Abstract
This study aimed to assess the association between chronic kidney disease (CKD) and the risk of pulmonary tuberculosis (TB) before initiating renal replacement therapy (RRT) in Taiwan.
Total 16,052 subjects newly diagnosed with CKD between 2000 and 2012 were included in the CKD group, and 31,949 randomly selected subjects who did not have CKD formed the non-CKD group. Subjects with a history of pulmonary TB or RRT, including dialysis and renal transplantation, before the index date were excluded. We determined the incidence of pulmonary TB at the end of 2013. A multivariable Cox proportional hazards regression model was used to assess the hazard ratio (HR) and 95% confidence interval (CI) for the risk of developing pulmonary TB associated with CKD.
The overall incidence of pulmonary TB was 1.47-fold greater in the CKD group compared to that in the non-CKD group (4.94 vs 3.35 per 1000 person-years, 95% CI 1.39, 1.56). Multivariable Cox proportional hazards regression analysis showed that the adjusted HR of pulmonary TB was 1.45-fold higher in the CKD group (95% CI 1.27, 1.64) than in the non-CKD group. Male sex (adjusted HR 2.04), age (increase per one year, adjusted HR 1.05), chronic obstructive pulmonary disease (adjusted HR 1.54), and diabetes mellitus (adjusted HR 1.34) were also associated with pulmonary TB.
CKD is associated with an increased risk of developing pulmonary TB before the initiation of RRT.
Keywords: chronic kidney disease, cohort study, epidemiology, pulmonary tuberculosis, renal replacement therapy
1. Introduction
The worldwide incidence of end-stage renal disease (ESRD) has remained relatively high for several decades, particularly in Taiwan.[1–4] Thus, more than 2 million ESRD patients worldwide required dialysis or transplantation in 2010.[5] Patients with diabetes mellitus (DM), hypertension, and chronic kidney disease (CKD) are at a high risk of developing ESRD,[5] and this trend is also observed in Taiwan.[6] Thus, it is crucial to perform early detection and prevention of CKD before the development of ESRD, a condition that poses a considerable global health care burden.
CKD is a significant global health issue, with an estimated worldwide prevalence of 8% to 16%.[7,8] Tuberculosis (TB) is responsible for >1.7 million annual deaths worldwide as per the most recent World Health Organization (WHO) report.[9] It is also related to conditions that increase the risk of active TB, including human immunodeficiency virus (HIV) infection and DM.[8] The association of CKD with altered cellular immunity, including malnutrition, hypoalbuminemia, uremia, and immunosuppression status, has been established.[10] The prevalence of DM, the most common risk factor for the progression to active TB, including pulmonary TB, is rapidly increasing in the world.[11] Individuals with concurrent HIV infection and abnormal kidney function are at a >30-fold higher risk of pulmonary TB.[8]
Many studies have summarized and confirmed a 6.9- to 52.5-fold higher risk of TB in patients with CKD, particularly in those undergoing dialysis, than in the general population.[12] However, the exact relationship between CKD and pulmonary TB remains unclear, and few studies have reported on the high prevalence of TB among patients with CKD before dialysis, especially in Taiwan. We aimed to bridge this research gap by conducting a population-based cohort study using the claims data from the National Health Insurance database in Taiwan to explore the association between older Taiwanese patients with CKD who are yet to undergo dialysis and pulmonary TB, the influence of other comorbidities on the risk of pulmonary TB, and the difference in the risk of pulmonary TB in patients with only CKD and in those with CKD and other comorbidities.
2. Methods
2.1. Design and data source
Taiwan is a sovereign and independent country with more than 23 million residents. This population-based cohort study used the database of the Taiwan National Health Insurance Program. This insurance program began on March 1, 1995, and it has covered approximately 99% of the 23 million residents living in Taiwan.[13–18] The details of the program have been well reported in previous studies.[19–21] This study was approved by the Ethics Review Board of China Medical University in Taiwan (CMUH-104-REC2-115).
2.2. Participants
We selected subjects aged 20 to 84 years with newly diagnosed CKD from 2000 to 2012 as the CKD group [the International Classification of Diseases (ICD) 9th Revision, ICD-9 codes 585–586]. To increase statistical power, for each subject with CKD, 2 subjects without CKD were randomly selected as the non-CKD group. The index date was defined as the date of diagnosis of CKD. Both CKD and non-CKD groups were matched for sex, age (every 5-year interval), and comorbidities. Subjects who had a history of pulmonary TB or who currently received renal replacement therapy (RRT), including dialysis and renal transplantation before the index date, were excluded from the study.
2.3. Comorbidities
Comorbidities that could be potentially related to pulmonary TB were included as follows: alcohol-related diseases, asbestosis, chronic obstructive pulmonary disease (COPD), DM, HIV infection, gastrectomy, pneumoconiosis, and splenectomy, as well as chronic liver diseases including cirrhosis, hepatitis B infection, hepatitis C infection, and other chronic hepatitis. All comorbidities were diagnosed with ICD-9 codes. The accuracy of ICD-9 codes has been examined in previous studies.[22–26]
2.4. Major outcome
The major outcome was a new diagnosis of pulmonary TB (ICD-9 codes 010, 011, 012, and 018) during the follow-up period. All study subjects were followed until they were diagnosed with pulmonary TB or to the end of 2013.
2.5. Statistical analysis
The distributions of sex, age, and comorbidities were compared between the CKD and non-CKD groups using the Chi-square test for categorized variables and the t-test for continuous variables. The incidence of pulmonary TB was estimated as the event number of pulmonary TB identified during the follow-up period, divided by the total follow-up person-years for each group. Initially, all variables were included in the univariable model. Next, variables found to be significant in the univariable model were further included in the multivariable model. The multivariable Cox proportional hazards regression model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the risk of pulmonary TB associated with CKD and other comorbidities. All analyzes were performed by using SAS software version 9.2 (SAS Institute Inc, Cary, NC).
3. Results
3.1. Baseline characteristics of the study population
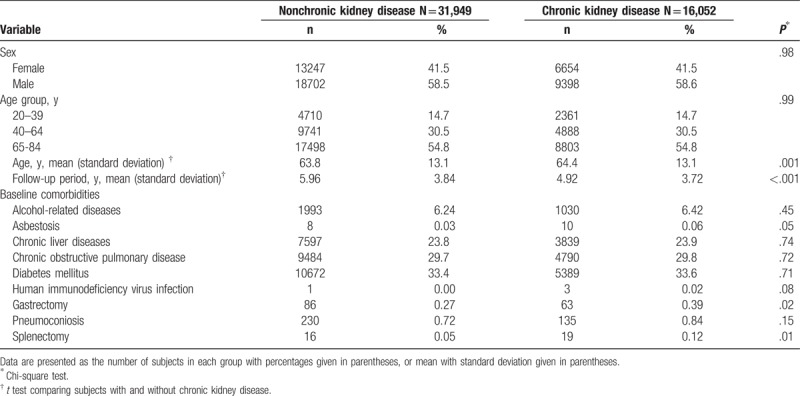
Table 1 discloses the baseline characteristics of the study population. There were 16,052 subjects in the CKD group and 31,949 subjects in the non-CKD group, with a similar sex distribution. The mean ages (standard deviation) of the study subjects were 64.4 ± 13.1 years for the CKD group and 63.8 ± 13.1 years for the non-CKD group. The CKD group had higher proportions of gastrectomy and splenectomy than the non-CKD group (chi-square test, P = .02 and P = .01, respectively).
Table 1.
Baseline characteristics between chronic kidney disease group and non-chronic kidney disease group.
3.2. Incidence of pulmonary tuberculosis of the study population stratified by sex and age
Table 2 discloses that the overall incidence of pulmonary TB was 1.47-fold greater in the CKD group than in the non-CKD group (4.94 vs 3.35 per 1000 person-years, 95% CI 1.39, 1.56). The incidences of pulmonary TB, stratified by sex and age, were all higher in the CKD group than those in the non-CKD group. The CKD group aged 65 to 84 years had the highest incidence of pulmonary TB (7.1 per 1000 person-years).
Table 2.
Incidence of pulmonary tuberculosis estimated by sex and age between chronic kidney disease group and nonchronic kidney disease group.
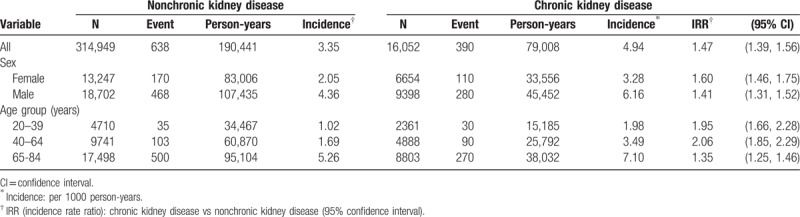
Figure 1 discloses that the Kaplan-Meier cumulative incidence of pulmonary TB was higher in the CKD group than the non-CKD group (4.72% vs 3.79% at the end of follow-up; P < .001).
Figure 1.
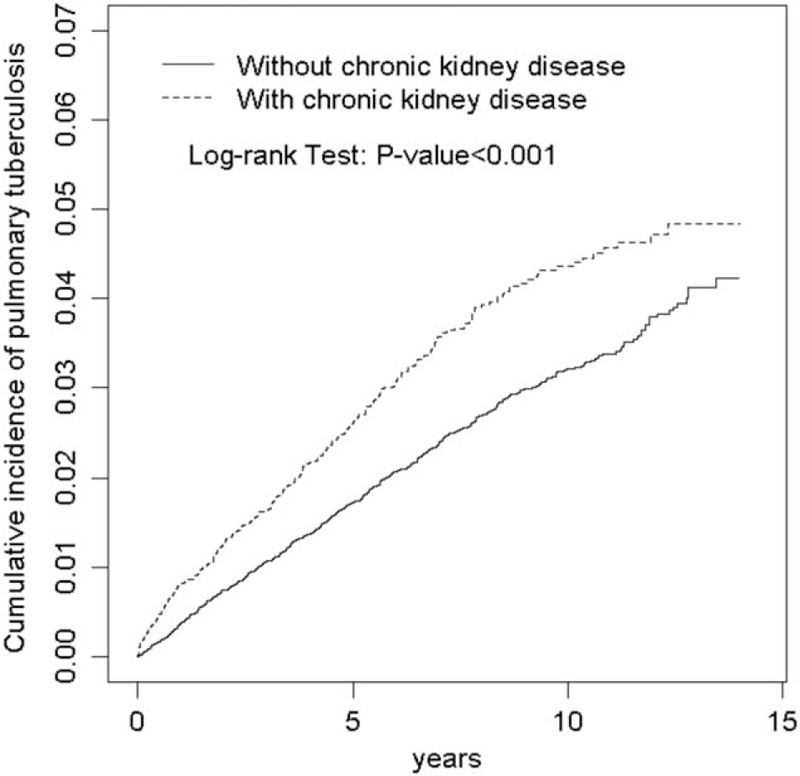
Cumulative incidence of pulmonary tuberculosis for subjects with chronic kidney disease and without chronic kidney disease (4.72% vs 3.79% at the end of follow-up; P < .001).
3.3. Pulmonary tuberculosis associated with chronic kidney disease and other comorbidities
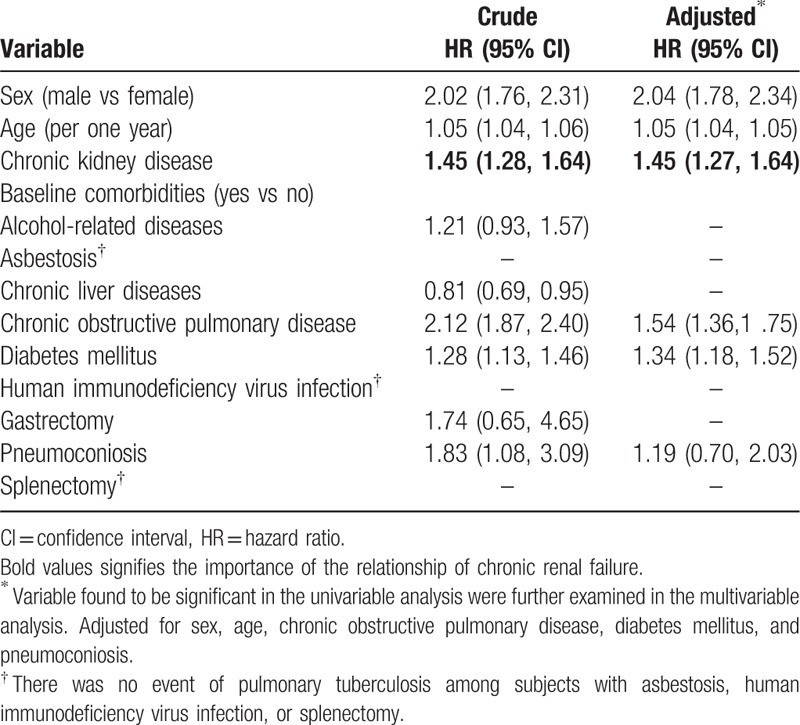
Table 3 discloses the risk of pulmonary TB associated with CKD and other comorbidities. Variables found to be significant in the univariable model were further included in the multivariable model. After adjustment for covariables, the multivariable Cox proportional hazards regression analysis disclosed that the adjusted HR of pulmonary TB was 1.45 for the CKD group (95% CI 1.27, 1.64), compared with the non-CKD group. Male (adjusted HR 2.04, 95% CI 1.78, 2.34), age (increase per one year, adjusted HR 1.05, 95% CI 1.04, 1.05), COPD (adjusted HR 1.54, 95% CI 1.36, 1.75), and DM (adjusted HR 1.34, 95% CI 1.18, 1.52) were other factors that could be related to pulmonary TB.
Table 3.
Cox model measured hazard ratio and 95% confidence interval of pulmonary tuberculosis associated with chronic kidney disease and comorbidities.
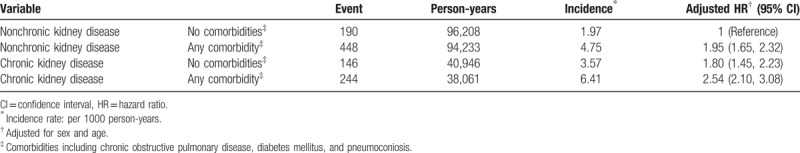
3.4. Risk of pulmonary tuberculosis stratified by chronic kidney disease and comorbidities
Table 4 discloses the risk of pulmonary TB stratified by CKD and comorbidities. To reduce the potential confounding effects of comorbidities studied, as a reference for subjects without CKD and without comorbidities, the adjusted HR for pulmonary TB was 1.80 for subjects with CKD alone and without comorbidities (95% CI 1.45, 2.23). This finding indicates that not requiring the presence of comorbidities, CKD may have a unique role in the risk of pulmonary TB. In addition, the adjusted HR would increase to 2.54 for subjects with CKD and comorbid with comorbidities (95% CI 2.10, 3.08). There seems to be a synergistic effect of CKD and comorbidities on the risk of pulmonary TB.
Table 4.
Risk of pulmonary tuberculosis stratified by chronic kidney disease and comorbidities.
4. Discussion
We found that the overall incidence of pulmonary TB was 1.47-fold higher in the CKD group compared to that in the non-CKD group. This result is in agreement with previous reports; the incidence of TB in the CKD population is higher than that in the general population in China,[27] showing a relative risk (RR) of 31.4. Two other retrospective studies in Jeddah revealed the same conditions in 1999 and 2000,[28,29] with emphasis on patients with chronic renal failure who have not yet undergone dialysis. In contrast, >50 articles were reviewed by Milburn et al[12,30] and Hussein et al[12] that discussed the high incidence rate of TB in dialysis and post renal transplant patients.
The reasons for the high incidence of pulmonary TB in advanced patients with CKD involve their acquired immunodeficiency status resulting from abnormal functioning of the T cells, B cells, neutrophils, monocytes, and natural killer cells.[30,31] A 2012 study not only explored the associations between T-cell-mediated immune response and TB, but also determined the superior method that clinical physicians can use to distinguish active TB from latent infection. Moreover, vitamin D deficiency impairs monocyte function, reducing the production of cathelicidin, a peptide that is capable of destroying Mycobacterium TB[30,32] and is commonly observed in stage 3 to 5 CKD. According to the guidelines of the National Institute for Health and Clinical Excellence (United Kingdom), the RR of developing TB is 10 to 25 in patients with CKD.[33] Patients with CKD who are yet to undergo dialysis and those on RRT, including dialysis and renal transplants, may be at a higher risk of developing pulmonary TB than those with ESRD.
Moreover, as per a study, the number of patients in the earlier stages of renal disease (stages 1–4) are likely to exceed by as much as 50 times those reaching ESRD (stage 5)[34]; therefore, the potential global health burden along the entire spectrum of CKD is considerably underestimated. The diagnosis of pulmonary TB is usually delayed in populations with renal disease, especially in earlier CKD stages because of atypical and nonspecific clinical manifestations. Thus, more attention should be paid to the risk of pulmonary TB in patients with CKD, particularly those who were not in ESRD and before dialysis.
It is noteworthy that patients aged 65 to 84 years in the CKD group had the highest incidence of pulmonary TB (7.1 per 1000 person-years) in the cohort. The elder patients with CKD were infected with pulmonary TB more easily than the younger patients, indicating the strong influence of age in CKD. This phenomenon revealed that the elderly were immunocompromised, and therefore, more susceptible to TB infection. Clinical physicians should be alert and attempt to prevent pulmonary TB infection in older or hospitalized CKD.[10,35]
In the present study, we also found that the risk of pulmonary TB was associated with CKD and other comorbidities. It is well known that immunity is compromised with age; therefore, older patients with CKD are at a higher risk of pulmonary TB. Men are affected more frequently than women, with a male/female ratio of 1.9 ± 0.6 for the worldwide case notification rate[36]; this result was in agreement with our findings. Smoking, alcohol consumption, drug use, and air pollution may be attributable for this condition.[36,37]
COPD is also a major public health problem. It is the fourth leading cause of chronic morbidity and mortality in the United States and is estimated to rank fifth in 2020 in the list of global burden of disease as per a study by the World Bank/WHO.[38] COPD poses a similar health burden in China, the most populated country in the world.[39] Several studies have examined pulmonary TB and COPD individually, whereas few have discussed the associations between them. Furthermore, most articles have focused on the Western countries and were conducted approximately 40 to 50 years previously,[40,41] were not representative, and did not match the current trends in the diagnosis and management of TB and COPD.
A nationwide survey on 13,826 adults in South Africa suggested that the strongest predictor of COPD was a history of pulmonary TB.[42] In a large population-based study conducted in 5 Latin American cities, the prevalence of COPD was 30.7% in patients with a history of pulmonary TB compared with 13.9% in those without such a history.[43] The 2 large-scale studies revealed results to our findings and likely meet current management demands; however, they did not focus on patients from the Eastern countries. Thus, owing to the above-mentioned reasons and due to the cumulative global healthcare burden, further investigations should be carried out in the future.
The final comorbidity, DM, showed a greater risk of pulmonary TB in patients with CKD compared to that in those without CKD. Thus is attributable to the fact that in DM patients, kidney function deteriorates with time. The natural course of DM leads to a higher infection rate of pulmonary TB in CKD populations than in those without CKD. Few studies conducted in Russia and other countries support this theory.[11,44] Further studies on the mechanism and other aspects of DM management in TB patients are necessary worldwide.
Of all baseline characteristics in our study, similar distributions of sex and age were observed between the CKD and non-CKD groups. The CKD group had higher proportions of gastrectomy and splenectomy compared to the non-CKD group. However, few studies have reported that patients who underwent gastrectomy or splenectomy had an increased risk of pulmonary TB.[45–48] Furthermore, 1 study was noted in Taiwan.[48] The above results indicate that physicians should be alert about the association of CKD and pulmonary TB in patients who have undergone gastrectomy and splenectomy.
Based on the results of the present study, the adjusted HR of pulmonary TB was 1.80 for patients with CKD without comorbidities (95% CI 1.45, 2.23). Thus, CKD may play an independent role in the risk of pulmonary TB before the appearance of comorbidities. Local clinicians, especially neurologists, must focus on the prevention of pulmonary TB infection in patients with CKD who have yet to undergo dialysis or in the ESRD stage. After considering other comorbidities, the adjusted HR increased to 2.54 (95% CI 2.10, 3.08) for subjects with the CKD and at least one of the other factors that we considered. The synergistic effect of CKD and comorbidities on the risk of pulmonary TB may be an indication for all healthcare professionals. We should pay more attention to lowering the infection rate of pulmonary TB in patients with CKD not only in Taiwan, but also worldwide, before they develop ESRD.
5. Limitations
The insurance claims data did not provide the serum creatinine level and microalbuminuria, and we were unable to evaluate the risk of pulmonary TB based on the kidney function. Early-stage patients with CKD might not have been included in the study by physicians’ coding alone. Alternatively, more accurate tools, such as chest radiography and sputum culture are necessary for establishing a definite diagnosis of pulmonary TB. Thus, the actual incidence of pulmonary TB in patients with CKD may have been underestimated or overestimated. Second, the diagnoses of CKD and pulmonary TB require long-term observation because the clinical manifestation of the symptoms occurs late. Thus, the shorter observation period of our study may have been insufficient to estimate the risk of pulmonary TB compared with the substantially longer observation periods for natural history in clinics or hospitals. The associated variable measures may thus have been underestimated. In addition, this study included the baseline comorbidity information that was collected before establishing the study cohorts. Comorbidities that develop during the follow-up period may vary, and this may have influenced the estimated pulmonary TB risk.
6. Conclusions
We evaluated the risk of pulmonary TB in association with CKD and several medical conditions in the Taiwanese population using representative population-based data. We demonstrated that patients with CKD had a significantly higher risk of pulmonary TB than those without CKD. Other comorbidities may interact with CKD and exert a synergistic effect on the risk of pulmonary TB. CKD and pulmonary TB are common disorders; therefore, they have been studied in several countries in recent decades. Further studies on patients with CKD are warranted, not only for the prevention of ESRD, but also for decreasing the probability of developing infected pulmonary TB before ESRD. The understanding of comorbidities may yield important preventive and detective methods for reducing the infection rate of pulmonary TB. We believe that our findings will enable earlier intervention and protection from pulmonary TB in patients with CKD and should also be used worldwide.
Author contributions
Kao-Chi Cheng and Kuan-Fu Liao participated in data interpretation, revised the article, and contributed equally to the article.
Chiu-Shong Liu and Cheng-Li Lin conducted data analysis.
Shih-Wei Lai contributed to the conception of the article, initiated the draft of the article, and revised the article.
Conceptualization: Kao-Chi Cheng.
Data curation: Kuan-Fu Liao.
Methodology: Cheng-Li Lin.
Supervision: Chiu-Shong Liu, Shih-Wei Lai.
Writing – original draft: Kao-Chi Cheng.
Writing – review and editing: Kao-Chi Cheng.
Footnotes
Abbreviations: CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, ESRD = end-stage renal disease, HIV = human immunodeficiency virus, ICD-9 code = International Classification of Diseases, 9th Revision, Clinical Modification, TB = tuberculosis, RR = relative risk, RRT = renal replacement therapy, WHO = World Health Organization.
This study was supported in part by the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital, Taiwan (DMR-107-192), Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106–2321-B-039–005–003), Tseng-Lien Lin Foundation at Taichung in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Hwang SJ, Tsai JC, Chen HC. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology 2010;15:3–9. [DOI] [PubMed] [Google Scholar]
- [2].Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis 2005;45:A5–7. [DOI] [PubMed] [Google Scholar]
- [3].Abbasi MA, Chertow GM, Hall YN. End-stage renal disease. BMJ 2010;2010: [PMC free article] [PubMed] [Google Scholar]
- [4].Cheng K-C, Chen Y-L, Lai S-W, et al. Patients with chronic kidney disease are at an elevated risk of dementia: a population-based cohort study in Taiwan. BMC Nephrol 2012;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [6].Hsu C-C, Hwang S-J, Wen C-P, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis 2006;48:727–38. [DOI] [PubMed] [Google Scholar]
- [7].Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- [8].Al-Efraij K, Mota L, Lunny C, et al. Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2015;19:1493–9. [DOI] [PubMed] [Google Scholar]
- [9].World Health Organization. Global Tuberculosis Control 2009: Epidemiology, Strategy, Financing. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- [10].Klote MM, Agodoa LY, Abbott K. Mycobacterium tuberculosis infection incidence in hospitalized renal transplant patients in the United States, 1998–2000. Am J Transplant 2004;4:1523–8. [DOI] [PubMed] [Google Scholar]
- [11].Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease. In: Seminars in Dialysis. Wiley Online Library; 2003:38–44. [DOI] [PubMed] [Google Scholar]
- [13].National Health Insurance Research Database. Taiwan. Available at: http://nhird.nhri.org.tw/en/index.html. [cited on February 8, 2017, English version]. [Google Scholar]
- [14].Yang J-S, Peng Y-R, Tsai S-C, et al. The molecular mechanism of contrast-induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM). Biomedicine 2018;8:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Sheu JJ-C, Lai M-T, et al. RSF-1 overexpression determines cancer progression and drug resistance in cervical cancer. Biomedicine 2018;8:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang MD, Lin KC, Lu MC, et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei) 2017;7:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang JS, Lu CC, Kuo SC, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) 2017;7:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai S-W, Lin C-L, Liao K-F. Association between oral corticosteroid use and pyogenic liver abscesses in a case-control study. Biomedicine 2018;8:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010;89:295–9. [DOI] [PubMed] [Google Scholar]
- [20].Lin H-F, Liao K-F, Chang C-M, et al. Population-based cohort study examining the association between splenectomy and empyema in adults in Taiwan. BMJ Open 2017;7:e015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng KC, Liao KF, Lin CL, et al. Increased risk of pulmonary tuberculosis in patients with depression: a cohort study in Taiwan. Front Psychiatry 2017;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liao KF, Cheng KC, Lin CL, et al. Etodolac and the risk of acute pancreatitis. Biomedicine (Taipei) 2017;7:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hung SC, Liao KF, Hung HC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract 2018;35:166–71. [DOI] [PubMed] [Google Scholar]
- [24].Cheng KC, Liao KF, Lin CL, et al. Gastrectomy correlates with increased risk of pulmonary tuberculosis: a population-based cohort study in Taiwan. Medicine 2018;97:e11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin HF, Liao KF, Chang CM, et al. Anti-diabetic medication reduces risk of pulmonary tuberculosis in diabetic patients: a population-based cohort study in Taiwan. Kuwait Med J 2017;49:22–8. [Google Scholar]
- [26].Lai SW, Lin CL, Liao KF. Population-based cohort study examining the association between weight loss and pulmonary tuberculosis in adults. Biomedicine (Taipei) 2018;8:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yuan F-h, Guang L-x, Zhao S-j. Clinical comparisons of 1,498 chronic renal failure patients with and without tuberculosis. Ren Fail 2005;27:149–53. [PubMed] [Google Scholar]
- [28].Al Shohaib S. Tuberculosis in chronic renal failure in Jeddah. J Infect 2000;40:150–3. [DOI] [PubMed] [Google Scholar]
- [29].Al Shohaib S. Tuberculosis in chronic renal failure in Jeddah. Int Urol Nephrol 1999;31:571–5. [DOI] [PubMed] [Google Scholar]
- [30].Milburn H, Ashman N, Davies P, et al. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax 2010;65:559–70. [DOI] [PubMed] [Google Scholar]
- [31].Gibbons RA, Martinez OM, Garovoy MR. Altered monocyte function in uremia. Clin Immunol Immunopathol 1990;56:66–80. [DOI] [PubMed] [Google Scholar]
- [32].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [33].National Collaborating Centre for Chronic Conditions (UK); Centre for Clinical Practice at NICE (UK). Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. In: 2006: Royal College of Physicians; 2006. [Google Scholar]
- [34].Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–2. [DOI] [PubMed] [Google Scholar]
- [35].Khan IH, Catto GR, MacLeod A, et al. Influence of coexisting disease on survival on renal-replacement therapy. Lancet 1993;341:415–8. [DOI] [PubMed] [Google Scholar]
- [36].Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009;6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lin H-H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007;4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;187:643–50. [DOI] [PubMed] [Google Scholar]
- [39].Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753–60. [DOI] [PubMed] [Google Scholar]
- [40].Birath G, Caro J, Malmberg R, et al. Airways obstruction in pulmonary tuberculosis. Scand J Respir Dis 1966;47:27–36. [PubMed] [Google Scholar]
- [41].Snider GL, Doctor L, Demas TA, et al. Obstructive airway disease in patients with treated pulmonary tuberculosis 1–4. American Review of Respiratory Disease 1971;103:625–40. [DOI] [PubMed] [Google Scholar]
- [42].Ehrlich R, White N, Norman R, et al. Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis 2004;8:369–76. [PubMed] [Google Scholar]
- [43].Menezes AMB, Perez-Padilla R, Jardim JB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005;366:1875–81. [DOI] [PubMed] [Google Scholar]
- [44].Coker R, McKee M, Atun R, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ 2006;332:85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Davies P, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis 2008;12:1226–34. [PubMed] [Google Scholar]
- [46].Mileno MD, Bia FJ. The compromised traveler. Infect Dis Clin North Am 1998;12:369–412. [DOI] [PubMed] [Google Scholar]
- [47].Sugiyama Y. A case of pulmonary tuberculosis with false negative quantiferon (TB-2G test). Kekkaku 2012;87:9–13. [PubMed] [Google Scholar]
- [48].Lai SW, Wang IK, Lin CL, et al. Splenectomy correlates with increased risk of pulmonary tuberculosis: a case–control study in Taiwan. Clin Microbiol Infect 2014;20:764–7. [DOI] [PubMed] [Google Scholar]


