Abstract
The lung is a delicate organ with a large surface area that is continuously exposed to the external environment, and is therefore highly vulnerable to exogenous sources of oxidative stress. In addition, each of its approximately 40 cell types can also generate reactive oxygen species (ROS), as byproducts of cellular metabolism and in a more regulated manner by NOX enzymes with functions in host defense, immune regulation, and cell proliferation or differentiation. To effectively regulate the biological actions of exogenous and endogenous ROS, various enzymatic and non-enzymatic antioxidant defense systems are present in all lung cell types to provide adequate protection against their injurious effects and to allow for appropriate ROS-mediated biological signaling. Acute and chronic lung diseases are commonly thought to be associated with increased oxidative stress, evidenced by altered cellular or extracellular redox status, increased irreversible oxidative modifications in proteins or DNA, mitochondrial dysfunction, and altered expression or activity of NOX enzymes and antioxidant enzyme systems. However, supplementation strategies with generic antioxidants has proven minimally successful in prevention or treatment of lung disease, most likely due to their inability to distinguish between harmful and beneficial actions of ROS. Recent studies have attempted to identify specific redox-based mechanisms that may mediate chronic lung disease, such as allergic asthma or pulmonary fibrosis, which provide opportunities for selective redox-based therapeutic strategies that may be useful in treatment of these diseases.
Keywords: epithelium, asthma, fibrosis, NOX, sulfenylation, S-glutathionylation, ER stress
1. Introduction
The lung represents the human body’s largest interface with the external environment, with an estimated surface area of ~150 m2. This large surface area is critical for effective uptake and delivery of oxygen (O2) to all organs, but also renders the lung vulnerable to airborne pathogens and pollutants, and the respiratory tract is therefore equipped with elaborate antimicrobial and antioxidant defense systems to minimize infection or injury and maintain appropriate lung function. Epithelial cells that coat the entire lung surface form a critical component of such defense, as they form a physical barrier that prevents entry of inhaled antigens and pathogens and also act as a source of antimicrobial factors, high-molecular weight glycoproteins (mucins), iron-binding proteins (e.g. lactoferrin and transferrin), and various secreted antioxidant enzymes and low-molecular weight antioxidant molecules (e.g. ascorbic acid, GSH), which are deposited into the airway surface fluids to provide a first-line defense network against inhaled pathogens or oxidant pollutants. While lung host defense against most airborne pathogens largely relies on resident inflammatory cell types, such as alveolar macrophages, dendritic cells, and innate lymphoid cells, the respiratory epithelium also plays a critical role in mediating acute responses to these pathogens by regulating innate or adaptive inflammatory responses to these common environmental challenges (Holgate, Holloway et al. 2004, Hammad and Lambrecht 2008). Chronic diseases of the lung, such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, have diverse etiologies but also share common traits in that they often involve repetitive cycles of injury to the respiratory epithelium (due to inhaled pollutants such as tobacco smoke or pathogens), combined with dysregulated epithelial repair pathways and chronic activation of inflammatory processes, collectively leading to inappropriate production of airway mucus, increased fibroblast activation, myofibroblast differentiation, smooth muscle proliferation, etc, thereby resulting in airway remodeling and decline in lung function. In addition, these various processes are further compromised by diverse genetic factors and chronic bacterial or viral infections due to loss of host defense pathways.
Many commonly encountered environmental pollutants, such as tobacco smoke, particulate matter, or ozone or nitrogen dioxide from photochemical smog, are oxidizing in nature and are thus thought to cause lung injury by oxidative stress. In addition, reactive oxygen species (ROS) are produced by phagocytic cells by activation of NADPH oxidase (NOX) enzymes in their efforts to kill pathogens, and inappropriate ROS production during acute or chronic lung inflammation is commonly thought to contribute to impaired lung cell function and progression of lung disease (Andreadis, Hazen et al. 2003, Jacobsen, Taranova et al. 2007, Shaw, Berry et al. 2007, Fahy 2009, Wedes, Khatri et al. 2009). Mitochondria, present in all lung cell types, also produce ROS during incomplete reduction of O2 to water (H2O) in the electron transport chain, and mitochondrial ROS formation is typically increased due to mitochondrial dysfunction during chronic disease, potentially contributing to disease pathology. Contrasting these presumed damaging effects of NOX- or mitochondria-derived ROS, NOX-family enzymes are widely expressed in other lung cells, and participate in other cell functions such as cell proliferation, differentiation, etc., illustrating more widespread physiological properties of ROS (van der Vliet 2011, Segal, Grimm et al. 2012). Similarly, mitochondrial ROS (mtROS) are also increasingly appreciated to serve important functions in cell biology, for example in basal and adaptive responses that control homeostasis and promote health span (Sena and Chandel 2012, Ristow 2014, Shadel and Horvath 2015). Based on these considerations, the mechanisms by which ROS contribute to lung disease (as well as diseases of other organs) may be related to dysregulated redox signaling rather than by non-discriminate oxidative biomolecular damage (“oxidative stress”). Because of the diverse biological functions of ROS in many diverse cell types, in both physiological and pathological outcomes, it is perhaps not surprising that generic antioxidant-based approaches to quench ROS have so far been minimally effective in mitigating chronic disease, including lung disease, and have in some cases even promoted adverse outcomes (Nadeem, Masood et al. 2008, Fortmann, Burda et al. 2013, Kirkham and Barnes 2013, Ristow 2014, Sayin, Ibrahim et al. 2014, Ghezzi, Jaquet et al. 2017). Therefore, recent research efforts have focused on identifying specific redox-based post-translational mechanisms that may mediate lung disease pathology, by addressing the involvement of specific cellular ROS sources and by characterizing critical protein targets and their redox-dependent modifications that induce functional alterations and mediate adverse biological outcomes. This review will briefly summarize the current evidence supporting dysfunctional ROS homeostasis in lung disease, and discuss some recent developments illustrating how selective targeting of specific ROS sources or redox signaling events may be more beneficial in combating chronic lung disease.
2. Sources of ROS in the lung – Location matters
2.1. Mitochondria
All cells in the body derive at least some of their energy from oxidative phosphorylation (OXPHOS) in mitochondria, during which O2 is reduced to H2O, typically leading to production of intermediate ROS (O2•– and H2O2) at complex I and III in the mitochondrial respiratory chain, the major site of cellular O2 consumption (Drose and Brandt 2012, Lenaz 2012, Wong, Dighe et al. 2017). Since the lung is the organ exposed to the highest O2 concentrations, such mitochondrial ROS (mtROS) production may be especially relevant to lung biology. The lung contains over 40 different cell types, which all contain varying mitochondrial densities. Among these, ciliated epithelial cells and secretory club cells that line the airways and alveolar type II cells that secrete surfactant are highly metabolically active cells and rich in mitochondria (Agrawal and Mabalirajan 2016). Most lung cells depend on aerobic glycolysis to supply carbon in the form of pyruvate to support OXPHOS and provide ATP as a constant supply of energy. Oxygen consumption rates in the lung are comparable to those in most other organs, and ATP content is also similar to that of other organs and mostly dependent on mitochondrial sources (Cloonan and Choi 2016, Piantadosi and Suliman 2017). Well-oxygenated tissues such as lung contain high levels of a unique isoform of the electron transport chain cytochrome oxidase complex IV subunit, COX IV isoform 2, which affects oxygen sensitivity, although the precise mechanisms and consequences are still not clear (Huttemann, Lee et al. 2012, Pierron, Wildman et al. 2012, Sommer, Huttemann et al. 2017).
Production of mtROS has typically been considered as a leak resulting from incomplete reduction of O2, but more recent studies indicate that such mtROS release may function as a cytosolic signal to support mitochondrial integrity and organismal homeostasis (Sena and Chandel 2012, Shadel and Horvath 2015, Topf, Suppanz et al. 2018). The diverse nature of mtROS production, which originates from up to 11 different sites depending on bioenergetic conditions (Wong, Dighe et al. 2017), further illustrates the complex and variable biological functions of mtROS. Moreover, mitochondria are engaged in dynamic networks throughout the cell, and factors that impact on mitochondrial fission/fusion dynamics or subcellular mitochondrial trafficking dictate the subcellular location of mitochondrial ATP or ROS to support specific cell functions (Schuler, Lewandowska et al. 2017, Lopez-Domenech, Covill-Cooke et al. 2018). Indeed, mitochondria in airway epithelial cells are not distributed randomly, but are localized primarily near the apical surface, and also in the basolateral region, indicating their specific functions in localized responses to e.g. external (apical) triggers (Ribeiro, Paradiso et al. 2003, Xu, Janocha et al. 2014). Thus, production of mtROS is a highly localized event, and likely effects cellular outcomes in a specific manner depending on location.
2.2. NADPH oxidases
A well-known major source of ROS during conditions of infection and inflammation is the activation of the so-called respiratory burst in phagocytic cells and other immune cells, due to assembly and activation of the NADPH oxidase complex within their phagosomes to generate intraphagosomal ROS to kill ingested microorganisms by oxidative mechanisms (Babior, Lambeth et al. 2002, Winterbourn, Hampton et al. 2006, Bedard and Krause 2007). We now know that multiple homologs of NADPH oxidase (NOX) exist that are more widely distributed across virtually all cell types, with a variety of biological functions beyond host defense (Bedard and Krause 2007, van der Vliet 2008). Similarly, NOX enzymes are widely distributed throughout the lung, with major roles in inflammatory/immune cells (alveolar macrophages, dendritic cells, T and B lymphocytes) as well as structural cell types (airway and alveolar epithelial cells, endothelial cells, fibroblasts, smooth muscle cells), each with distinct functional properties (van der Vliet 2011, Bernard, Hecker et al. 2014). Activation of NOX enzymes generates superoxide anion (O2•–) and/or hydrogen peroxide (H2O2) as primary products (Geiszt and Leto 2004, Ameziane-El-Hassani, Morand et al. 2005, Martyn, Frederick et al. 2006), which in some specialized cases interact with locally secreted heme peroxidases (e.g. myeloperoxidase, lactoperoxidase) to generate secondary oxidants (e.g. nitrogen dioxide, hypochlorous acid, hypobromous acid) (Wijkstrom-Frei, El-Chemaly et al. 2003, Geiszt and Leto 2004, El Hassani, Benfares et al. 2005). In some cases, O2•– or H2O2 can also react directly with alternative susceptible cell targets, including redox-sensitive proteins, by which NOX enzymes control a variety of biological processes ranging from cell proliferation, differentiation, to inflammatory signaling and immune regulation. NOX-mediated signaling mechanisms are often related to regulation of common signaling processes involving protein phosphorylation and Ca2+ signaling (Finkel 2003, Forman, Fukuto et al. 2004, Terada 2006, Janssen-Heininger, Mossman et al. 2008, van der Vliet 2008). Specificity in such oxidative signaling is typically achieved by strict spatial localization of NOX activation and its target proteins, e.g. in endosomes or membrane lipid rafts (Terada 2006, van der Vliet 2008, Ushio-Fukai 2009). In fact, ROS can have opposing effects on biological processes, depending on the location or extent of ROS production, as illustrated by the ability of ROS to promote as well as inhibit activation of transcription factors such as nuclear factor (NF)-κB or HIF-1 (Brar, Kennedy et al. 2003, Li, Harraz et al. 2006, Janssen-Heininger, Mossman et al. 2008).
Recent observations also indicate the presence of reciprocal interactions between different NOX enzymes (e.g. (Heppner, Hristova et al. 2016, Kim, Kim et al. 2017) and also between NOX enzymes and mitochondria (Dikalov 2011, Kroller-Schon, Steven et al. 2014), as examples of the more broadly known concept of ROS-induced ROS release (Zandalinas and Mittler 2017). These interactions imply that site-specific actions of specific NOX enzymes or mtROS can also induce more widespread oxidative events by more indirect mechanisms.
2.3. Other ROS sources
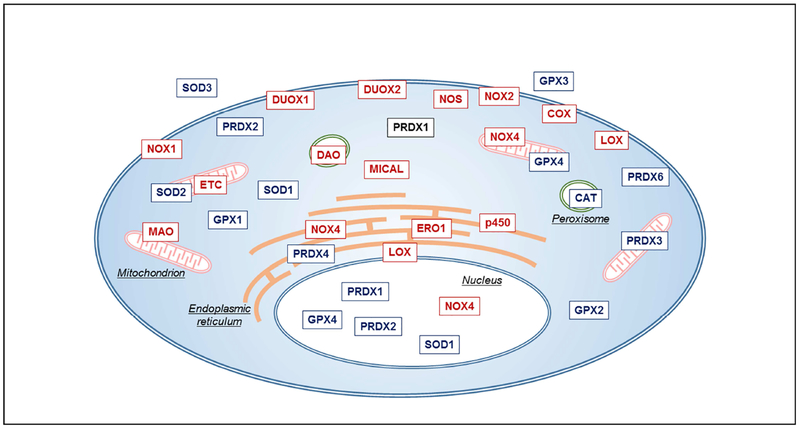
Other cellular sources of endogenous ROS production include cytochrome P450 enzymes, nitric oxide (NO•) synthases, as well as various metabolic enzymes involved in e.g. lipid biosynthesis and β-oxidation of amino acids (Schrader and Fahimi 2006, Antonenkov, Grunau et al. 2010), oxidative lipid metabolism by cyclooxygenases and lipoxygenases (Bae, Oh et al. 2011), enzymes involved in oxidative protein folding within the ER (e.g. endoplasmic reticulum oxireductin 1; ERO1) (Zito 2015), or the family of MICAL (for “molecule interacting with CasL”) oxidoreductases (Fremont, Romet-Lemonne et al. 2017), and are typically countered by (organelle-specific) antioxidant enzymes, such as e.g. catalase (CAT) in peroxisomes (Fig. 1). The biological significance of ROS production from these latter sources is much less appreciated and studies of biological ROS-mediated signaling have focused primarily on regulated ROS production by NOX enzymes or mitochondria. Nevertheless, it should be evident from Figure 1 that cellular ROS-mediated events are highly localized and likely have widely distinct cellular consequences. Also, this implies that generic antioxidant-based therapeutic approaches lacking specificity towards specific cellular ROS events are not necessarily useful.
Figure 1: Distribution of various ROS sources and antioxidant systems throughout the cell.
All cells (as well as all lung cells) contain actively respiring mitochondria that generate ROS at their electron transfer chain (ETC), as well as various NOX/DUOX isoform distributed across different organelles. Other cytoplasmic or organelle-specific ROS sources include nitric oxide synthase (NOS), cyclooxygenase (COX), lipoxygenase (LOX), monoamine oxidase (MAO, D-amino acid oxidase (DAO), MICAL, ERO1, and others. Moreover, all cells contain various isoforms of (organelle-specific) antioxidant enzymes that metabolize ROS. Please refer to manuscript text for further clarifications.
3. Oxidative stress and chronic lung disease – What is the evidence?
3.1. Direct evidence of oxidative stress in lung disease
Over the years, a large volume of studies has attempted to associate various measures of oxidative stress with disease progression, and this is also true for various chronic lung diseases such as asthma, COPD, pulmonary fibrosis, etc. Such approaches have largely been based on measurements of overall redox status (e.g. GSH redox status), lipid oxidation products, or analysis of stable oxidation products in proteins (e.g. protein carbonyls, nitrotyrosine, etc.) or DNA (e.g. 8-oxo-guanine), and have often shown an association of these outcomes with disease status (Andreadis, Hazen et al. 2003, Rahman, Biswas et al. 2006, Fitzpatrick, Park et al. 2014). However, clinical studies of antioxidant supplementation typically do not assess any of these oxidative markers. Therefore, evidence that any of these stable oxidative modifications actually contribute to disease progression is sparse.
3.2. Mitochondrial dysfunction
Increasing evidence indicates that mitochondrial integrity and function is impaired or altered in various chronic lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and lung cancer (Cloonan and Choi 2016, Piantadosi and Suliman 2017). Electron microscopy has revealed ultrastructural changes in airway epithelial mitochondria in mouse models of asthma or biopsies from human subjects with asthma, associated with biochemical features of mitochondrial dysfunction such as increased oxidative damage or activation of apoptotic pathways (Agrawal and Mabalirajan 2016). More recent studies also indicated increased mitochondrial number in airway epithelia of asthmatics, which has been linked with metabolic alterations (Xu, Ghosh et al. 2016). Mitochondrial abnormality has also been described in epithelial cells and airway smooth muscle cells from patients with COPD (Agrawal and Mabalirajan 2016, Cloonan and Choi 2016), and leukocytes of smokers with COPD have almost 50% lower mtDNA copy number compared to healthy controls (Liu, Kuo et al. 2015). Mitochondrial dysfunction in alveolar type II cells from patients with idiopathic pulmonary fibrosis (IPF) was found to be associated with upregulation of ER stress markers and reduced expression of PTEN-induced putative kinase 1 (PINK1), an enzyme that regulates mitochondrial maintenance by mitophagy of damaged mitochondria (Bueno, Lai et al. 2015). The importance of mitochondria in lung disease is also supported by genetic evidence, for example by reported associations of the mitochondrial haplogroup U with increased IgE and asthma (Zifa, Daniil et al. 2012, Flaquer, Heinzmann et al. 2014), and genetic associations of genes of mitochondrially targeted proteins involved in metabolism or ROS regulation with COPD (Agrawal and Mabalirajan 2016). For example, the iron-regulatory protein IRP2 (critical in regulating cellular iron homeostasis) was identified as a major COPD susceptibility gene, and its overexpression in the lungs of COPD patients was associated with mitochondrial dysfunction (Cloonan, Glass et al. 2016). Enhanced production of mtROS is often considered a central feature of mitochondrial dysfunction. Therefore, in attempts to address the specific role of mtROS in lung pathology, various experimental approaches to target mtROS (e.g. mitoQ, mitoTEMPO) have been used in animal studies of allergic airways disease or COPD (Jaffer, Carter et al. 2015, Wiegman, Michaeloudes et al. 2015, Agrawal and Mabalirajan 2016). Mitochondrially-targeted iron chelators have also been shown to attenuate inflammation and mucociliary defects in experimental models of COPD (Cloonan, Glass et al. 2016), which may further point to a role for mtROS in COPD pathology given the importance of iron overload in ROS biochemistry. Administration of the mitochondria-targeted antioxidant mitoQ has also been shown to attenuate hypoxia-induced pulmonary vasoconstriction, thus suggesting a role for mtROS in pulmonary hypertension (Pak, Scheibe et al. 2018).
3.3. Dysregulated NOX expression or activation
The most widely appreciated source of ROS in the context of acute or chronic lung inflammation is NOX2 present in infiltrating monocytes, macrophages, and/or granulocytes following their activation. In addition to their direct antimicrobial roles, NOX2-derived ROS also contribute to pro-inflammatory signaling (Forman and Torres 2002, Rinna, Torres et al. 2006, Cruz, Rinna et al. 2007), antigen processing and cross-presentation (Jancic, Savina et al. 2007, Rybicka, Balce et al. 2010, Rybicka, Balce et al. 2012), and regulation of e.g. neutrophil apoptosis (Carneiro, Roma et al. 2018). The latter also explains why genetic deficiency of NOX2 or its cofactors often results in enhanced inflammation and injury in response to infection or other stimuli (Morgenstern, Gifford et al. 1997, Gao, Standiford et al. 2002, Kassim, Fu et al. 2005, Segal, Davidson et al. 2007), and leads to chronic granulomatous disease (CGD), illustrating the importance of NOX2 in regulating inflammation and its resolution (Singel and Segal 2016). The respiratory epithelium also represents an important source for extracellular ROS in response to inhaled pathogens or allergens or inflammatory stimuli, originating primarily from the main epithelial NADPH oxidase isoforms DUOX1 and DUOX2 (Geiszt, Witta et al. 2003, Harper, Xu et al. 2006, Moskwa, Lorentzen et al. 2007, Wesley, Bove et al. 2007, Gattas, Forteza et al. 2009). DUOX1 is mostly localized at the apical surface of tracheobronchial and alveolar epithelial cells, and DUOX2 is primarily found in salivary and submucosal glands (Geiszt, Witta et al. 2003, Schwarzer, Machen et al. 2004, Forteza, Salathe et al. 2005, Harper, Xu et al. 2005, Fischer, Gonzales et al. 2007). Airway DUOX2 is highly inducible by various bacterial and viral stimuli (Harper, Xu et al. 2005, Gattas, Forteza et al. 2009), and was also found to be elevated in severe asthma (Voraphani, Gladwin et al. 2014). DUOX1 is the main constitutive isoform in airway and alveolar epithelia, is inducible by Th2 cytokines, and is enhanced in allergic asthma (Hristova, Habibovic et al. 2016, Wan, Hollins et al. 2016). In contrast, airway DUOX1 (and to a lesser extent DUOX2) are suppressed in lung epithelial cells from smokers with COPD (Nagai, Betsuyaku et al. 2008) and in various lung cancers (Little, Sulovari et al. 2017). Airway and alveolar epithelial cells also express other NOX isoforms such as NOX1 and NOX4 which may be enhanced during acute lung injury (Carnesecchi, Deffert et al. 2009), neutrophilic asthma (Wan, Hollins et al. 2016), or pulmonary fibrosis (Carnesecchi, Deffert et al. 2011). NOX4 is also expressed in smooth muscle cells and pulmonary fibroblasts, and is enhanced in lung tissues from patients with pulmonary arterial hypertension (Mittal, Roth et al. 2007) and pulmonary fibrosis (Hecker, Vittal et al. 2009, Amara, Goven et al. 2010). Collectively, lung pathologies are associated with variable changes in different NOX isoforms, which potentially unique contributing roles to disease pathology. Selective NOX inhibitors are largely lacking (van der Vliet, Danyal et al. 2018), but a semiselective NOX1/NOX4 inhibitor was found to attenuate and reverse pulmonary fibrosis in experimental models (Hecker, Logsdon et al. 2014).
3.4. Alterations in antioxidant systems
As a fourth line of evidence for ROS imbalance as a potential causative factor in lung pathology, several studies indicate altered expression or activity of various ROS-metabolizing enzymes during chronic lung disease. For example, the cytosolic isoform of superoxide dismutase (Cu,Zn-SOD; SOD1), abundantly expressed in most lung cell types (Zelko, Mariani et al. 2002, Kinnula and Crapo 2003), is inactivated in asthmatic airways (based on loss of overall SOD activity, which is primarily accounted for by SOD1) (Smith, Shamsuddin et al. 1997, Comhair, Xu et al. 2005, Ghosh, Willard et al. 2013). The mitochondrial MnSOD isoform SOD2 is induced in the bronchial and alveolar epithelium of e.g. smokers with or without COPD (Harju, Kaarteenaho-Wiik et al. 2004), but oxidative modifications within SOD2 have been detected in asthmatic airways, which may contribute to the loss of SOD activity in these patients (Comhair, Xu et al. 2005). The third isoform, extracellular (EC)-SOD (SOD3) is also induced in the lungs of COPD patients (Folz, Guan et al. 1997), and SOD3 haplotypes have been associated with development of COPD (Young, Hopkins et al. 2006, Bentley, Emrani et al. 2008). Evidence also suggests that oxidative mechanisms during asthma contribute to loss of lung activity of the H2O2-metabolizing enzyme catalase (Ghosh, Janocha et al. 2006, Comhair and Erzurum 2010). In contrast, some isoforms of GSH peroxidase (GPX), such as GPX2 and the extracellular isoform GPX3, are enhanced during allergic airway inflammation (Comhair and Erzurum 2005, Dittrich, Meyer et al. 2010). Finally, several isoforms of peroxiredoxin (PRDX), especially PDRX1, PRDX5, and PRDX6, are inducible by oxidative stress or during inflammation and are often elevated in several lung diseases including lung cancer, mesothelioma and sarcoidosis (Kinnula, Lehtonen et al. 2002, Aracena-Parks, Goonasekera et al. 2006, Rostila, Puustinen et al. 2012). Overall, the various changes in antioxidant enzyme expression or activity can be expected to contribute to dysregulated cellular responses to ROS. In fact, upregulation of mitochondrial thioredoxin (TRX) and PRDX3 in cancer cells is thought to contribute to dysregulated signaling and energy metabolism, and may represent attractive targets for therapeutic management of various intractable human cancers (Cunniff, Newick et al. 2015).
Collectively, these variable lines of evidence clearly establish that lung ROS-antioxidant status is frequently altered in chronic lung disease. However, given the diverse biological actions of ROS on both physiological processes and cellular dysfunction, it is difficult to translate these various findings into relevant mechanisms of disease. Indeed, one inherent problem is that reported ROS-antioxidant imbalances are typically based on global analyses of tissues, cells or body fluids, which does not offer insight into localized redox events within cells (e.g. in NOX-containing signaling complexes or near mitochondria) that are likely more relevant for cell signaling or biological outcomes.
4. Oxidative stress versus redox signaling – Basic concepts
4.1. Redox signaling is localized
The tendency to describe biological systems in terms of redox balance or oxidative status (ie. based on measurements of cell or tissue GSH/GSSG ratios or estimations of steady-state levels of ROS) has been helpful in appreciating how such parameters change e.g. with increasing age or during ongoing disease (Fitzpatrick, Park et al. 2014, Jones 2015), but such concepts fail to recognize the importance of subcellular location of specific and dynamic redox events occurring throughout a cell. It is also important to point out that the ability of ROS to diffuse freely throughout a cell (because of their small size) and cause global alterations of redox status within a cell is often overestimated. If one considers the complex architecture of a cell, containing dense networks of membrane-rich mitochondrial and ER structures, protein complexes such as actin and tubulin fibers, and a gel-like cytoplasm whose physicochemical properties are highly distinct from dilute aqueous buffer solutions, it should be easy to appreciate that even small molecules such as H2O2 (with physical properties similar to water) are unlikely to distribute randomly throughout a cell and may even require transport mechanisms (such as aquaporins that facilitate transmembrane transport of H2O and also H2O2). It follows that biological actions of ROS must be dictated primarily by their site of production (e.g. activation of NOX, extracellular source, etc.), which is further assured by the widespread presence of diverse ROS-metabolizing enzymes within cells that help constrain locally generated ROS and prevent their ability to induce more widespread changes throughout the cell (Heppner, Janssen-Heininger et al. 2017). Observed changes in overall redox status in the cell due to e.g. metabolic alterations during aging or chronic disease, might reflect shifts in overall metabolic status determined by an imbalance between cellular ROS production and adaptive responses leading to induction of antioxidant enzyme, but may not necessarily affect local redox signaling events due to dynamic local fluctuations in e.g. H2O2 production by specific NOX enzymes. From this perspective, it is not difficult to understand why general antioxidant supplementation strategies in an effort to alter global redox status may be relatively incapable of inhibiting such localized ROS production by e.g. NOX activation. This is fortunate, since such strategies would otherwise also interfere with beneficial effects of NOX in e.g. host defense functions and thus increase risk of infectious disease. In fact, supplementation with ascorbate has paradoxically been demonstrated to enhance ROS production and bacterial killing by phagocytes, rather than inhibit it (Carr and Maggini 2017), and might similarly affect activation of other NOX isoforms as well.
4.2. General mechanisms of redox signaling
The original concept of oxidative stress as leading to molecular and tissue damage as a causative factor in disease pathology has been largely supplanted by the phenomenon of redox signaling, which involves reversible redox modifications of susceptible protein targets as a mechanism to regulate protein function and affect many physiological aspects of cell biology, and the premise that pathology arises primarily from dysregulation of such redox signaling processes. The most common sites in proteins that are subject to such reversible redox modifications include redox-active transition metal ion centers (e.g. heme groups, iron-sulfur centers, zinc-thiolate centers) and oxidant-sensitive amino acid side chains (primarily cysteine, selenocysteine, and methionine residues). Oxidative modifications of these sites can either enhance or inhibit enzyme activity (e.g. through oxidation of catalytically active cysteines), but can also result in altered protein-protein interactions, subcellular trafficking, protein turnover, etc.
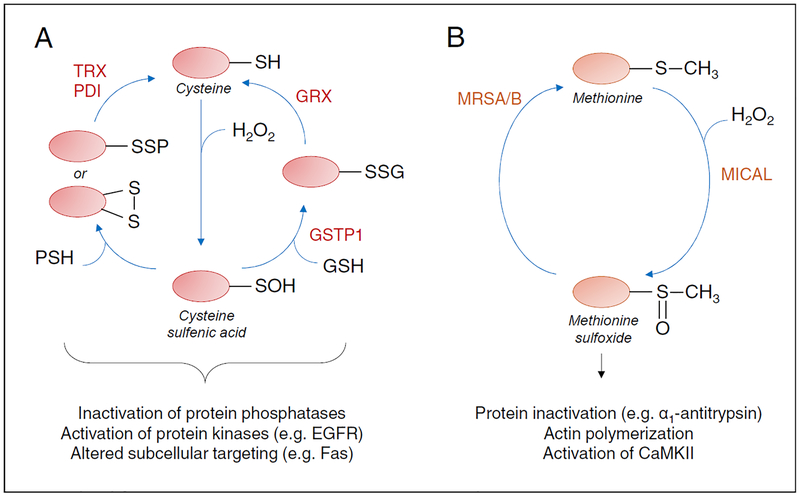
The field of redox-based signaling has mostly focused on reversible oxidation of cysteine residues, since cysteine residues are often highly conserved in proteins and the relative abundance of protein cysteines has increased with evolution of complex multicellular organisms, in apparent coordination with increased diversity of NOX enzymes in these higher organisms (van der Vliet 2008, Jones and Sies 2015). Protein tyrosine phosphatases were among the first proteins recognized to be subject to inactivation by reversible cysteine oxidation during e.g. growth factor-mediated signaling, but with development of improved detection tools and proteomics approaches many examples exist of proteins that are also oxidized on cysteines as a result of cellular ROS production (Janssen-Heininger, Mossman et al. 2008, Yang, Gupta et al. 2014, Topf, Suppanz et al. 2018). In addition to the fact that many protein phosphatases are subject to inhibition by reversible oxidative modification, more recent evidence indicates that various protein kinases (including both protein tyrosine kinases and serine/threonine-specific protein kinases) can be activated by oxidation of conserved cysteine residues, thus implying that redox mechanisms are closely intertwined with cell signaling pathways involving protein phosphorylation. An important consideration in this regard is the fact that oxidative cysteine modifications can be highly diverse (e.g. sulfenic acids vs. mixed disulfides with other proteins or small molecules such as GSH) (Figure 2A), with potentially variable functional or structural consequences (Heppner, Hristova et al. 2016, Truong, Ung et al. 2016, Moffett, Bender et al. 2017). Protein S-glutathionylation, the formation of mixed disulfides with GSH, has received special attention as a signaling mechanism, supported by the fact that enzymatic systems exist that can catalyze protein S-glutathionylation (e.g. glutathione S-transferases) and reverse it (glutaredoxins) (Janssen-Heininger, Mossman et al. 2008, Zhang, Ye et al. 2018). Also, because of the rather bulky and charged nature of GSH, protein S-glutathionylation is thought to induce significant structural and functional alterations within proteins (e.g. (Moffett, Bender et al. 2017)). Protein glutathionylation is also believed to protect against irreversible cysteine oxidation of thioredoxins and peroxiredoxins, which can be restored by the action of glutaredoxins (Zhang, Du et al. 2014, Peskin, Pace et al. 2016), thus demonstrating complex and intricate interplay between these various redox systems. Dysregulation of these enzyme systems during disease can therefore impact on this important mode of redox signaling.
Figure 2: Schematic illustration of reversible oxidation of protein cysteine or methionine residues in redox-based signaling.
(A) Oxidation of protein cysteines (P-SH) by e.g. H2O2 results in initial formation of sulfenic acid (P-SOH), which can subsequently react with other protein cysteines to form inter- or intra-molecular disulfides, or with GSH (potentially catalyzed by GSTP1) to form S-glutationylated proteins (P-SSG). These disulfides can then be reduced by thioredoxins (TRX), protein disulfide isomerases (PDI) or glutaredoxins (GRX). (B) Oxidation of methionine by H2O2 (potentially catalyzed by MICAL) results in formation of methionine sulfoxide, which can be reduced by methionine sulfoxide reductases (MRSA and MRSB).
In contrast to the rapidly growing literature addressing cysteine oxidation, methionine oxidation has been less well recognized as a potential signaling mechanism. This is largely due to the fact that biochemical reagents are lacking to assess methionine oxidation in biological systems, and only MS approaches can successfully demonstrate methionine sulfoxidation. Sulfoxidation of methionine residues markedly increases the hydrophilic properties of methionine and can thus significantly alter the physicochemical properties of proteins, thereby either enhancing or inhibiting protein activity (Veredas, Canton et al. 2017). Also, similar to reversible cysteine oxidation, enzymatic systems have been identified that can both enhance and reverse methionine sulfoxidation (Manta and Gladyshev 2017) (Figure 2B). Intriguingly, recent proteomic analyses have revealed that methionine oxidation tends to be highly favorable in phosphorylation motifs, suggesting intricate crosstalk between methionine oxidation and protein phosphorylation pathways (Veredas, Canton et al. 2017).
5. Redox mechanisms in pulmonary disease
It should be evident from the previous sections that the field of redox biology has become exceedingly complex and continues to evolve. Indeed, reports are rapidly accumulating in the biomedical literature that address new aspects of redox-based signaling and its diverse impact on cell biology, and it would be beyond the scope of this review to adequately and cohesively summarize all available literature in this area. Indeed, a simple PubMed search using the title/abstract search terms “redox” and “lung” yields >1500 publications. Moreover, although ROS have been implicated in many diverse lung pathologies, our understanding of these cell-specific aspects of redox signaling in lung biology and diverse chronic lung disease is still rather various lung diseases. Therefore, rather than attempting to exhaustively cover the diverse literature that addresses redox-based mechanisms in various lung diseases, which has been covered in excellent recent reviews (e.g. (Sundar, Yao et al. 2013, Sommer, Strielkov et al. 2016, Boukhenouna, Wilson et al. 2018)), in the following paragraphs we will instead highlight some recent advances with respect to specific redox-based mechanisms in the context of two major lung pathologies, allergic asthma and idiopathic pulmonary fibrosis, which form the basis of new and unique redox-based therapeutic approaches that could be developed to treat these diseases. Given the fact that other chronic lung diseases, such as COPD, may also include overlapping features of chronic inflammation or remodeling similar to those seen in asthma or pulmonary fibrosis, these targeted approaches might also be applicable to other chronic lung pathologies.
5.1. NOX4 in pulmonary fibrosis
Pulmonary fibrosis is a debilitating disease characterized by progressive development of excess fibrous tissue, leading to lung “scarring” and rapidly diminishing lung function. In many cases, the cause of pulmonary fibrosis is unknown, which is known as idiopathic pulmonary fibrosis (IPF). Common features of pulmonary fibrosis are recurrent and persistent injury of the alveolar epithelium, combined with disordered deposition of extracellular matrix and accumulation of myofibroblasts in so-called fibroblastic foci. As mentioned previously, the NADPH oxidase homolog NOX4 was found to be overexpressed in the lungs of patients with IPF, primarily in myofibroblasts in fibroblastic foci and remodeled blood vessels, but also in epithelial cells associated with aberrant bronchiolization, and this NOX4 induction is largely mediated by production of the pro-fibrotic growth factor transforming growth factor beta (TGF-β) (Hecker, Vittal et al. 2009, Amara, Goven et al. 2010, Pache, Carnesecchi et al. 2011). Curiously, NOX4 apoptotic cell death in epithelial cells (Carnesecchi, Deffert et al. 2011) but inducing fibroblast differentiation towards and apoptosis-resistant myofibroblast phenotype (Bernard, Hecker et al. 2014), and thus appears to contribute to both critical features of progressive lung fibrosis. The molecular mechanisms by which NOX4-dependent production of H2O2 contributes to these functional outcomes are not clear, but various recent studies indicate an interaction of NOX4 with mitochondrial function. NOX4 contains an N-terminal mitochondrial localization signal, and can localize to the inner mitochondrial membrane and promote mtROS production (Block, Gorin et al. 2009, Graham, Kulawiec et al. 2010, Case, Li et al. 2013). Metabolic profiling of IPF lung tissues has suggested altered metabolic signatures in IPF consistent with increased glycolysis, reduced GSH biosynthesis, and increased ATP degradation (Kang, Lee et al. 2016), which may be associated with mitochondrial dysfunction. Studies in human or murine fibroblasts in which NOX4 was genetically deleted highlighted a suppressive effect of NOX4 on mitochondrial biogenesis and bioenergetics (Bernard, Logsdon et al. 2017), and indicated that NOX4 silencing resulted in upregulation of mitochondrial transcription factor A (TFAM) by nuclear factor erythroid-derived 2-like 2 (Nrf2). Intriguingly, ATP was recently identified as a negative allosteric regulator of NOX4 (Shanmugasundaram, Nayak et al. 2017) suggesting that ATP degradation during IPF may contribute to enhanced NOX4 activation, thus further perpetuating this progressive disease. Based on these various observations, pharmacologic inhibition of NOX4 has been proposed as a potential treatment for IPF, and preclinical studies have suggested efficacy of a recently development NOX inhibitor with some selectivity towards NOX4 (Hecker, Logsdon et al. 2014).
5.2. Redox regulation of epithelial apoptosis by S-glutathionylation in pulmonary fibrosis
IPF is often characterized by chronic injury and loss of bronchiolar and alveolar epithelial cells, and targeted injury to alveolar type II cells can induce fibrosis (Sisson, Mendez et al. 2010). One major contributor to such epithelial loss is the production of the ligand for the death receptor Fas (CD95) by myofibroblasts, which induces alveolar epithelial apoptosis (Golan-Gerstl, Wallach-Dayan et al. 2007) whereas myofibroblasts are typically resistant to Fas-induced apoptosis (Wynes, Edelman et al. 2011). It is widely known that apoptosis is associated with dramatic changes in cellular GSH status, and GSH status is also impaired in IPF (Anathy, Roberson et al. 2012), although approaches to restore GSH redox status using e.g. N-acetylcysteine have not been successful in improving IPF in humans (Idiopathic Pulmonary Fibrosis Clinical Research, Martinez et al. 2014, Sun, Liu et al. 2016). Recent studies by our group indicated that Fas-induced apoptosis in epithelial cells is associated with S-glutathionylation of the Fas receptor on Cys294, and this S-glutathionylation was found to promote cell surface expression of Fas, as well as its aggregation and activation, leading to downstream caspase signaling (Anathy, Roberson et al. 2012). In addition, Fas-mediated caspase activation was found to promote cleavage and inactivation of glutaredoxin 1 (GLRX, the main cytoplasmic glutaredoxin isoform that reverses S-glutathionylation), and overexpression of GLRX could prevent Fas S-glutathionylation and attenuate apoptosis (Anathy, Aesif et al. 2009). Subsequent studies indicated that initial Fas stimulation promotes oxidative processing of ER-localized Fas, mediated by interactions with the ER-resident protein disulfide isomerase ERp57 and glutathione S-transferase P1 (GSTP1), a known catalyst of S-glutathionylation, and that such interactions between GSTP1 and Fas were also observed in experimental models of pulmonary fibrosis (Anathy, Roberson et al. 2012) as well as lung tissues from human IPF patients (McMillan, van der Velden et al. 2016). Since GSTP1 was also found to be increased in IPF lungs, pharmacological inhibition of GSTP1 S-glutathionylation could potentially inhibit the progression of pulmonary fibrosis by minimizing Fas S-glutathionylation and subsequent caspase activation and alveolar apoptosis. Indeed, experimentally-induced lung fibrosis in mice was strongly attenuated by genetic deletion of Gstp, and by administration of TLK177, the active metabolite of the clinically applicable GSTP inhibitor TLK199 (McMillan, van der Velden et al. 2016). Conversely, approaches to augment the activity of GLRX may also be similar efficacious in treating IPF. Indeed, we recently observed that increases in protein S-glutathionylation in fibrotic lung tissues are largely associated with significant decrease in GLRX activity, and that administration of catalytically active recombinant GRLX into the airways of mice with ongoing experimental pulmonary fibrosis can in fact reverse increases in collagen deposition, thus illustrating the therapeutic potential of exogenous GLRX in treating lung fibrosis (Anathy, Lahue et al. 2018). These various approaches to modify S-glutathionylation could affect fibrosis by attenuation Fas activation, but could also affect S-glutathionylation of other target proteins which may also contribute to disease pathology. Hence, further characterization of these additional S-glutathionylation events would be necessary to advance our understanding of this redox signaling axis in pulmonary fibrosis. An alternative redox mechanism involved in apoptosis involves the protein disulfide isomerase isoform PDIA3 (also known as ERp57), which is known to catalyze inter-molecular disulfide bonds in pro-apoptotic BAK and thereby enhances intrinsic apoptosis (Zhao, Lu et al. 2015), which was demonstrated in airway epithelial cells in the context of allergen induced peri-bronchiolar fibrosis (Hoffman, Tully et al. 2013, Hoffman, Chapman et al. 2016). Airway epithelial specific deletion of ERp57 was shown to decrease allergen induced apoptosis and peri-bronchiolar fibrosis in mice (Hoffman, Chapman et al. 2016).
5.3. Redox-dependent activation of tyrosine kinases in allergic asthma
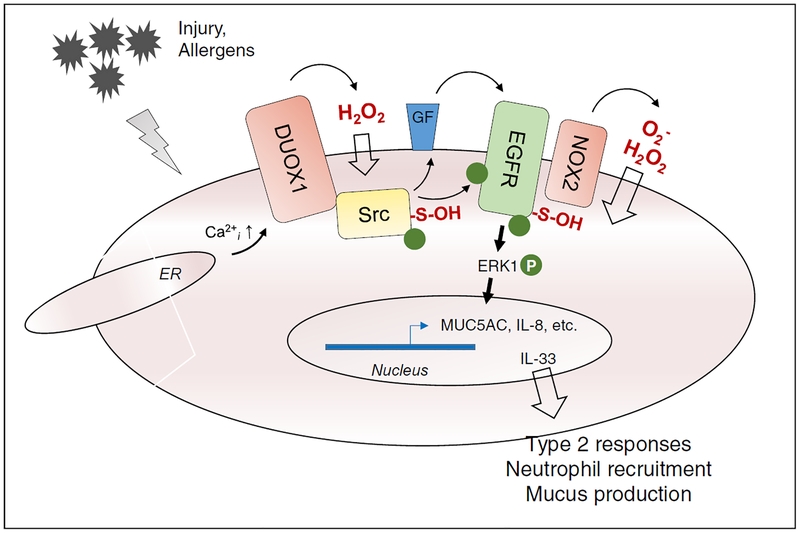
Asthma is a chronic inflammatory disease that is associated with hyperactive airways and variable airflow obstruction. Because asthma is typically characterized by activation of T-helper 2 (Th2) cells and eosinophilic inflammation, it is widely assumed that asthma pathology is largely driven by adaptive immune responses to allergens. However, a significant subgroup of non-allergic asthmatics also exists, and increasing evidence points to the importance of innate responses to epithelial injury in asthma pathogenesis (Hammad and Lambrecht 2008, Holgate 2011). Indeed, the respiratory epithelium forms a major source of cytokines that can activate type 2 inflammation, such as IL-25, IL-33, and TSLP, and asthma is also characterized by epithelial remodeling in favor of secretory epithelial cells (Goblet cells) that generate mucus which contributes to airflow obstruction. Epithelial signaling through tyrosine kinases such as the epidermal growth factor receptor (EGFR) has been identified as a convergent pathway in innate epithelial responses to infectious and other noxious stimuli, and contributes to epithelial repair pathways, as well as production of mucins (such as MUC5AC) and the chemokine IL-8 (Burgel and Nadel 2008). Increased expression and activation of EGFR is observed in the airways of asthmatic subjects (Polosa, Puddicombe et al. 2002, Hamilton, Puddicombe et al. 2005, Habibovic, Hristova et al. 2016), and experimental studies have shown that pharmacological EGFR inhibition can prevent many features of allergic asthma (Le Cras, Acciani et al. 2011). Unfortunately, the clinical significance is limited, and recent studies showed poor tolerance of an inhaled EGFR inhibitor (BIBW 2948 BS) in COPD patients, which also had minimal effect on mucus secretion (Woodruff, Wolff et al. 2010). As mentioned previously, EGFR-dependent signaling is subject to redox-dependent regulation, through oxidative inactivation of protein tyrosine phosphatases (Lee, Kwon et al. 1998), but more recent studies indicate that EGFR itself can also be oxidized on a conserved cysteine within its kinase domain, which results in enhanced kinase activity (Paulsen, Truong et al. 2011, Heppner, Hristova et al. 2016, Truong, Ung et al. 2016). During ligand-mediated EGFR activation this oxidative mechanism relies on NOX2, which interacts directly with EGFR (Paulsen, Truong et al. 2011), but in the context of EGFR transactivation by diverse injurious stimuli, DUOX1 was found to be primarily responsible for EGFR activation, through initial redox-dependent activation of Src non-receptor tyrosine kinase (Sham, Wesley et al. 2013, Heppner, Hristova et al. 2016) (Figure 3). Molecular studies indicate that during EGFR/Src activation, both proteins are successively oxidized to a sulfenic acid and then S-glutathionylated, but that sulfenylation is primarily responsible for increasing kinase activity (Paulsen, Truong et al. 2011, Heppner, Hristova et al. 2016, Truong, Ung et al. 2016). Supporting the importance of these redox mechanisms, DUOX1 was found to be responsible for EGFR-mediated activation of MUC5AC as well as IL-8 in response to various external triggers (Shao and Nadel 2005, Boots, Hristova et al. 2009). Moreover, through similar EGFR-dependent mechanisms, DUOX1 was also found to mediate of epithelial production of IL-33 and IL-25 in response to common airborne allergens that are associated with allergic asthma (Hristova, Habibovic et al. 2016). Indeed, oxidative EGFR activation has been observed in nasal epithelial cells of asthmatic subjects, and in the airways of mice with experimental allergic asthma, and genetic or pharmacological inhibition of DUOX1 was found to suppress EGFR activation, mucus metaplasia and airway remodeling, features known to be related to enhanced epithelial EGFR signaling (Habibovic, Hristova et al. 2016). These and other findings implicating DUOX1 in asthma pathology (Chang, Linderholm et al. 2013) suggest that pharmacological targeting of DUOX1 may be an attractive strategy to treat severe asthma (van der Vliet, Danyal et al. 2018). It is important to note that other NOX isoforms can also contribute to asthma pathology, although their actions may be mostly involved in other cell types such as T helper cell or dendritic cells (van der Vliet 2011, Sevin, Newcomb et al. 2013).
Figure 3: Schematic illustration of NOX-dependent activation of protein tyrosine kinases in epithelial responses to injury or allergens.
Injurious stimuli typically trigger Ca2+ mobilization or influx, which activates DUOX1 to produce H2O2. This contributes to oxidative activation of Src, and release of ligands for the epidermal growth factor receptor (EGFR). EGFR can also be oxidatively activated, which appears to depend on activation of NOX2. These collective events contribute to induction of mucus genes and IL-8, and release of alarmins such as IL-33.
5.4. Redox-dependent activation of CaMKII by methionine oxidation
Another interesting example of oxidant-mediated enzyme activation is the calcium/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII), which is typically activated by binding of Ca2+/calmodulin but can also be activated independently of Ca2+ by oxidation of a pair of methionine residues (Met281/Met282) (Erickson, Joiner et al. 2008). Oxidant-mediated CaMKII activation is also subject to reversal by methionine sulfoxide reductase A (MsrA). CaMKII is a multifunctional serine/threonine kinase that is ubiquitously expressed, but is most widely recognized for its role in cardiovascular biology (Hund and Mohler 2015). Recent studies highlighted the presence of oxidatively activated CaMKII in the bronchial epithelium of patients with asthma, suggesting a potential mechanism by which ROS contribute to asthma pathology. Indeed, the degree of oxidative CaMKII activation correlated with severity of allergic inflammation in animal models, based on studies with mice deficient in NOX activity or MsrA, and pharmacological targeting of CaMKII, using a small molecule inhibitor (KN-93) or an inhibitory peptide (AC3-I), was to inhibit inflammation and disease severity in a model of experimental asthma (Sanders, Koval et al. 2013). Subsequent studies indicated an association between oxidative CaMKII activation and mtROS production, suggesting that CaMKII is also localized in mitochondria and contributes to mtROS production in response to e.g. IL-13, an asthma-relevant cytokine. Moreover, a mitochondria-targeted CaMKII inhibitor was found to abrogate allergen-induced cytokine production, eosinophilic inflammation, and airways hyperresponsiveness in experimental models of allergic asthma (Sebag, Koval et al. 2017). The role of oxidative CaMKII activation in allergic asthma was also demonstrated by using a CaMKII knock-in mouse in which the two methionines were replaced with valine (Qu, Do et al. 2017). Also, these latter studies indicated an important function for oxidized CaMKII in mast cell accumulation during experimental asthma, suggesting wider roles for CaMKII oxidation in asthma pathology beyond functions in the airway epithelium (Qu, Do et al. 2017). While these studies suggest the potential use of CaMKII inhibitors in treatment of asthma, the ubiquitous presence of CaMKII and its importance in e.g. cardiac function (Hund and Mohler 2015) may complicate such approaches.
6. Summary and future perspectives
The lung represents the largest surface in contact with the external environment, and thus needs to effectively cope with the often oxidative nature of environmental triggers. At the same time, all lung cells also utilize ROS derived from mitochondria and a family of NOX enzymes in many biological functions, such as appropriate immune responses, and maintenance of cell and tissue homeostasis. Therefore, lung pathologies cannot simply be attributed to general increases in ROS, but more likely result from dysregulated redox homeostasis or redox signaling pathways. Likewise, rather than non-discriminatory inhibition of ROS by supplementation with antioxidant molecules, more selective approaches that target specific dysfunctional redox processes in lung diseases are likely more effective in treating these diseases. This issue also applies to considerations of other factors, such as aging or metabolic alterations associated with e.g. obesity, which are commonly associated with altered redox homeostasis and potentially unique redox alterations that may contribute to chronic lung disease. Recent efforts to develop immunotargeting approaches to deliver antioxidant enzymes to specific cell types (Han, Shuvaev et al. 2012) may be more effective, but since ROS are considered poor druggable targets (Ghezzi, Jaquet et al. 2017) it would be more sensible to develop approaches to target specific ROS sources or specific cellular redox events. Unfortunately, in spite of considerable progress, we still are lacking in specific pharmacological approaches that selectively inhibit individual NOX isoforms (van der Vliet, Danyal et al. 2018) or specific mitochondrial sources of ROS (in spite of encouraging results with mitochondria-targeted antioxidants), and further development of such specific inhibitors would be desirable. Alternatively, it is worthwhile to consider developing selective approaches to target other redox enzymes (e.g. GSTP1, ERp57, etc.) or specific redox-modifications in proteins, for example towards sulfenylated forms of EGFR, which would potentially avoid unwanted side effects of current EGFR tyrosine kinase inhibitors. The field of redox biology has relatively recently emerged. Judging from the newly discovered insights in reactions that govern redox biochemistry and new tools to measure and target redox perturbations in biological molecules, it is likely that important new information will be forthcoming about the mechanisms whereby redox-based processes regulate lung homeostasis, and their dysregulation facilitates disease. Such insights will be paramount towards the development of targeted redox-based drugs.
Acknowledgements:
The author wishes to thank NHLBI for their generous research support (grants R01 HL085646, R01 HL138708, R01 HL122383 and R35 HL135828).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal A and Mabalirajan U (2016). “Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria.” Am J Physiol Lung Cell Mol Physiol 310(2): L103–113. [DOI] [PubMed] [Google Scholar]
- Amara N, Goven D, Prost F, Muloway R, Crestani B and Boczkowski J (2010). “NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts.” Thorax 65(8): 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A and Dupuy C (2005). “Dual Oxidase-2 Has an Intrinsic Ca2+-dependent H2O2-generating Activity.” J Biol Chem 280(34): 30046–30054. [DOI] [PubMed] [Google Scholar]
- Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC and Janssen-Heininger YM (2009). “Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas.” J Cell Biol 184(2): 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM, McMillan DH, Jones JT, Nolin JD, Abdalla S, Schneider R, Seward DJ, Roberson EC, Liptak MD, Cousins ME, Butnor KJ, Taatjes DJ, Budd RC, Irvin CG, Ho YS, Hakem R, Brown KK, Matsui R, Bachschmid MM, Gomez JL, Kaminski N, van der Vliet A and Janssen-Heininger YMW (2018). “Reducing protein oxidation reverses lung fibrosis.” Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD and Janssen-Heininger YM (2012). “Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis.” Mol Cell Biol 32(17): 3464–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anathy V, Roberson EC, Guala AS, Godburn KE, Budd RC and Janssen-Heininger YM (2012). “Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death.” Antioxid Redox Signal 16(6): 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis AA, Hazen SL, Comhair SA and Erzurum SC (2003). “Oxidative and nitrosative events in asthma.” Free Radic Biol Med 35(3): 213–225. [DOI] [PubMed] [Google Scholar]
- Antonenkov VD, Grunau S, Ohlmeier S and Hiltunen JK (2010). “Peroxisomes are oxidative organelles.” Antioxid Redox Signal 13(4): 525–537. [DOI] [PubMed] [Google Scholar]
- Aracena-Parks P, Goonasekera SA, Gilman CP, Dirksen RT, Hidalgo C and Hamilton SL (2006). “Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1.” J Biol Chem 281(52): 40354–40368. [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD and Nauseef W (2002). “The neutrophil NADPH oxidase.” Arch Biochem Biophys 397(2): 342–344. [DOI] [PubMed] [Google Scholar]
- Bae YS, Oh H, Rhee SG and Yoo YD (2011). “Regulation of reactive oxygen species generation in cell signaling.” Mol Cells 32(6): 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K and Krause KH (2007). “The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology.” Physiol Rev 87(1): 245–313. [DOI] [PubMed] [Google Scholar]
- Bentley AR, Emrani P and Cassano PA (2008). “Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: a systematic review.” Thorax 63(11): 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Hecker L, Luckhardt TR, Cheng G and Thannickal VJ (2014). “NADPH oxidases in lung health and disease.” Antioxid Redox Signal 20(17): 2838–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM and Thannickal VJ (2017). “NADPH Oxidase 4 (Nox4) Suppresses Mitochondrial Biogenesis and Bioenergetics in Lung Fibroblasts via a Nuclear Factor Erythroid-derived 2-like 2 (Nrf2)-dependent Pathway.” J Biol Chem 292(7): 3029–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Gorin Y and Abboud HE (2009). “Subcellular localization of Nox4 and regulation in diabetes.” Proc Natl Acad Sci U S A 106(34): 14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A and van der Vliet A (2009). “ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli.” J Biol Chem 284(26): 17858–17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhenouna S, Wilson MA, Bahmed K and Kosmider B (2018). “Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease.” Oxid Med Cell Longev 2018: 5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar SS, Kennedy TP, Quinn M and Hoidal JR (2003). “Redox signaling of NF-kappaB by membrane NAD(P)H oxidases in normal and malignant cells.” Protoplasma 221(1–2): 117–127. [DOI] [PubMed] [Google Scholar]
- Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT and Mora AL (2015). “PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis.” J Clin Invest 125(2): 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel PR and Nadel JA (2008). “Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases.” Eur Respir J 32(4): 1068–1081. [DOI] [PubMed] [Google Scholar]
- Carneiro MBH, Roma EH, Ranson AJ, Doria NA, Debrabant A, Sacks DL, Vieira LQ and Peters NC (2018). “NOX2-Derived Reactive Oxygen Species Control Inflammation during Leishmania amazonensis Infection by Mediating Infection-Induced Neutrophil Apoptosis.” J Immunol 200(1): 196–208. [DOI] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C and Krause KH (2011). “A key role for NOX4 in epithelial cell death during development of lung fibrosis.” Antioxid Redox Signal 15(3): 607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH and Barazzone Argiroffo C (2009). “NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice.” Am J Respir Crit Care Med 180(10): 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AC and Maggini S (2017). “Vitamin C and Immune Function.” Nutrients 9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case AJ, Li S, Basu U, Tian J and Zimmerman MC (2013). “Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons.” Am J Physiol Heart Circ Physiol 305(1): H19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H and Harper R (2013). “Dual oxidase regulates neutrophil recruitment in allergic airways.” Free Radic Biol Med 65: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan SM and Choi AM (2016). “Mitochondria in lung disease.” J Clin Invest 126(3): 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, Pabon MA, Konrad C, Polverino F, Siempos II, Perez E, Mizumura K, Ghosh MC, Parameswaran H, Williams NC, Rooney KT, Chen ZH, Goldklang MP, Yuan GC, Moore SC, Demeo DL, Rouault TA, D’Armiento JM, Schon EA, Manfredi G, Quackenbush J, Mahmood A, Silverman EK, Owen CA and Choi AM (2016). “Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice.” Nat Med 22(2): 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA and Erzurum SC (2005). “The regulation and role of extracellular glutathione peroxidase.” Antioxid Redox Signal 7(1–2): 72–79. [DOI] [PubMed] [Google Scholar]
- Comhair SA and Erzurum SC (2010). “Redox control of asthma: molecular mechanisms and therapeutic opportunities.” Antioxid Redox Signal 12(1): 93–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comhair SA, Xu W, Ghosh S, Thunnissen FB, Almasan A, Calhoun WJ, Janocha AJ, Zheng L, Hazen SL and Erzurum SC (2005). “Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity.” Am J Pathol 166(3): 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM and Ojcius DM (2007). “ATP Activates a Reactive Oxygen Species-dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages.” J Biol Chem 282(5): 2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniff B, Newick K, Nelson KJ, Wozniak AN, Beuschel S, Leavitt B, Bhave A, Butnor K, Koenig A, Chouchani ET, James AM, Haynes AC, Lowther WT, Murphy MP, Shukla A and Heintz NH (2015). “Disabling Mitochondrial Peroxide Metabolism via Combinatorial Targeting of Peroxiredoxin 3 as an Effective Therapeutic Approach for Malignant Mesothelioma.” PLoS One 10(5): e0127310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S (2011). “Cross talk between mitochondria and NADPH oxidases.” Free Radic Biol Med 51(7): 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich AM, Meyer HA, Krokowski M, Quarcoo D, Ahrens B, Kube SM, Witzenrath M, Esworthy RS, Chu FF and Hamelmann E (2010). “Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice.” Eur Respir J 35(5): 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drose S and Brandt U (2012). “Molecular mechanisms of superoxide production by the mitochondrial respiratory chain.” Adv Exp Med Biol 748: 145–169. [DOI] [PubMed] [Google Scholar]
- El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A and Dupuy C (2005). “Dual oxidase2 is expressed all along the digestive tract.” Am J Physiol Gastrointest Liver Physiol 288(5): G933–942. [DOI] [PubMed] [Google Scholar]
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ and Anderson ME (2008). “A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation.” Cell 133(3): 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV (2009). “Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies.” Proc Am Thorac Soc 6(3): 256–259. [DOI] [PubMed] [Google Scholar]
- Finkel T (2003). “Oxidant signals and oxidative stress.” Curr Opin Cell Biol 15(2): 247–254. [DOI] [PubMed] [Google Scholar]
- Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B and Ballard PL (2007). “Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells.” Am J Physiol Lung Cell Mol Physiol 292(6): L1506–1514. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AM, Park Y, Brown LA and Jones DP (2014). “Children with severe asthma have unique oxidative stress-associated metabolomic profiles.” J Allergy Clin Immunol 133(1): 258–261 e251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaquer A, Heinzmann A, Rospleszcz S, Mailaparambil B, Dietrich H, Strauch K and Grychtol R (2014). “Association study of mitochondrial genetic polymorphisms in asthmatic children.” Mitochondrion 14(1): 49–53. [DOI] [PubMed] [Google Scholar]
- Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ and Crapo JD (1997). “Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization.” Am J Respir Cell Mol Biol 17(4): 393–403. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto JM and Torres M (2004). “Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers.” Am J Physiol Cell Physiol 287(2): C246–256. [DOI] [PubMed] [Google Scholar]
- Forman HJ and Torres M (2002). “Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling.” Am J Respir Crit Care Med 166(12 Pt 2): S4–8. [DOI] [PubMed] [Google Scholar]
- Forteza R, Salathe M, Miot F and Conner GE (2005). “Regulated hydrogen peroxide production by Duox in human airway epithelial cells.” Am J Respir Cell Mol Biol 32(5): 462–469. [DOI] [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS and Whitlock EP (2013). “Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force.” Ann Intern Med 159(12): 824–834. [DOI] [PubMed] [Google Scholar]
- Fremont S, Romet-Lemonne G, Houdusse A and Echard A (2017). “Emerging roles of MICAL family proteins - from actin oxidation to membrane trafficking during cytokinesis.” J Cell Sci 130(9): 1509–1517. [DOI] [PubMed] [Google Scholar]
- Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH and Malik AB (2002). “Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice.” J Immunol 168(8): 3974–3982. [DOI] [PubMed] [Google Scholar]
- Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M and Conner GE (2009). “Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli.” Free Radic Biol Med 47(10): 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M and Leto TL (2004). “The Nox family of NAD(P)H oxidases: host defense and beyond.” J Biol Chem 279(50): 51715–51718. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Witta J, Baffi J, Lekstrom K and Leto TL (2003). “Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense.” Faseb J. [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Jaquet V, Marcucci F and Schmidt H (2017). “The oxidative stress theory of disease: levels of evidence and epistemological aspects.” Br J Pharmacol 174(12): 1784–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, Zheng L, Kaveti S, Kinter M, Hazen SL and Erzurum SC (2006). “Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation.” J Immunol 176(9): 5587–5597. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Willard B, Comhair SA, Dibello P, Xu W, Shiva S, Aulak KS, Kinter M and Erzurum SC (2013). “Disulfide bond as a switch for copper-zinc superoxide dismutase activity in asthma.” Antioxid Redox Signal 18(4): 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan-Gerstl R, Wallach-Dayan SB, Amir G and Breuer R (2007). “Epithelial cell apoptosis by fas ligand-positive myofibroblasts in lung fibrosis.” Am J Respir Cell Mol Biol 36(3): 270–275. [DOI] [PubMed] [Google Scholar]
- Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D and Singh KK (2010). “NADPH oxidase 4 is an oncoprotein localized to mitochondria.” Cancer Biol Ther 10(3): 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibovic A, Hristova M, Heppner DE, Danyal K, Ather JL, Janssen-Heininger YM, Irvin CG, Poynter ME, Lundblad LK, Dixon AE, Geiszt M and van der Vliet A (2016). “DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma.” JCI Insight 1(18): e88811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LM, Puddicombe SM, Dearman RJ, Kimber I, Sandstrom T, Wallin A, Howarth PH, Holgate ST, Wilson SJ and Davies DE (2005). “Altered protein tyrosine phosphorylation in asthmatic bronchial epithelium.” Eur Respir J 25(6): 978–985. [DOI] [PubMed] [Google Scholar]
- Hammad H and Lambrecht BN (2008). “Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma.” Nat Rev Immunol 8(3): 193–204. [DOI] [PubMed] [Google Scholar]
- Han J, Shuvaev VV and Muzykantov VR (2012). “Targeted interception of signaling reactive oxygen species in the vascular endothelium.” Ther Deliv 3(2): 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harju T, Kaarteenaho-Wiik R, Sirvio R, Paakko P, Crapo JD, Oury TD, Soini Y and Kinnula VL (2004). “Manganese superoxide dismutase is increased in the airways of smokers’ lungs.” Eur Respir J 24(5): 765–771. [DOI] [PubMed] [Google Scholar]
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H and Wu R (2005). “Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium.” FEBS Lett 579(21): 4911–4917. [DOI] [PubMed] [Google Scholar]
- Harper RW, Xu C, McManus M, Heidersbach A and Eiserich JP (2006). “Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium.” FEBS Lett 580(22): 5150–5154. [DOI] [PubMed] [Google Scholar]
- Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY and Thannickal VJ (2014). “Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance.” Sci Transl Med 6(231): 231ra247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ and Thannickal VJ (2009). “NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury.” Nat Med 15(9): 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner DE, Hristova M, Dustin CM, Danyal K, Habibovic A and van der Vliet A (2016). “The NADPH Oxidases DUOX1 and NOX2 Play Distinct Roles in Redox Regulation of Epidermal Growth Factor Receptor Signaling.” J Biol Chem 291(44): 23282–23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner DE, Janssen-Heininger YMW and van der Vliet A (2017). “The role of sulfenic acids in cellular redox signaling: Reconciling chemical kinetics and molecular detection strategies.” Arch Biochem Biophys 616: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SM, Chapman DG, Lahue KG, Cahoon JM, Rattu GK, Daphtary N, Aliyeva M, Fortner KA, Erzurum SC, Comhair SA, Woodruff PG, Bhakta N, Dixon AE, Irvin CG, Janssen-Heininger YM, Poynter ME and Anathy V (2016). “Protein disulfide isomerase-endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness.” J Allergy Clin Immunol 137(3): 822–832 e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SM, Tully JE, Nolin JD, Lahue KG, Goldman DH, Daphtary N, Aliyeva M, Irvin CG, Dixon AE, Poynter ME and Anathy V (2013). “Endoplasmic reticulum stress mediates house dust mite-induced airway epithelial apoptosis and fibrosis.” Respir Res 14: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST (2011). “The sentinel role of the airway epithelium in asthma pathogenesis.” Immunol Rev 242(1): 205–219. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S and Davies DE (2004). “Epithelialmesenchymal communication in the pathogenesis of chronic asthma.” Proc Am Thorac Soc 1(2): 93–98. [DOI] [PubMed] [Google Scholar]
- Hristova M, Habibovic A, Veith C, Janssen-Heininger YM, Dixon AE, Geiszt M and van der Vliet A (2016). “Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses.” J Allergy Clin Immunol 137(5): 1545–1556 e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund TJ and Mohler PJ (2015). “Role of CaMKII in cardiac arrhythmias.” Trends Cardiovasc Med 25(5): 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, Aras S, Sommer N, Sanderson TH, Tost M, Neff F, Aguilar-Pimentel JA, Becker L, Naton B, Rathkolb B, Rozman J, Favor J, Hans W, Prehn C, Puk O, Schrewe A, Sun M, Hofler H, Adamski J, Bekeredjian R, Graw J, Adler T, Busch DH, Klingenspor M, Klopstock T, Ollert M, Wolf E, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Weissmann N, Doan JW, Bassett DJ and Grossman LI (2012). “Cytochrome c oxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology.” FASEB J 26(9): 3916–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idiopathic Pulmonary Fibrosis Clinical Research, Martinez N,FJ, de Andrade JA, Anstrom KJ, King TE Jr. and Raghu G (2014). “Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis.” N Engl J Med 370(22): 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen EA, Taranova AG, Lee NA and Lee JJ (2007). “Eosinophils: singularly destructive effector cells or purveyors of immunoregulation?” J Allergy Clin Immunol 119(6): 1313–1320. [DOI] [PubMed] [Google Scholar]
- Jaffer OA, Carter AB, Sanders PN, Dibbern ME, Winters CJ, Murthy S, Ryan AJ, Rokita AG, Prasad AM, Zabner J, Kline JN, Grumbach IM and Anderson ME (2015). “Mitochondrial-targeted antioxidant therapy decreases transforming growth factor-beta-mediated collagen production in a murine asthma model.” Am J Respir Cell Mol Biol 52(1): 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC and Amigorena S (2007). “Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes.” Nat Cell Biol 9(4): 367–378. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG and van der Vliet A (2008). “Redox-based regulation of signal transduction: principles, pitfalls, and promises.” Free Radic Biol Med 45(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP (2015). “Redox theory of aging.” Redox Biol 5: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP and Sies H (2015). “The Redox Code.” Antioxid Redox Signal 23(9): 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, Lee WJ, Choi CW, Shin HK, Kim DJ, Koh ES, Park CS, Kwon SW and Park SW (2016). “Metabolic Profiling Regarding Pathogenesis of Idiopathic Pulmonary Fibrosis.” J Proteome Res 15(5): 1717–1724. [DOI] [PubMed] [Google Scholar]
- Kassim SY, Fu X, Liles WC, Shapiro SD, Parks WC and Heinecke JW (2005). “NADPH oxidase restrains the matrix metalloproteinase activity of macrophages.” J Biol Chem 280(34): 30201–30205. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T and Ushio-Fukai M (2017). “ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis.” Am J Physiol Cell Physiol 312(6): C749–C764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL and Crapo JD (2003). “Superoxide dismutases in the lung and human lung diseases.” Am J Respir Crit Care Med 167(12): 1600–1619. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Lehtonen S, Kaarteenaho-Wiik R, Lakari E, Paakko P, Kang SW, Rhee SG and Soini Y (2002). “Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis.” Thorax 57(2): 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham PA and Barnes PJ (2013). “Oxidative stress in COPD.” Chest 144(1): 266–273. [DOI] [PubMed] [Google Scholar]
- Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T and Daiber A (2014). “Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models.” Antioxid Redox Signal 20(2): 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA and Hershey GK (2011). “Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma.” Am J Physiol Lung Cell Mol Physiol 300(3): L414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR and Rhee SG (1998). “Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor.” J Biol Chem 273(25): 15366–15372. [DOI] [PubMed] [Google Scholar]
- Lenaz G (2012). “Mitochondria and reactive oxygen species. Which role in physiology and pathology?” Adv Exp Med Biol 942: 93–136. [DOI] [PubMed] [Google Scholar]
- Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B and Engelhardt JF (2006). “Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes.” Mol Cell Biol 26(1): 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, Sulovari A, Danyal K, Heppner DE, Seward DJ and van der Vliet A (2017). “Paradoxical roles of dual oxidases in cancer biology.” Free Radic Biol Med 110: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SF, Kuo HC, Tseng CW, Huang HT, Chen YC, Tseng CC and Lin MC (2015). “Leukocyte Mitochondrial DNA Copy Number Is Associated with Chronic Obstructive Pulmonary Disease.” PLoS One 10(9): e0138716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Domenech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N and Kittler JT (2018). “Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution.” EMBO J 37(3): 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta B and Gladyshev VN (2017). “Regulated methionine oxidation by monooxygenases.” Free Radic Biol Med 109: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC and Knaus UG (2006). “Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases.” Cell Signal 18(1): 69–82. [DOI] [PubMed] [Google Scholar]
- McMillan DH, van der Velden JL, Lahue KG, Qian X, Schneider RW, Iberg MS, Nolin JD, Abdalla S, Casey DT, Tew KD, Townsend DM, Henderson CJ, Wolf CR, Butnor KJ, Taatjes DJ, Budd RC, Irvin CG, van der Vliet A, Flemer S, Anathy V and Janssen-Heininger YM (2016). “Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase pi.” JCI Insight 1(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH and Weissmann N (2007). “Hypoxia-Dependent Regulation of Nonphagocytic NADPH Oxidase Subunit NOX4 in the Pulmonary Vasculature.” Circ Res 101: 258–267. [DOI] [PubMed] [Google Scholar]
- Moffett AS, Bender KW, Huber SC and Shukla D (2017). “Allosteric Control of a Plant Receptor Kinase through S-Glutathionylation.” Biophys J 113(11): 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern DE, Gifford MA, Li LL, Doerschuk CM and Dinauer MC (1997). “Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus.” J Exp Med 185(2): 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB Jr., Nauseef WM, Dupuy C and Banfi B (2007). “A novel host defense system of airways is defective in cystic fibrosis.” Am J Respir Crit Care Med 175(2): 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem A, Masood A and Siddiqui N (2008). “Oxidant--antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options.” Ther Adv Respir Dis 2(4): 215–235. [DOI] [PubMed] [Google Scholar]
- Nagai K, Betsuyaku T, Suzuki M, Nasuhara Y, Kaga K, Kondo S and Nishimura M (2008). “Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease.” Antioxid Redox Signal 10(4): 705–714. [DOI] [PubMed] [Google Scholar]
- Pache JC, Carnesecchi S, Deffert C, Donati Y, Herrmann FR, Barazzone-Argiroffo C and Krause KH (2011). “NOX-4 is expressed in thickened pulmonary arteries in idiopathic pulmonary fibrosis.” Nat Med 17(1): 31–32; author reply 32–33. [DOI] [PubMed] [Google Scholar]
- Pak O, Scheibe S, Esfandiary A, Gierhardt M, Sydykov A, Logan A, Fysikopoulos A, Veit F, Hecker M, Kroschel F, Quanz K, Erb A, Schafer K, Fassbinder M, Alebrahimdehkordi N, Ghofrani HA, Schermuly RT, Brandes RP, Seeger W, Murphy MP, Weissmann N and Sommer N (2018). “Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension.” Eur Respir J. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE and Carroll KS (2011). “Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity.” Nat Chem Biol 8(1): 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin AV, Pace PE, Behring JB, Paton LN, Soethoudt M, Bachschmid MM and Winterbourn CC (2016). “Glutathionylation of the Active Site Cysteines of Peroxiredoxin 2 and Recycling by Glutaredoxin.” J Biol Chem 291(6): 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA and Suliman HB (2017). “Mitochondrial Dysfunction in Lung Pathogenesis.” Annu Rev Physiol 79: 495–515. [DOI] [PubMed] [Google Scholar]
- Pierron D, Wildman DE, Huttemann M, Markondapatnaikuni GC, Aras S and Grossman LI (2012). “Cytochrome c oxidase: evolution of control via nuclear subunit addition.” Biochim Biophys Acta 1817(4): 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Puddicombe SM, Krishna MT, Tuck AB, Howarth PH, Holgate ST and Davies DE (2002). “Expression of c-erbB receptors and ligands in the bronchial epithelium of asthmatic subjects.” J Allergy Clin Immunol 109(1): 75–81. [DOI] [PubMed] [Google Scholar]
- Qu J, Do DC, Zhou Y, Luczak E, Mitzner W, Anderson ME and Gao P (2017). “Oxidized CaMKII promotes asthma through the activation of mast cells.” JCI Insight 2(1): e90139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Biswas SK and Kode A (2006). “Oxidant and antioxidant balance in the airways and airway diseases.” Eur J Pharmacol 533(1–3): 222–239. [DOI] [PubMed] [Google Scholar]
- Ribeiro CM, Paradiso AM, Livraghi A and Boucher RC (2003). “The mitochondrial barriers segregate agonist-induced calcium-dependent functions in human airway epithelia.” J Gen Physiol 122(4): 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinna A, Torres M and Forman HJ (2006). “Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B.” Free Radic Biol Med 41(1): 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M (2014). “Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits.” Nat Med 20(7): 709–711. [DOI] [PubMed] [Google Scholar]
- Rostila A, Puustinen A, Toljamo T, Vuopala K, Lindstrom I, Nyman TA, Oksa P, Vehmas T and Anttila SL (2012). “Peroxiredoxins and tropomyosins as plasma biomarkers for lung cancer and asbestos exposure.” Lung Cancer 77(2): 450–459. [DOI] [PubMed] [Google Scholar]
- Rybicka JM, Balce DR, Chaudhuri S, Allan ER and Yates RM (2012). “Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner.” Embo J 31(4): 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicka JM, Balce DR, Khan MF, Krohn RM and Yates RM (2010). “NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes.” Proc Natl Acad Sci U S A 107(23): 10496–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PN, Koval OM, Jaffer OA, Prasad AM, Businga TR, Scott JA, Hayden PJ, Luczak ED, Dickey DD, Allamargot C, Olivier AK, Meyerholz DK, Robison AJ, Winder DG, Blackwell TS, Dworski R, Sammut D, Wagner BA, Buettner GR, Pope RM, Miller FJ Jr., Dibbern ME, Haitchi HM, Mohler PJ, Howarth PH, Zabner J, Kline JN, Grumbach IM and Anderson ME (2013). “CaMKII is essential for the proasthmatic effects of oxidation.” Sci Transl Med 5(195): 195ra197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P and Bergo MO (2014). “Antioxidants accelerate lung cancer progression in mice.” Sci Transl Med 6(221): 221ra215. [DOI] [PubMed] [Google Scholar]
- Schrader M and Fahimi HD (2006). “Peroxisomes and oxidative stress.” Biochim Biophys Acta 1763(12): 1755–1766. [DOI] [PubMed] [Google Scholar]
- Schuler MH, Lewandowska A, Caprio GD, Skillern W, Upadhyayula S, Kirchhausen T, Shaw JM and Cunniff B (2017). “Miro1-mediated mitochondrial positioning shapes intracellular energy gradients required for cell migration.” Mol Biol Cell 28(16): 2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Machen TE, Illek B and Fischer H (2004). “NADPH oxidase-dependent acid production in airway epithelial cells.” J Biol Chem 279(35): 36454–36461. [DOI] [PubMed] [Google Scholar]
- Sebag SC, Koval OM, Paschke JD, Winters CJ, Jaffer OA, Dworski R, Sutterwala FS, Anderson ME and Grumbach IM (2017). “Mitochondrial CaMKII inhibition in airway epithelium protects against allergic asthma.” JCI Insight 2(3): e88297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BH, Davidson BA, Hutson AD, Russo TA, Holm BA, Mullan B, Habitzruther M, Holland SM and Knight PR 3rd (2007). “Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice.” Am J Physiol Lung Cell Mol Physiol 292(3): L760–768. [DOI] [PubMed] [Google Scholar]
- Segal BH, Grimm MJ, Khan AN, Han W and Blackwell TS (2012). “Regulation of innate immunity by NADPH oxidase.” Free Radic Biol Med 53(1): 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA and Chandel NS (2012). “Physiological roles of mitochondrial reactive oxygen species.” Mol Cell 48(2): 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevin CM, Newcomb DC, Toki S, Han W, Sherrill TP, Boswell MG, Zhu Z, Collins RD, Boyd KL, Goleniewska K, Huckabee MM, Blackwell TS and Peebles RS Jr. (2013). “Deficiency of gp91phox inhibits allergic airway inflammation.” Am J Respir Cell Mol Biol 49(3): 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel GS and Horvath TL (2015). “Mitochondrial ROS signaling in organismal homeostasis.” Cell 163(3): 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham D, Wesley UV, Hristova M and van der Vliet A (2013). “ATP-Mediated Transactivation of the Epidermal Growth Factor Receptor in Airway Epithelial Cells Involves DUOX1-Dependent Oxidation of Src and ADAM17.” PLoS One 8(1): e54391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D, Rodriguez R and Block K (2017). “NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance.” Nat Commun 8(1): 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao MX and Nadel JA (2005). “Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells.” Proc Natl Acad Sci U S A 102(3): 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, Brightling CE, Wardlaw AJ and Pavord ID (2007). “Association between neutrophilic airway inflammation and airflow limitation in adults with asthma.” Chest 132(6): 1871–1875. [DOI] [PubMed] [Google Scholar]
- Singel KL and Segal BH (2016). “NOX2-dependent regulation of inflammation.” Clin Sci (Lond) 130(7): 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES, Thannickal VJ, Moore BB, Christensen PJ and Simon RH (2010). “Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis.” Am J Respir Crit Care Med 181(3): 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Shamsuddin M, Sporn PH, Denenberg M and Anderson J (1997). “Reduced superoxide dismutase in lung cells of patients with asthma.” Free Radic Biol Med 22(7): 1301–1307. [DOI] [PubMed] [Google Scholar]
- Sommer N, Huttemann M, Pak O, Scheibe S, Knoepp F, Sinkler C, Malczyk M, Gierhardt M, Esfandiary A, Kraut S, Jonas F, Veith C, Aras S, Sydykov A, Alebrahimdehkordi N, Giehl K, Hecker M, Brandes RP, Seeger W, Grimminger F, Ghofrani HA, Schermuly RT, Grossman LI and Weissmann N (2017). “Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing.” Circ Res 121(4): 424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer N, Strielkov I, Pak O and Weissmann N (2016). “Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction.” Eur Respir J 47(1): 288–303. [DOI] [PubMed] [Google Scholar]
- Sun T, Liu J and Zhao de W (2016). “Efficacy of N-Acetylcysteine in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis.” Medicine (Baltimore) 95(19): e3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar IK, Yao H and Rahman I (2013). “Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases.” Antioxid Redox Signal 18(15): 1956–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada LS (2006). “Specificity in reactive oxidant signaling: think globally, act locally.” J Cell Biol 174(5): 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topf U, Suppanz I, Samluk L, Wrobel L, Boser A, Sakowska P, Knapp B, Pietrzyk MK, Chacinska A and Warscheid B (2018). “Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species.” Nat Commun 9(1): 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TH, Ung PM, Palde PB, Paulsen CE, Schlessinger A and Carroll KS (2016). “Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase.” Cell Chem Biol 23(7): 837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M (2009). “Compartmentalization of redox signaling through NADPH oxidase-derived ROS.” Antioxid Redox Signal 11(6): 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A (2008). “NADPH oxidases in lung biology and pathology: host defense enzymes, and more.” Free Radic Biol Med 44(6): 938–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A (2011). “Nox enzymes in allergic airway inflammation.” Biochim Biophys Acta 1810(11): 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A, Danyal K and Heppner DE (2018). “Dual oxidase: a novel therapeutic target in allergic disease.” Br J Pharmacol 175(9): 1401–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veredas FJ, Canton FR and Aledo JC (2017). “Methionine residues around phosphorylation sites are preferentially oxidized in vivo under stress conditions.” Sci Rep 7: 40403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Ray A, Ray P, Erzurum SC, Busse WW, Zhao J, Trudeau JB and Wenzel SE (2014). “An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma.” Mucosal Immunol 7(5): 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan WY, Hollins F, Haste L, Woodman L, Hirst RA, Bolton S, Gomez E, Sutcliffe A, Desai D, Chachi L, Mistry V, Szyndralewiez C, Wardlaw A, Saunders R, O’Callaghan C, Andrew PW and Brightling CE (2016). “NADPH Oxidase-4 Overexpression Is Associated With Epithelial Ciliary Dysfunction in Neutrophilic Asthma.” Chest 149(6): 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SA, Wenzel S, Teague WG, Israel E, Erzurum SC and Hazen SL (2009). “Noninvasive markers of airway inflammation in asthma.” Clin Transl Sci 2(2): 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley UV, Bove PF, Hristova M, McCarthy S and van der Vliet A (2007). “Airway Epithelial Cell Migration and Wound Repair by ATP-mediated Activation of Dual Oxidase 1.” J Biol Chem 282(5): 3213–3220. [DOI] [PubMed] [Google Scholar]
- Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, Bittner A, Rao N, Murphy MP, Kirkham PA, Chung KF, Adcock IM and Copdmap (2015). “Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease.” J Allergy Clin Immunol 136(3): 769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M and Conner GE (2003). “Lactoperoxidase and Human Airway Host Defense.” Am J Respir Cell Mol Biol 29(2): 206–212. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB, Livesey JH and Kettle AJ (2006). “Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing.” J Biol Chem 281(52): 39860–39869. [DOI] [PubMed] [Google Scholar]
- Wong HS, Dighe PA, Mezera V, Monternier PA and Brand MD (2017). “Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions.” J Biol Chem 292(41): 16804–16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff PG, Wolff M, Hohlfeld JM, Krug N, Dransfield MT, Sutherland ER, Criner GJ, Kim V, Prasse A, Nivens MC, Tetzlaff K, Heilker R and Fahy JV (2010). “Safety and efficacy of an inhaled epidermal growth factor receptor inhibitor (BIBW 2948 BS) in chronic obstructive pulmonary disease.” Am J Respir Crit Care Med 181(5): 438–445. [DOI] [PubMed] [Google Scholar]
- Wynes MW, Edelman BL, Kostyk AG, Edwards MG, Coldren C, Groshong SD, Cosgrove GP, Redente EF, Bamberg A, Brown KK, Reisdorph N, Keith RC, Frankel SK and Riches DW (2011). “Increased cell surface Fas expression is necessary and sufficient to sensitize lung fibroblasts to Fas ligation-induced apoptosis: implications for fibroblast accumulation in idiopathic pulmonary fibrosis.” J Immunol 187(1): 527–537. [DOI] [PubMed] [Google Scholar]
- Xu W, Ghosh S, Comhair SA, Asosingh K, Janocha AJ, Mavrakis DA, Bennett CD, Gruca LL, Graham BB, Queisser KA, Kao CC, Wedes SH, Petrich JM, Tuder RM, Kalhan SC and Erzurum SC (2016). “Increased mitochondrial arginine metabolism supports bioenergetics in asthma.” J Clin Invest 126(7): 2465–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Janocha AJ, Leahy RA, Klatte R, Dudzinski D, Mavrakis LA, Comhair SA, Lauer ME, Cotton CU and Erzurum SC (2014). “A novel method for pulmonary research: assessment of bioenergetic function at the air-liquid interface.” Redox Biol 2: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Gupta V, Carroll KS and Liebler DC (2014). “Site-specific mapping and quantification of protein S-sulphenylation in cells.” Nat Commun 5: 4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RP, Hopkins R, Black PN, Eddy C, Wu L, Gamble GD, Mills GD, Garrett JE, Eaton TE and Rees MI (2006). “Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function.” Thorax 61(5): 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI and Mittler R (2017). “ROS-induced ROS release in plant and animal cells.” Free Radic Biol Med. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ and Folz RJ (2002). “Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression.” Free Radic Biol Med 33(3): 337–349. [DOI] [PubMed] [Google Scholar]
- Zhang H, Du Y, Zhang X, Lu J and Holmgren A (2014). “Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal.” Antioxid Redox Signal 21(5): 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ye ZW, Singh S, Townsend DM and Tew KD (2018). “An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation.” Free Radic Biol Med 120: 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Lu H and Li C (2015). “Proapoptotic activities of protein disulfide isomerase (PDI) and PDIA3 protein, a role of the Bcl-2 protein Bak.” J Biol Chem 290(14): 8949–8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifa E, Daniil Z, Skoumi E, Stavrou M, Papadimitriou K, Terzenidou M, Kostikas K, Bagiatis V, Gourgoulianis KI and Mamuris Z (2012). “Mitochondrial genetic background plays a role in increasing risk to asthma.” Mol Biol Rep 39(4): 4697–4708. [DOI] [PubMed] [Google Scholar]
- Zito E (2015). “ERO1: A protein disulfide oxidase and H2O2 producer.” Free Radic Biol Med 83: 299–304. [DOI] [PubMed] [Google Scholar]