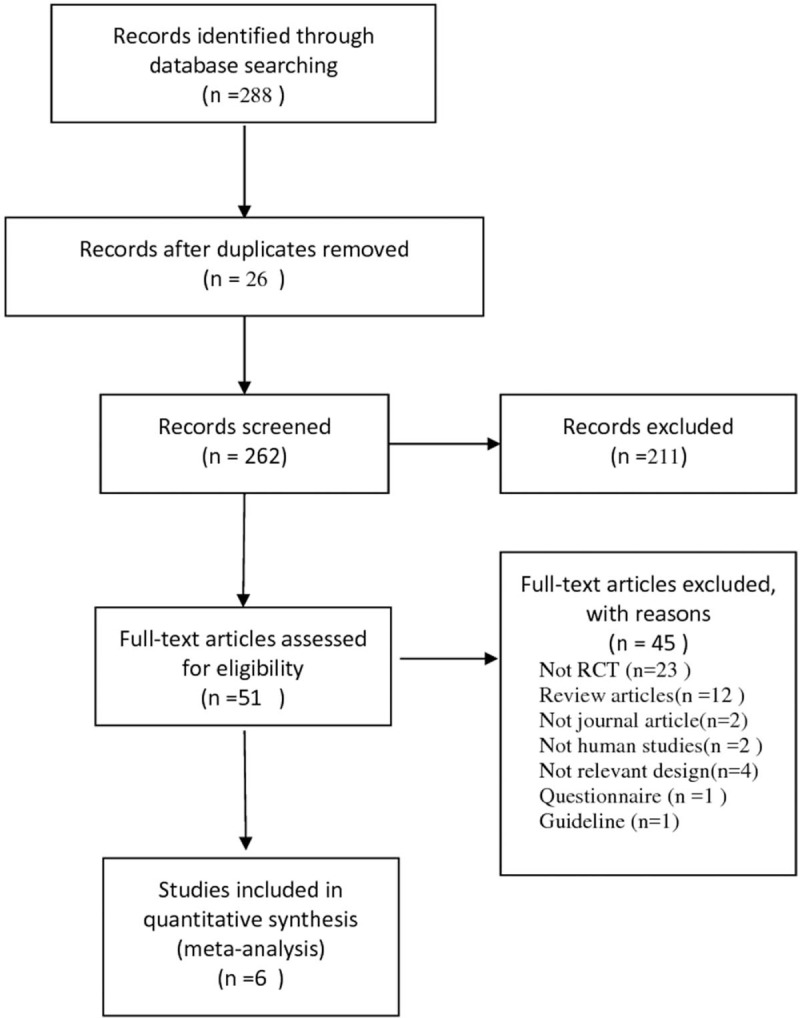
Flowchart for selection of studies—PRISMA flow diagram.
Table 1 lists the study characteristics and patients’ demographic data retrieved from each of the eight trials included in the review. The studies were published between 1989 and 2010, and the sample sizes ranged from 26 to 343. All the studies evaluated patients admitted with CO poisoning. The diagnosis of CO poisoning was generally established on the basis of a history of CO exposure and an elevated carboxyhemoglobin level. Two studies each had conducted 2 separate trials according to the severity of CO poisoning.[7,12] Baseline characteristics were balanced and similar between the 2 treatment groups in the 8 trials. The duration, timing, and dosages of both HBO and NBO treatments differed across the trials because of the use of different protocols.
Table 1.
Characteristics of included studies.
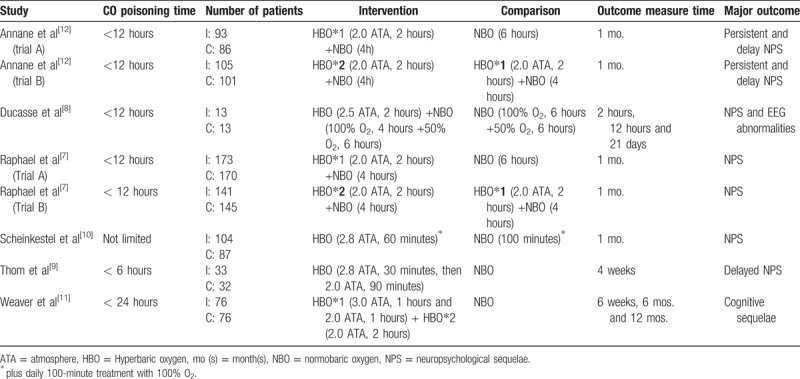
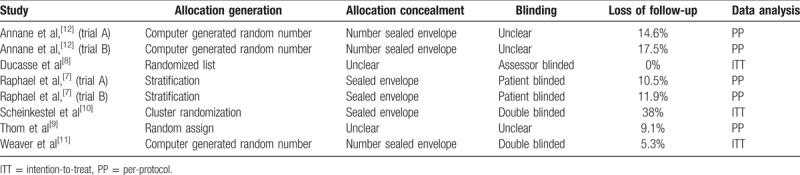
Table 2 details the methodological quality of the 8 trials. Of these, 7 trials clearly documented the use of random allocation.[7,8,10–12] Six trials described the concealment of patient allocation to different treatment groups.[7,10–12] Two trials reported the double blinding of participants and personnel,[10,11] whereas 2 trials reported only the blinding of patients.[7] One trial reported only the blinding of investigators who assessed outcomes.[8] Three studies conducted their analyses according to the ITT principle.[8,10,11] Loss to follow-up was acceptable (<20%) in all the studies, except in Scheinkestel et al,[10] in which the rate of loss to follow-up was 38%. The outcomes of neuropsychological sequelae were assessed 1 month after CO poisoning, except in 1 study that assessed the outcomes at 2 hours, 12 hours, and 21 days after HBO treatment.[8]
Table 2.
Methodological quality assessment of included trial.
3.2. Incidence of neuropsychological sequelae
3.2.1. HBO versus NBO treatment
Commonly reported neuropsychological sequelae included headache, memory impairment, difficulty concentrating, and disturbed sleep. Compared with patients treated with NBO, a lower percentage of patients treated with HBO reported headache (16.2% vs 16.5%, relative risk [RR] = 0.83, 95% CI = 0.38–1.80), memory impairment (18.2% vs 23.8%, RR = 0.80, 95% CI = 0.43–1.49), difficulty concentrating (15.0% vs 18.4%, RR = 0.86, 95% CI = 0.55–1.34), and disturbed sleep (14.7% vs 16.2%, RR = 0.91, 95% CI = 0.59–1.39). Moreover, a lower percentage of patients treated with HBO experienced delayed neurological sequelae (25.0% vs 31.1%, RR = 0.35, 95% CI = 0.02–5.97). Therefore, HBO therapy in CO poisoning patients had lower risk in neuropsychological sequelae including headache, memory impairment, difficulty concentrating, disturbed sleep and delayed neurological sequelae compared to those with NBO therapy (Fig. 2).
Figure 2.
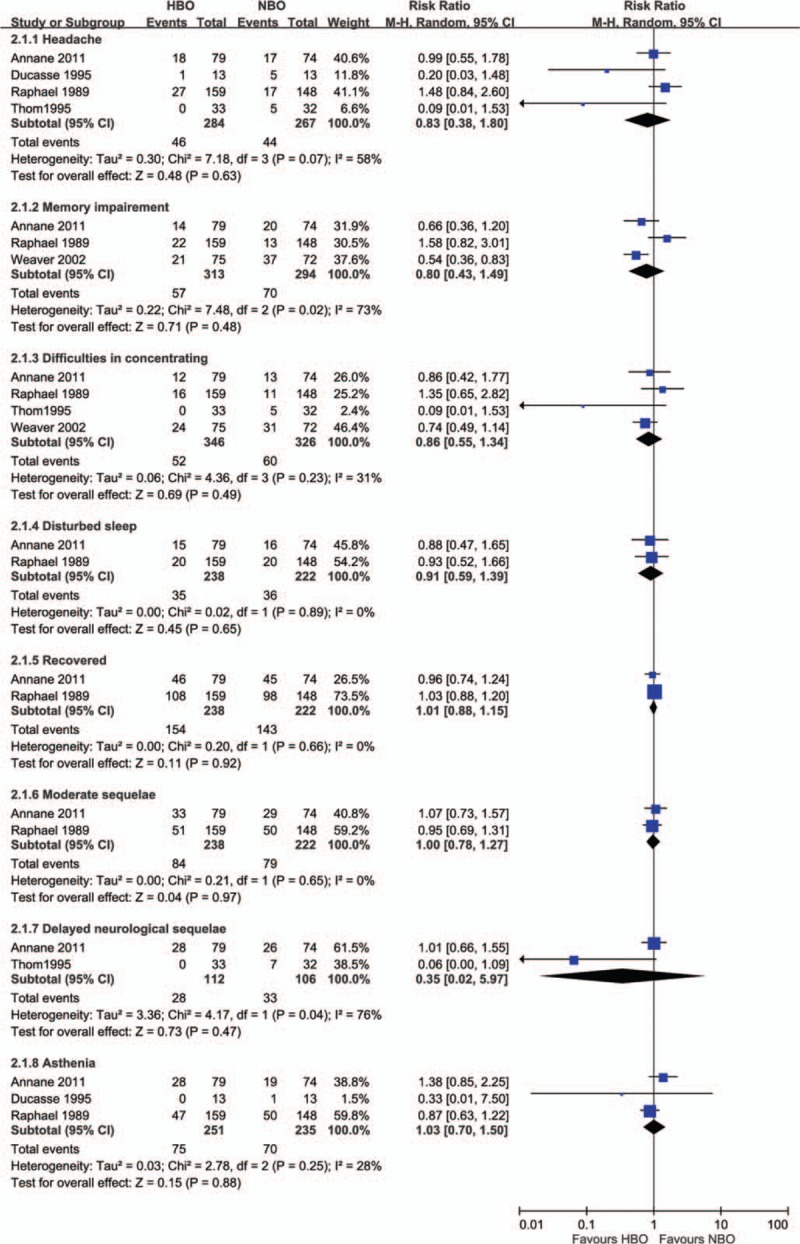
Forest plot for comparison of hyperbaric oxygen versus normobaric oxygen. Outcome: incidence of neuropsychological sequelae.
3.3. Two sessions versus one session of HBO treatment
Two trials that included a total of 492 patients compared 2 different HBO protocols in patients with CO poisoning who had initial loss of consciousness or were in coma.[7,12] The intervention group received NBO treatment for 4 hours and 2 sessions of HBO treatment (2.0 ATA, 2 hours) within an interval of 6 to 12 hours, and the control group received NBO treatment for 4 hours and one session of HBO treatment (2.0 ATA, 2 hours). Two sessions of HBO treatment exhibited no advantage over one session. Memory impairment (8.9% vs 18.3%, RR = 0.49, 95% CI = 0.30–0.80) and difficulty concentrating (6.1% vs 13.4%, RR = 0.46, 95% CI = 0.24–0.89) were significantly lower in the group that received one session of HBO treatment. Compared with patients who received 2 sessions of HBO treatment, a lower percentage of patients who received 1 session of HBO treatment experienced asthenia (28.5% vs 34.1%, RR = 0.78, 95% CI = 0.43–1.42), headache (16.3% vs 20.7%, RR = 0.77, 95% CI = 0.45–1.30), disturbed sleep (13.8% vs 16.7%, RR = 0.83, 95% CI = 0.55–1.27), behavioral impairment (6.9% vs 8.1%, RR = 0.81, 95% CI = 0.34–1.93), and moderate sequelae (37.8% vs 43.5%, RR = 0.82, 95% CI = 0.50–1.33). There is no significant benefit of neuropsychological sequence in CO poisoning patients with 2 session HBO treatment compared to one session treatment (Fig. 3).
Figure 3.
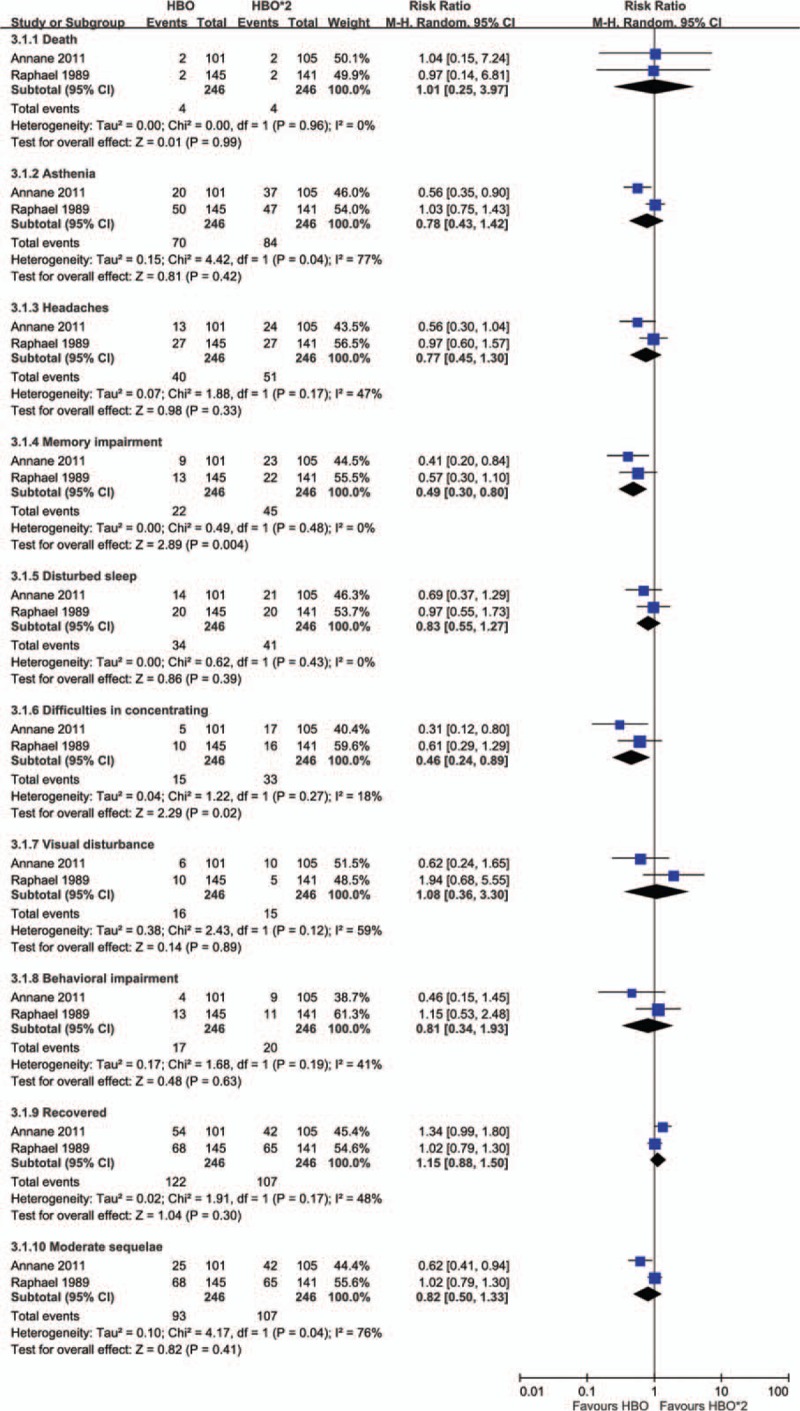
Forest plot for comparison of 2 sessions versus one session of hyperbaric oxygen treatment. Outcome: incidence of neuropsychological sequelae.
4. Discussion
4.1. Summary
This systematic review assessed the evidence from 6 RCTs reported between 1989 and 2010. The meta-analysis indicated that compared with patients treated with NBO, HBO treated patients have a lower incidence of neuropsychological sequelae, including headache, memory impairment, difficulty concentrating, disturbed sleep, and delayed neurological sequelae. Although the summarized effects of HBO on neuropsychological sequelae were not statistical significance, there is a trend of decreased proportion of neuropsychological sequelae after HBO therapy. Raphael et al and Annane et al compared neuropsychological sequelae in patients with more severe CO poisoning who had initial loss of consciousness or were in coma and were administered 1 or 2 sessions of HBO treatment.[7,12] Both studies reported that 2 sessions of HBO treatment had no advantage over 1 session. In the present meta-analysis, memory impairment (8.9% vs 18.3%) and difficulty concentrating (6.1% vs 13.4%) were significantly lower in the group that received one session of HBO treatment than in the group that received 2 sessions. However, these studies had relatively short follow-up durations and high percentage of loss of follow-up.
4.2. Strengths and limitations
The strengths of this review include the comprehensive search for eligible studies, systematic and explicit application of eligibility criteria, careful consideration of study quality, and a rigorous analytical approach. However, all meta-analyses are prone to certain limitations, some of which were evident in the present study. First, despite the comprehensive search strategy, the possibility of publication bias remains. Second, the sample sizes of the included studies were small, ranging from 13 to 173 patients per group, and high-quality data from RCTs were insufficient. Third, it is impossible to demonstrate the positive effects of HBO therapy in those with CO poisoning on mechanical ventilation. Scheinkestel et al reported no benefit of HBO therapy but the measurements of neuropsychological performance cannot be summarized with other studies. All the reviewed trials exhibited inadequate methodological rigor, as indicated by their descriptions of double blinding being unclear or nonexistent (Table 2).
The reviewed studies were highly heterogeneous, as demonstrated by the I2 value of 50%. However, the published RCTs were not in complete agreement, and their results were inconsistent. This could have resulted from heterogeneity in patient demographics and characteristics, study methods, inclusion and exclusion criteria, treatment durations, and HBO treatment protocols.
4.3. Comparison with existing literature
A delayed onset of neuropsychiatric symptoms has been reported 3 to 240 days after exposure to CO. The reported incidence of neuropsychiatric symptoms varies widely, and these symptoms are estimated to occur in 10% to 30% of patients with CO poisoning.[13,14] Our previous study indicated that the incidence rate of dementia was 26.15 per 10,000 person-years in patients with CO poisoning in Taiwan.[15] Lin et al[16] also reported the overall incidence of ischemic stroke was 2.5-fold greater for those with CO exposure. However, the underlying mechanisms remain unclear. Direct hypoxic effects, subsequent oxidative stress, and inflammatory responses leading to oxidative injury and damage of the vascular endothelium due to peroxynitrate deposition, excitotoxicity, and apoptosis have been linked to central nervous system damage.[17,18] The microvascular injury may exacerbate the progress of atherosclerosis and increased the risk of stroke.[19] Magnetic resonance imaging of the brain may reveal imaging abnormalities, including increased numbers of T2-weighted hyperintensities, basal-ganglia lesions, and atrophy of the hippocampi after CO poisoning, which are associated with an increased risk of early cognitive decline.[19–21] Utilizing HBO therapy to treat delayed neurological symptoms (DNS) patients is reasonable, because HBO improves the pathogeneses of DNS including decreasing oxidative stress, especially lipid peroxidation caused by tissue hypoxia and the resulting cascade of inflammatory changes.[22–24] Thom et al[25] also reported HBO could improve post-ischemic/inflammatory tissue survival by increasing reactive species to temporarily inhibit β2-integrin function of neutrophils as well as inducing antioxidant enzymes and anti-inflammatory proteins in many tissues.
Sources of harm such as fire, claustrophobia, barotraumas (including rupture of the tympanic membrane), sinus damage, pneumothorax, hyperoxic seizures, and gas emboli have been associated with HBO treatment.[5,6,26] Because of the limited availability of hyperbaric centers, lack of access to immediate medical care while in the hyperbaric chamber, and long-distance transfers, determining whether these harms outweigh the potential benefits of HBO treatment is difficult.
4.4. Implications for research and practice
CO elimination is related to minute ventilation, the duration of exposure, and the fraction of inspired oxygen. HBO treatment significantly reduces the half-life of carboxyhemoglobin.[27] Animal studies have suggested that HBO treatment exerts beneficial effects on brain cells traumatized by CO, including a reduction in lipid peroxidation, endothelial leukocyte migration, and other post-hypoxic events.[22,28] However, whether HBO treatment improves the prognosis or outcomes of patients with CO poisoning who have persistent or delayed neurological sequelae remains unclear, because different studies have reported conflicting conclusions. HBO therapy is especially advised for patients with transient or prolonged episodes of loss of consciousness, abnormal neurological signs, cardiovascular dysfunction, severe acidosis, pregnant women, when exposed for more than 24 hours, or those who have COHb levels of 25% or more.[29] The cost of one session of HBO treatment was found to be lower than that of 2 sessions. One session of HBO treatment may therefore be an economical option for patients with CO poisoning with high severity.
5. Conclusion
The results of the present meta-analysis indicated that the high incidence of neuropsychological sequelae after CO poisoning and HBO treatment may play a crucial role in lowering the occurrence of these sequelae. However, the advantages and disadvantages of using HBO to treat CO poisoning remain unclear. One session of HBO treatment significantly reduced neuropsychological sequelae in patients with severe CO poisoning who had initial loss of consciousness or were in coma. Therefore, one session of HBO treatment could be an economical option for patients with CO poisoning with high severity.
Author contributions
Conceptualization: Wei-Haiang Su, Jiann-Ruey Ong, Mei-Yi Wu, Chung-Shun Wong.
Data curation: Chun-Hung Lin.
Formal analysis: Chun-Hung Lin, Ying-Chun Chen, Po-Hao Feng, Mei-Yi Wu.
Investigation: Wei-Haiang Su, Ying-Chun Chen, Po-Hao Feng, Mei-Yi Wu.
Methodology: Chun-Hung Lin, Wei-Haiang Su, Ying-Chun Chen.
Project administration: Po-Hao Feng, Jiann-Ruey Ong.
Resources: Ying-Chun Chen, Po-Hao Feng.
Software: Wei-Haiang Su, Po-Hao Feng, Wan-Chen Shen.
Supervision: Jiann-Ruey Ong, Mei-Yi Wu, Chung-Shun Wong.
Validation: Po-Hao Feng, Wan-Chen Shen, Mei-Yi Wu.
Visualization: Wan-Chen Shen, Jiann-Ruey Ong, Mei-Yi Wu, Chung-Shun Wong.
Writing – original draft: Chun-Hung Lin.
Writing – review & editing: Mei-Yi Wu, Chung-Shun Wong.
Footnotes
Abbreviations: ATA = atmospheres absolute, CI = confidence intervals, CO = carbon monoxide, DNS = delayed neurological symptoms, HBO = hyperbaric oxygen, HBOT = hyperbaric oxygen therapy, ITT = intention-to-treat, NBO = normobaric oxygen, RCT = randomized controlled trials, RR = relative risk.
MYW and CSW contributed equally to this work.
The authors have no funding and no conflicts of interest to disclose.
The author(s) of this work have nothing to disclose.
References
- [1].Shie HG, Li CY. Population-based case-control study of risk factors for unintentional mortality from carbon monoxide poisoning in Taiwan. Inhal Toxicol 2007;19:905–12. [DOI] [PubMed] [Google Scholar]
- [2].Norris CR, Jr, Trench JM, Hook R. Delayed carbon monoxide encephalopathy: clinical and research implications. J Clin Psychiatry 1982;43:294–5. [PubMed] [Google Scholar]
- [3].Weaver LK, Hopkins RO, Elliott G. Carbon monoxide poisoning. N Engl J Med 1999;340:1290author reply 1292. [DOI] [PubMed] [Google Scholar]
- [4].Jay GD, McKindley DS. Alterations in pharmacokinetics of carboxyhemoglobin produced by oxygen under pressure. Undersea Hyperb Med 1997;24:165–73. [PubMed] [Google Scholar]
- [5].Hampson NB, Simonson SG, Kramer CC, et al. Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning. Undersea Hyperb Med 1996;23:215–9. [PubMed] [Google Scholar]
- [6].Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med 1996;334:1642–8. [DOI] [PubMed] [Google Scholar]
- [7].Raphael JC, Elkharrat D, Jars-Guincestre MC, et al. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet 1989;2:414–9. [DOI] [PubMed] [Google Scholar]
- [8].Ducasse JL, Celsis P, Marc-Vergnes JP. Non-comatose patients with acute carbon monoxide poisoning: hyperbaric or normobaric oxygenation? Undersea Hyperb Med 1995;22:9–15. [PubMed] [Google Scholar]
- [9].Thom SR, Taber RL, Mendiguren II, et al. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med 1995;25:474–80. [DOI] [PubMed] [Google Scholar]
- [10].Scheinkestel CD, Bailey M, Myles PS, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J Aust 1999;170:203–10. [DOI] [PubMed] [Google Scholar]
- [11].Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002;347:1057–67. [DOI] [PubMed] [Google Scholar]
- [12].Annane D, Chadda K, Gajdos P, et al. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: two randomized controlled trials. Intensive Care Med 2011;37:486–92. [DOI] [PubMed] [Google Scholar]
- [13].Sawa GM, Watson CP, Terbrugge K, et al. Delayed encephalopathy following carbon monoxide intoxication. Can J Neurol Sci 1981;8:77–9. [DOI] [PubMed] [Google Scholar]
- [14].Hart IK, Kennedy PG, Adams JH, et al. Neurological manifestation of carbon monoxide poisoning. Postgrad Med J 1988;64:213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong CS, Lin YC, Hong LY, et al. Increased long-term risk of dementia in patients with carbon monoxide poisoning: a population-based study. Medicine (Baltimore) 2016;95:e2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin CW, Chen WK, Hung DZ, et al. Association between ischemic stroke and carbon monoxide poisoning: a population-based retrospective cohort analysis. Eur J Intern Med 2016;29:65–70. [DOI] [PubMed] [Google Scholar]
- [17].Thom SR. Leukocytes in carbon monoxide-mediated brain oxidative injury. Toxicol Appl Pharmacol 1993;123:234–47. [DOI] [PubMed] [Google Scholar]
- [18].Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med 2009;360:1217–25. [DOI] [PubMed] [Google Scholar]
- [19].Parkinson RB, Hopkins RO, Cleavinger HB, et al. White matter hyperintensities and neuropsychological outcome following carbon monoxide poisoning. Neurology 2002;58:1525–32. [DOI] [PubMed] [Google Scholar]
- [20].Gale SD, Hopkins RO, Weaver LK, et al. MRI, quantitative MRI, SPECT, and neuropsychological findings following carbon monoxide poisoning. Brain Inj 1999;13:229–43. [DOI] [PubMed] [Google Scholar]
- [21].Pulsipher DT, Hopkins RO, Weaver LK. Basal ganglia volumes following CO poisoning: a prospective longitudinal study. Undersea Hyperb Med 2006;33:245–56. [PubMed] [Google Scholar]
- [22].Thom SR, Bhopale VM, Fisher D, et al. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci U S A 2004;101:13660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thom SR. Antagonism of carbon monoxide-mediated brain lipid peroxidation by hyperbaric oxygen. Toxicol Appl Pharmacol 1990;105:340–4. [DOI] [PubMed] [Google Scholar]
- [24].Lo CP, Chen SY, Chou MC, et al. Diffusion-tensor MR imaging for evaluation of the efficacy of hyperbaric oxygen therapy in patients with delayed neuropsychiatric syndrome caused by carbon monoxide inhalation. Eur J Neurol 2007;14:777–82. [DOI] [PubMed] [Google Scholar]
- [25].Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985) 2009;106:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sheffield PJ, Desautels DA. Hyperbaric and hypobaric chamber fires: a 73-year analysis. Undersea Hyperb Med 1997;24:153–64. [PubMed] [Google Scholar]
- [27].Pace N, Strajman E, Walker EL. Acceleration of carbon monoxide elimination in man by high pressure oxygen. Science 1950;111:652–4. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest 1992;90:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Betterman K, Patel S. Neurologic complications of carbon monoxide intoxication. Handb Clin Neurol 2014;120:971–9. [DOI] [PubMed] [Google Scholar]


