Supplemental Digital Content is available in the text
Keywords: coronary artery disease, disease severity, meta-analysis, neutrophil-to-lymphocyte ratio
Abstract
This study aimed to evaluate the association between blood neutrophil-to-lymphocyte ratio (NLR) and severity of coronary artery disease (CAD), and investigate the diagnostic ability and optimal cut-off value of NLR in predicting severe stenosis in CAD.
A systematic search was conducted in public databases to identify all relevant studies. Weighted mean difference (MD) and 95% confidence interval (CI) were pooled for continuous univariate data, and odds ratios (OR) and 95% CI were calculated for dichotomous multivariate data.
Seventeen studies were included in this meta-analysis with a total of 7017 CAD cases. For continuous univariate data, the cases with the highest stenosis category had a significantly higher NLR level than those with lowest stenosis category (MD: 1.57, 95% CI: 1.06–2.09; n = 17). After further classification according to the Gensini or SYNTAX score, the cases with severe stenosis demonstrated a higher NLR than those with mild stenosis (MD: 2.33, 95% CI: 1.22–3.43; n = 6) and moderate stenosis (MD: 1.92, 95% CI: 0.80–3.04; n = 6). Compared with mild stenosis, NLR was also higher in those with moderate-to-severe stenosis (MD: 1.34, 95% CI: 0.77–1.92; n = 6) and moderate stenosis (MD: 0.52, 95% CI: 0.36–0.68; n = 6). For dichotomous multivariate data, high NLR levels were recognized as an independent predictor for severe stenosis in CAD (OR: 1.50, 95% CI: 1.32–1.72; n = 11). NLR showed a diagnostic ability in predicting severe stenosis in CAD (area under receiver operating characteristics [ROC] curve [AUC]: 0.66, 95% CI: 0.64–0.68; n = 8), with the cut-off ranging from 1.95 to 3.97. Subgroup analysis and sensitivity analysis showed the results were robust. Begg's test detected no significant publication biases.
This study suggested that high blood NLR was associated with the severity of CAD, and it might be useful for predicting severe stenosis in CAD.
1. Introduction
Coronary artery disease (CAD) is recognized to be a global health threat, which accounts for a high proportion of mortality worldwide. As a complex inflammatory disease, atherosclerosis plays an important role in the onset and progression of CAD and its complications.[1] It has been found that elevated levels of inflammatory biomarkers are associated with increasing rates of cardiac events in CAD patients.[2] White blood cell (WBC) and its subtypes have been addressed in association with cardiovascular risk. Increased neutrophil count was shown to be associated with the presence and severity of coronary atherosclerosis.[3] High neutrophil levels could increase blood viscosity and hypercoagulability, interact with platelets and endothelium, and induce microvascular injury and reperfusion injury.[4–6] As a representative indicator of inflammation, a high neutrophil-to-lymphocyte ratio (NLR) was recognized as an independent risk factor for the progression of atheromatous plaque lesions, in-stent re-stenosis, cardiac death after percutaneous coronary intervention or coronary artery bypass surgery, and incidence of cardiac events in acute coronary syndrome (ACS).[7,8]
In recent years, several studies have investigated the association between NLR and CAD. However, most studies focused on the role of NLR in diagnosing CAD, instead of disease severity. Second, few studies failed to adjust the result by multivariate analysis and provide the optimal cut-off value of NLR in predicting severe CAD for further clinical practice. Thus, we conducted this meta-analysis to evaluate the association between NLR and CAD severity using both univariate and multivariate data, and then investigate the diagnostic ability and optimal cut-off value of NLR in predicting severe stenosis in CAD.
2. Materials and methods
2.1. Search strategy
The databases of PubMed, China Wanfang Database, China SinoMed Database and China Knowledge Resource Integrated Database (CNKI) were searched for relevant studies published up to June 6th, 2018, using the keywords including: “neutrophil” AND “lymphocyte” AND (“coronary” OR “coronary artery” OR “coronary atherosclerosis” OR “heart disease”). Studies in languages other than English or Chinese were excluded. Moreover, we also reviewed the references of related studies and reviews for undetected studies. This study was approved by the ethics committee of Affiliated Heping Hospital of Changzhi Medical College.
2.2. Study selection and exclusion
Two authors (XL and YJ) reviewed the studies independently. The inclusion criteria were as follows: focused on CAD patients; patients were divided into 2 or more groups according to the coronary stenosis severity determined by Gensini or SYNTAX scoring system; The NLR in each group was presented as mean ± standard error (SD) or median (interquartile range [IQR]). The exclusion criteria were as follows: abstracts without full texts, reviews, case reports, and animal studies.
2.3. Data extraction and quality assessment
Two authors (JK and NF) extracted the data by a standardized collection form. All differences were resolved by discussion. In each study, the following information was extracted: first author, publication year, study area, disease type, total cases, scoring system, groups divided by Gensini or SYNTAX score, number of cases per group, number of males per group, average age per group, average NLR per group, effect size, area under ROC curve, and optimal cut-off value. For studies from the same area, we also reviewed the medical center and investigating time to remove duplicate publications. The Newcastle–Ottawa scale (NOS) was used to assess the methodological quality of included studies.[9]
2.4. Statistical analysis
For continuous univariate data, weighted mean differences (MD) and 95% confidence intervals (CI) were pooled by the Inverse Variance method to evaluate the association between NLR levels and CAD severity. If the NLR level was presented as median and interquartile range (IQR), we regarded the median as the mean level, and converted IQR to standard error (SD) by dividing it by 1.35.[10] For dichotomous multivariate data, odds ratios (OR) and 95% CI were pooled by the Mantel–Haenszel method to evaluate the association between NLR levels and severe stenosis in CAD, and the same method was also adopted in integrating the area under receiver operating characteristics (ROC) curve (AUC) and 95% CI.[11] The heterogeneity among studies was estimated by Q test and I2 statistic.[12]I2 > 50% represented substantial heterogeneity, and the summary estimate was analyzed by a random-effects model. Otherwise, a fixed-effects model was applied. Sensitivity analysis was performed to estimate the stability of the meta-analysis by omitting one study at a time during repeated analyses. Subgroup analysis was conducted to assess the effect of confounding factors on the primary result. Publication bias was assessed by using funnel plots and Begg's test. Furthermore, we also conducted meta-regression analysis to evaluate the effect of NLR cut-off values in predicting severe stenosis in CAD.
Statistical analyses were performed using Review Manager 5.2 (RevMan, The Nordic Cochrane Center, The Cochrane Collaboration, 2012), and Begg's test meta-regression analysis was realized with software STATA version 12.0 (StataCorp LP, College Station, TX). P values < .05 were considered statistically significant.
3. Results
3.1. Study characteristics
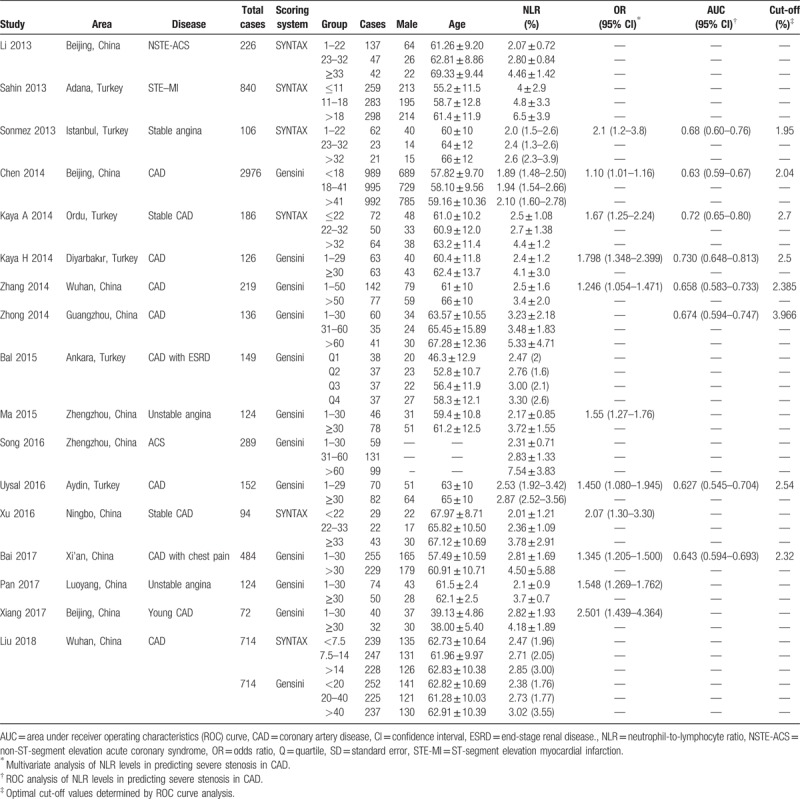
The search strategy resulted in 586 records: 179 from PubMed, 178 from Wanfang Database, 173 from SinoMed, and 56 from CNKI (Fig. 1). After excluding duplicated and irrelevant records, 17 studies were included in this meta-analysis with a total of 7017 CAD cases (Table 1).[13–29] Eleven studies were conducted in China, and the remaining 6 were performed in Turkey. The medical center and investigating time were reviewed in each included study, and no studies were duplicated. Twelve studies evaluated the severity by Gensini score, while 6 chose SYNTAX score. Liu et al study adopted both scores. Five studies presented NLR levels in the form of median (IQR). Eleven studies investigated the association by multivariate analysis, and eight studies reported the optimal cut-off value of NLR in predicting severe stenosis by ROC analysis. In quality assessment, the included studies had an average score of 7.53 (Table S1).
Figure 1.
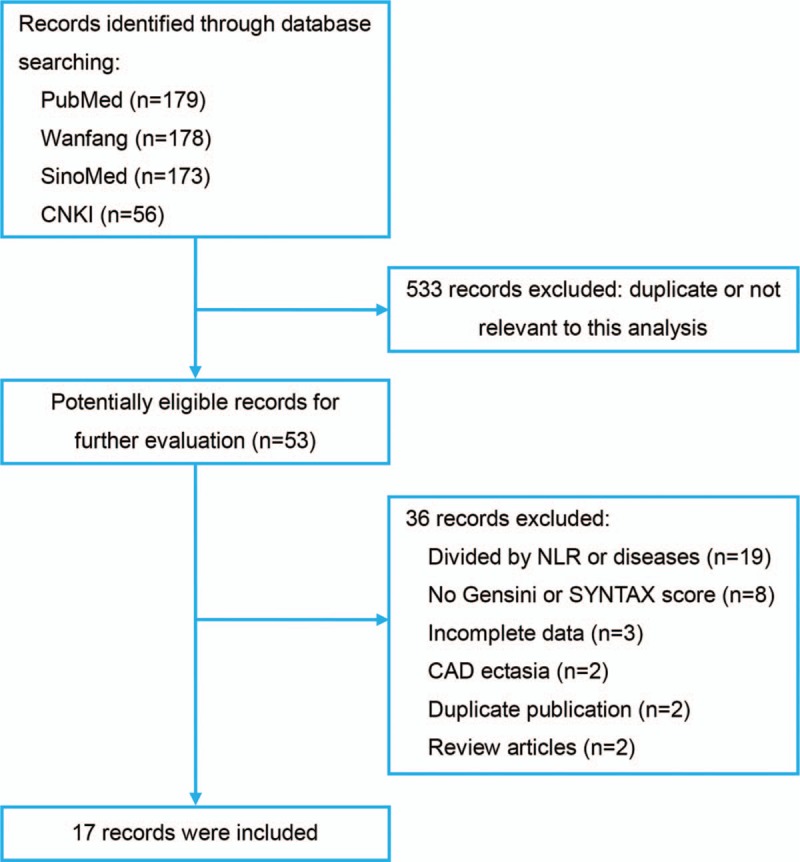
Flowchart of literature search.
Table 1.
Characteristics of included studies.
3.2. NLR levels and CAD severity (the highest stenosis category vs the lowest stenosis category)
All studies divided the cases into groups of different CAD severity according to the Gensini or SYNTAX score. The cases with the highest stenosis category had a significantly higher NLR level than those with lowest stenosis category (MD: 1.57, 95% CI: 1.06–2.09; I2 = 97%) (Fig. 2). Sensitivity analysis showed the result was robust. Begg's test detected no significant publication bias (P = .077, z = 1.77) (Fig. S1).
Figure 2.
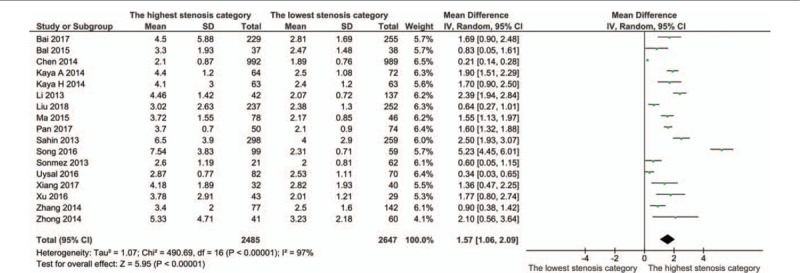
Meta-analysis of continuous univariate data on neutrophil-to-lymphocyte ratio and severity of coronary artery disease (the highest stenosis category vs the lowest stenosis category).
3.3. NLR levels and different CAD severity
The cases were divided into groups by Gensini or SYNTAX score, namely mild stenosis (Gensini score 1–30 or SYNTAX score 1–22), mild-to-moderate stenosis (Gensini score 1–60 or SYNTAX score 1–32), moderate stenosis (Gensini score 31–60 or SYNTAX score 23–32), moderate-to-severe stenosis (Gensini score > 30 or SYNTAX score > 22), and severe stenosis (Gensini score > 60 or SYNTAX score > 32). The cases with severe stenosis had a higher NLR level than those with mild stenosis (MD: 2.33, 95% CI: 1.22–3.43; n = 6, I2 = 95%; P for Begg's test = .452, z = 0.75) (Fig. 3; Fig. S2). The cases with moderate-to-severe stenosis had a higher NLR level than those with mild stenosis (MD: 1.34, 95% CI: 0.77–1.92; n = 6, I2 = 88%; P for Begg's test = .133, z = 1.50) (Fig. 4; Fig. S3). The cases with moderate stenosis had a higher NLR level than those with mild stenosis (MD: 0.52, 95% CI: 0.36–0.68; n = 6, I2 = 4%; P for Begg's test = 1.000, z = 0.00) (Fig. 5; Fig. S4). The cases with severe stenosis had a higher NLR level than those with moderate stenosis (MD: 1.92, 95% CI: 0.80–3.04; n = 6, I2 = 94%; P for Begg's test = .133, z = 1.50) (Fig. 6; Fig. S5). Sensitivity analysis showed the result was robust in each meta-analysis.
Figure 3.
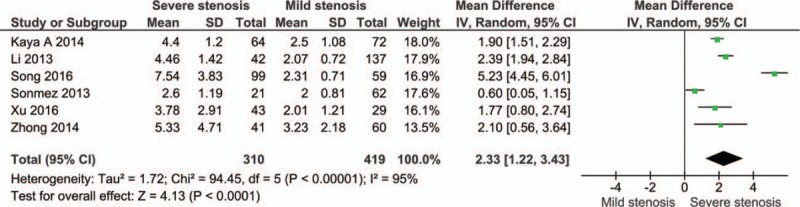
Meta-analysis of continuous univariate data on neutrophil-to-lymphocyte ratio and severity of coronary artery disease (severe stenosis vs mild stenosis).
Figure 4.
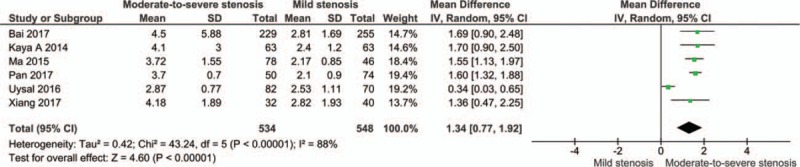
Meta-analysis of continuous univariate data on neutrophil-to-lymphocyte ratio and severity of coronary artery disease (moderate-to-severe stenosis vs mild stenosis).
Figure 5.
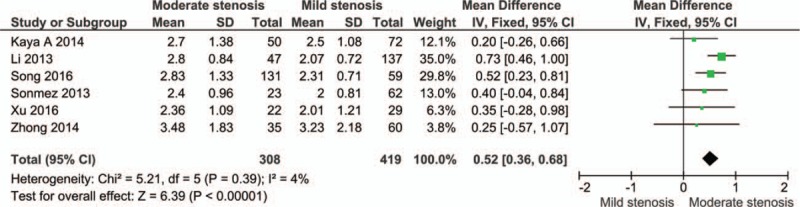
Meta-analysis of continuous univariate data on neutrophil-to-lymphocyte ratio and severity of coronary artery disease (moderate stenosis vs mild stenosis).
Figure 6.
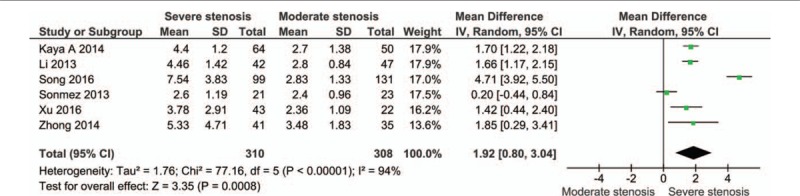
Meta-analysis of continuous univariate data on neutrophil-to-lymphocyte ratio and severity of coronary artery disease (severe stenosis vs moderate stenosis).
3.4. NLR levels and severe stenosis in CAD (based on dichotomous multivariate data)
Eleven studies conducted multivariate analyses of the association between NLR levels and severe stenosis in CAD. It was found that high NLR levels were related with severe stenosis in CAD (OR: 1.50, 95% CI: 1.32–1.72; I2 = 81%) (Fig. 7). Sensitivity analysis showed the result was robust. Begg's test detected no significant publication bias (P = .640, z = 0.47) (Fig. S6).
Figure 7.
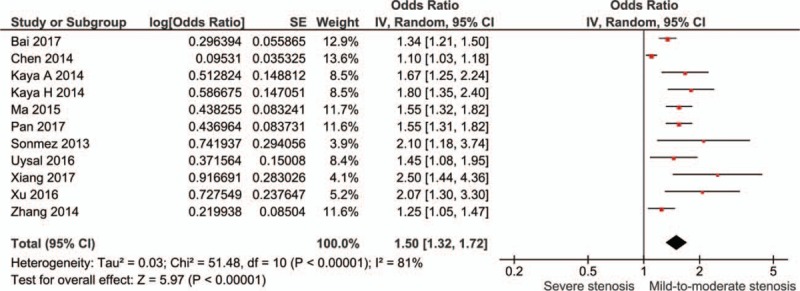
Meta-analysis of dichotomous multivariate data on neutrophil-to-lymphocyte ratio and severe stenosis in coronary artery disease.
3.5. Diagnostic ability of NLR levels in predicting severe stenosis in CAD
Eight studies evaluated the ability of NLR levels in predicting severe stenosis in CAD, and the pool AUC was 0.66 (95% CI: 0.64–0.68; I2 = 24%). Sensitivity analysis showed the result was robust. Begg's test detected no significant publication bias (P = .711, z = 0.37) (Fig. 8; Fig. S7).
Figure 8.
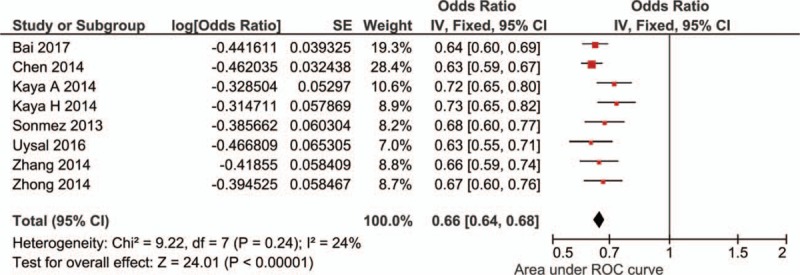
Meta-analysis of diagnostic ability of neutrophil-to-lymphocyte ratio in predicting severe stenosis in coronary artery disease.
3.6. The effect of NLR cut-off values in predicting severe stenosis in CAD
Eight studies reported the results of ROC analysis and the corresponding optimal cut-off value of NLR in predicting severe stenosis in CAD. Meta-regression analysis showed the cut-off values between 1.95 and 3.97 made no significant effect on the diagnostic ability (OR: 1.03, 95% CI: 0.94–1.13; P = .449) (Fig. 9).
Figure 9.
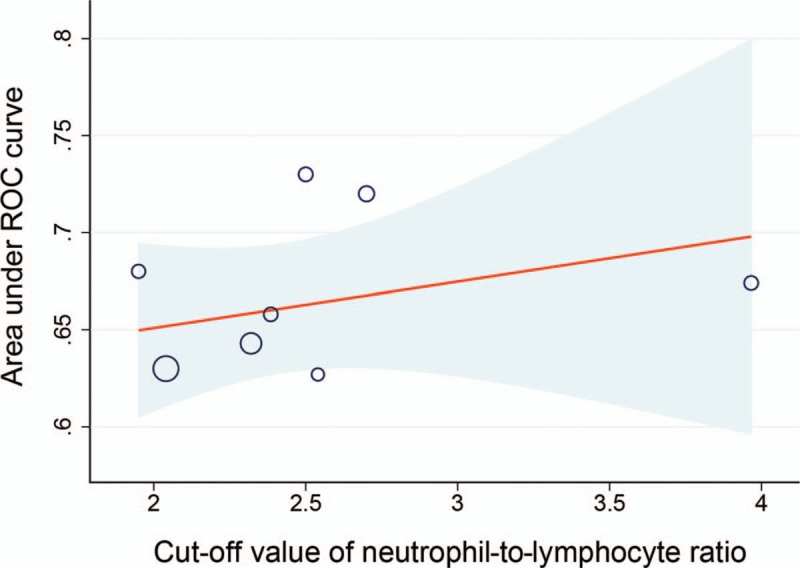
Meta-regression analysis of cut-off values of neutrophil-to-lymphocyte ratio in predicting severe stenosis in coronary artery disease.
3.7. Subgroup analysis
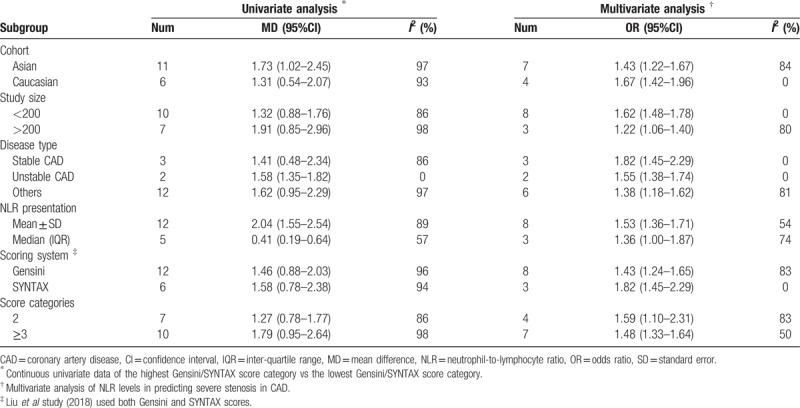
Subgroup analysis was conducted on the meta-analyses of both continuous univariate data and dichotomous multivariate data to evaluate the effect of confounding factors, including cohort, study size, disease type, NLR presentation, scoring system, and categories. No substantial changes of the primary result were found between subgroups (Table 2).
Table 2.
Subgroup analysis of neutrophil-to-lymphocyte ratio and severity of coronary artery disease.
4. Discussion
Currently, 2 scoring systems of Gensini and SYNTAX basing on coronary angiography were usually used to determine the severity of coronary artery stenosis.[30,31] The Gensini score system defines narrowing of the lumen of the coronary arteries as follows: 1 point for <25% stenosis, 2 for 26% to 50%, 4 for 51% to 75%, 8 for 76% to 90%, 16 for 91% to 99%, and 32 for total occlusion. Then, the score is multiplied by a factor representing the importance of the lesion's location in the coronary artery system. For the location scores, 5 points were given for left main lesion; 2.5 for proximal left anterior descending (LAD) or left circumflex (LCX) artery; 1.5 for the mid-segment LAD and LCX; 1 for the distal segment of LAD and LCX, first diagonal branch, first obtuse marginal branch, right coronary artery, posterior descending artery, and intermediate artery; 0.5 for the second diagonal and second obtuse marginal branches. CAD cases were usually divided into groups of different severity according to the Gensini score: low stenosis (1–30), moderate stenosis (31–60) and severe stenosis (>60).
The SYNTAX score is also an angiographic tool used in grading the severity of CAD. Each coronary lesion with a diameter stenosis > 50% in vessels > 1.5 mm is scored separately and added together to provide the cumulative score which is prospectively calculated using the score algorithm on the baseline diagnostic angiogram. Cases with a score of 1 to 22 were usually regarded as mild stenosis, while 23 to 32 for moderate stenosis and > 32 for severe stenosis.
In the present study, we demonstrated the independent association between NLR levels and CAD severity. Some studies divided the cases into different severity groups according to the tertiles of the Gensini or SYNTAX score. The cases with the highest category had a higher NLR levels than those with the lowest category. When adopting the same classification criteria, the NLR levels still increased with the disease severity. As for multivariate data, the association existed. NLR showed a diagnostic ability in predicting severe stenosis in CAD (AUC: 0.66, 95% CI: 0.64–0.68), with the cut-off ranging from 1.95 to 3.97. We thought NLR might be a useful biomarker in predicting CAD severity. For the CAD cases with a NLR level of more than 1.95 to 3.97, physicians should pay more attention, especially after exclusion of infectious diseases.
Several studies also evaluated the association using different criteria to classify the CAD severity. In Arbel et al study (n = 3005), CAD severity was divided into 4 categories according to the number of diseased vessels (0, 1, 2, 3), and the patients were divided into 3 groups according to the NLR value (<2, 2–3 and > 3).[32] Patients with NLR > 3 had more advanced obstructive CAD (P<.001) and worse prognosis, with a higher rate of major CVD events during up to 3 years of follow-up (P = .01). In the studies of both Iranirad et al (n = 500) and Datta et al (n = 110), patients grouped by NLR levels had a significant different distribution in the number of diseased coronary vessels.[33,34] In Demir et al[35] study, NLR levels were significantly higher in the group of chronic coronary total occlusion (n = 75) than in the group with coronary stenosis > 50% (n = 75) (P<.001). In Ates et al[36] study (n = 684), NLR was found to be an independent predictor of critical coronary plaques detected by multi-detector computed tomography (MDCT) (P<.001). The criteria based on the number of diseased vessels or maximum stenosis in single vessels could not reflect the disease severity systematically. However, the 2 most popular criteria of Gensini score and SYNTAX score considered the effects of lesion location, stenosis in single vessels and the number of diseased vessels, which could assess the CAD severity systematically and quantitatively. Thus, we adopted studies with these 2 criteria, and the meta-analysis showed a consistent result with the studies using other criteria, indicating that high blood NLR was associated with the CAD severity. Furthermore, NLR was also reported with the ability in diagnosing CAD.[37–39]
The inflammatory process played a key role in pathogenesis of atherosclerosis, and multiple studies have demonstrated a strong correlation between various inflammatory biomarkers and CAD.[40] Furthermore, NLR is a combination of 2 independent inflammatory biomarkers.[41] Neutrophils could reflect the ongoing nonspecific inflammation, and lymphocytes acted as a marker of the regulatory pathway. A higher NLR level suggested a higher inflammatory level.[42] Thus, NLR could reveal more information that was not evident from the total leukocyte count. The NLR was also associated with arterial stiffness and coronary calcium score.[43] Unlike other inflammatory biomarkers and bioassays, NLR was an inexpensive and easily available marker that provided an additional level of risk scores in predicting the severity of coronary artery stenosis. NLR levels were also associated with the severity of multiple infectious and inflammatory diseases.[44–46] This indicated NLR might not be a specific biomarker in the diagnosis of certain diseases, but it was a good indicator of disease severity. In other words, it might be more meaningful among the patients who have been diagnosed as CAD. After excluding the etiology of infection, inflammation and cancers, physicians should pay more attention to the CAD patients with high NLR levels.[47,48]
This meta-analysis had several strengths. First, to the best of our knowledge, this is the first meta-analysis to evaluate the association between NLR and CAD severity. Second, the estimates based on univariate and multivariate data were pooled respectively, which made the results more reliable. Third, AUC was also pooled to evaluate the diagnostic ability of NLR in predicting severe stenosis in CAD, and the effect of cut-off values was also considered. Fourth, sensitivity analysis and Bgger’ test were conducted to estimate the stability of pooled results and potential publication bias. However, several limitations in this study should be considered. First, the number of cases and controls in part studies was relatively small. Second, the obvious heterogeneity between studies was observed. For this, we conducted both sensitivity analysis and subgroup analysis to evaluate the stability of pooled results.
5. Conclusions
In conclusion, this study suggested that high-blood NLR was associated with the severity of CAD, and it might be useful for predicting severe stenosis in CAD.
Author contributions
Data curation: Xiaoli Li, Jinhua Kang, Ning Fang.
Formal analysis: Xiaoli Li, Jinhua Kang, Ning Fang.
Methodology: Xiaoli Li, Jinhua Kang, Ning Fang.
Software: Yanli Ji.
Supervision: Jinhua Kang, Ning Fang.
Visualization: Yanli Ji.
Writing – original draft: Xiaoli Li, Ning Fang.
Writing – review & editing: Ning Fang.
Supplementary Material
Footnotes
Abbreviations: AUC = area under receiver operating characteristics (ROC) curve, CAD = coronary artery disease, CI = confidence interval, ESRD = end-stage renal disease, IQR = interquartile range, LAD = left anterior descending, LCX = left circumflex, MD = mean difference, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle–Ottawa scale, NSTE-ACS = non-ST-segment elevation acute coronary syndrome, OR = odds ratio, Q = quartile, SD = standard error, STE-MI = ST-segment elevation myocardial infarction.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- [2].Hatmi ZN, Saeid AK, Broumand MA, et al. Multiple inflammatory prognostic factors in acute coronary syndromes: a prospective inception cohort study. Acta Med Iran 2010;48:51–7. [PubMed] [Google Scholar]
- [3].Kawaguchi H, Mori T, Kawano T, et al. Band neutrophil count and the presence and severity of coronary atherosclerosis. Am Heart J 1996;132:9–12. [DOI] [PubMed] [Google Scholar]
- [4].Gibson PH, Cuthbertson BH, Croal BL, et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 2010;105:186–91. [DOI] [PubMed] [Google Scholar]
- [5].Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol 1997;58:95–117. [DOI] [PubMed] [Google Scholar]
- [6].Sheridan FM, Cole PG, Ramage D. Leukocyte adhesion to the coronary microvasculature during ischemia and reperfusion in an in vivo canine model. Circulation 1996;93:1784–7. [DOI] [PubMed] [Google Scholar]
- [7].Kalay N, Dogdu O, Koc F, et al. Hematologic parameters and angiographic progression of coronary atherosclerosis. Angiology 2012;63:213–7. [DOI] [PubMed] [Google Scholar]
- [8].Turak O, Ozcan F, Isleyen A, et al. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am J Cardiol 2012;110:1405–10. [DOI] [PubMed] [Google Scholar]
- [9].Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011; Available online: http://www.ohri.ca. [Google Scholar]
- [10].Zheng XX, Xu YL, Li SH, et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr 2011;94:601–10. [DOI] [PubMed] [Google Scholar]
- [11].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analysis. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sahin DY, Elbasan Z, Gür M, et al. Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology 2013;64:423–9. [DOI] [PubMed] [Google Scholar]
- [14].Sönmez O, Ertaş G, Bacaksiz A, et al. Relation of neutrophil-to-lymphocyte ratio with the presence and complexity of coronary artery disease: an observational study. Anadolu Kardiyol Derg 2013;13:662–7. [DOI] [PubMed] [Google Scholar]
- [15].Kaya A, Kurt M, Tanboga IH, et al. Relation of neutrophil to lymphocyte ratio with the presence and severity of stable coronary artery disease. Clin Appl Thromb Hemost 2014;20:473–7. [DOI] [PubMed] [Google Scholar]
- [16].Chen J, Chen MH, Li S, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting the severity of coronary artery disease: a Gensini score assessment. J Atheroscler Thromb 2014;21:1271–82. [DOI] [PubMed] [Google Scholar]
- [17].Bal Z, Bal U, Okyay K, et al. Hematological parameters can predict the extent of coronary artery disease in patients with end-stage renal disease. Int Urol Nephrol 2015;47:1719–25. [DOI] [PubMed] [Google Scholar]
- [18].Uysal HB, Dağli B, Akgüllü C, et al. Blood count parameters can predict the severity of coronary artery disease. Korean J Intern Med 2016;31:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kaya H, Ertaş F, İslamoğlu Y, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost 2014;20:50–4. [DOI] [PubMed] [Google Scholar]
- [20].Zhang GY, Chen M, Yu ZM, et al. Relation between neutrophil-to-lymphocyte ratio and severity of coronary artery stenosis. Genet Mol Res 2014;13:9382–9. [DOI] [PubMed] [Google Scholar]
- [21].Li Y, Ji HH. Association of the neutrophil to lymphocyte ratio and the non-ST-elevation acute coronary syndrome and its severity. Chin J Clinicians (Electronic Edition) 2013;7:40–3. [Google Scholar]
- [22].Xu WF, Chen ZK, Jiang QJ, et al. The correlation of neutrophil to lymphocyte ratio and red blood cel distribution width with the severity of coronary lesion in stable coronary artery disease. J Electrocardiol Circ 2016;35:156–9. [Google Scholar]
- [23].Liu ZM, Zhao FF, Li KY, et al. Relationship between neutrophil-to-lymphocyte ratio and coronary artery disease. Med J Wuhan Univ 2018;1:114–9. [Google Scholar]
- [24].Zhong Y, Tian CW, Ye J, et al. Correlation between neutrophil-to-lymphocyte ratio and severity of coronary artery disease. Acad J Guangzhou Med Univ 2014;42:34–8. [Google Scholar]
- [25].Ma LF, Zhang FF, Qiu CG, et al. Association between LDL/HDL, neutrophil to lymphocyte ratio and severity of the coronary artery lesion in patients with unstable angina pectoris. J Clin Cardiol 2015;10:1057–60. [Google Scholar]
- [26].Song YX, Zhao XY, Wang XF, et al. Relationship between neutrophils to lymphocyte ratio, D-dimer and coro-nary artery stenosis degree in patients with acute coronary syndrome. J Zhengzhou Univ (Medical Sciences) 2016;51:780–3. [Google Scholar]
- [27].Xiang W, Wang JH, Lan YH, et al. Correlation between the neutrophil-to-lymphocyte ratio and the severity of coronary stenosis in young patients with coronary atherosclerotic disease. Int J Cardiovasc Dis 2017;44:373–6. [Google Scholar]
- [28].Pan GJ. The relationship among LDL/HDL and neutrophil/lymphocyte ratio and the severity of coronary artery disease in patients with angina pectoris. J Qiqihar Univ Med 2017;13:1527–9. [Google Scholar]
- [29].Bai HY, Wang XQ, Wang TZ, et al. Early identification of neutrophil-to-lymphocyte ratio for severe coronary artery stenosis in patients with chest pain. J Wenzhou Med Univ 2017;47:282–5. [Google Scholar]
- [30].Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 2009;5:50–6. [DOI] [PubMed] [Google Scholar]
- [31].Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- [32].Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456–60. [DOI] [PubMed] [Google Scholar]
- [33].Iranirad L, Sadeghi M, Ahmadi M, et al. Relationship between neutrophil-to-lymphocyte ratio and the severity of coronary artery disease in patients undergoing cardiac catheterization. J Cardiothorac Med 2018;6:246–50. [Google Scholar]
- [34].Datta RK, Rashid MM, Azam MG, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease in chronic stable angina. Cardiovasc J 2018;10:164–70. [Google Scholar]
- [35].Demir K, Avci A, Altunkeser BB, et al. The relation between neutrophil-to-lymphocyte ratio and coronary chronic total occlusions. BMC Cardiovasc Disord 2014;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ateş AH, Aytemir K, Koçyiğit D, et al. Association of neutrophil-to-lymphocyte ratio with the severity and morphology of coronary atherosclerotic plaques detected by multidetector computerized tomography. Acta Cardiol Sin 2016;32:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kizilarslanoğlu Mc, Kuyumcu Me, Kiliç Mk, et al. Neutrophil to lymphocyte ratio may predict coronary artery disease in geriatric patients. Acta Medica 2015;46:58–63. [Google Scholar]
- [38].Sharma K, Patel AK, Shah KH, et al. Is Neutrophil-to-lymphocyte ratio a predictor of coronary artery disease in Western Indians? Int J Inflam 2017;2017:4136126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jagadish HR, Divyaprakash M, Manjunath R, et al. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Int J Adv Med 2018;21:265–70. [Google Scholar]
- [40].Zanardo V, Fanelli T, Weiner G, et al. Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int 2011;80:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol 2010;106:470–6. [DOI] [PubMed] [Google Scholar]
- [42].Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park BJ, Shim JY, Lee HR, et al. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chim Acta 2011;412:925–9. [DOI] [PubMed] [Google Scholar]
- [44].Xie X, Feng S, Tang Z, et al. Neutrophil-to-lymphocyte ratio predicts the severity of incarcerated groin hernia. Med Sci Monit 2017;23:5558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Celikbilek M, Dogan S, Ozbakir O, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal 2013;27:72–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Guclu M, Faruq Agan A. Association of severity of helicobacter pylori infection with peripheral blood neutrophil to lymphocyte ratio and mean platelet volume. Euroasian J Hepatogastroenterol 2017;7:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [48].Wang X, Zhang G, Jiang X, et al. Neutrophil to lymphocyte ratio in relation to risk of all-cause mortality and cardiovascular events among patients undergoing angiography or cardiac revascularization: a meta-analysis of observational studies. Atherosclerosis 2014;234:206–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


