Abstract
Background:
We theorized that modafinil, an atypical psychomotor stimulant, utilized to improve daytime somnolence in patients with obstructive sleep apnea, would improve functional recovery after general anesthesia by improving time to extubation, post-anesthesia care unit (PACU) length of stay and subjective recovery after general anesthesia.
Methods:
A double blind, randomized, placebo-controlled pilot study was performed. 102 patients with the diagnosis of obstructive sleep apnea (OSA) were randomized to receive either 200 mg of modafinil or placebo before general anesthesia. The trial was terminated for futility. The primary outcome was PACU length of stay between groups. Secondary functional metrics of improved post-anesthesia recovery were compared between groups.
Results:
No difference between groups was found on the primary outcome of PACU length of stay (PACULOS). Emergence from general anesthesia was not significantly different when assessed by the time period between termination of volatile anesthetic and extubation. Similarly, no difference between groups was found in intraoperative bispectral index (BIS) values, postoperative pain scores or narcotic consumption (morphine equivalent units). In the post-anesthesia care unit, respiratory rate was increased and mean arterial pressure was lower in the modafinil group.
Conclusions:
Our results suggest that the use of single-dose preoperative modafinil may not improve functional recovery after general anesthesia in patients with the diagnosis of OSA. Further research is needed before use of atypical psychomotor stimulants in this surgical population.
Keywords: anesthesia recovery, atypical psychomotor stimulant, emergence, modafinil, obstructive sleep apnea, OSA
1. Introduction
Obstructive sleep apnea (OSA) is a common disorder of upper airway obstruction resulting in intermittent periods of hypopnea, apnea, disordered sleep architecture and deleterious long-term cardiopulmonary effects. Postoperatively, OSA has been associated with increased apnea-hypopnea index score, frequency of oxygen desaturations, and in-hospital respiratory complications compared to non-OSA patients.[1–5] In addition, postoperative patients with OSA have been found to have higher rates of postoperative oxygen therapy, postoperative noninvasive ventilation, reintubation, and unplanned intensive care unit admissions.[6] Furthermore, perioperative complications associated with OSA are being increasingly reported as the precipitant of malpractice suits.[7] Given these findings, developing treatment modalities to improve functional recovery after general anesthesia in this population is of utmost importance. Modafinil, a long acting wakefulness-promoting agent (half-life (t1/2): 15 h), has demonstrated effectiveness in the treatment of daytime somnolence associated with OSA, narcolepsy and third worker shift syndrome.[8–12] In contrast to psychomotor stimulants such as methylphenidate, it has a lower incidence of adverse cardiopulmonary effects and abuse potential.[13–15] Its mechanism of action is partly unknown, though studies have demonstrated increased central monoamine (dopamine, serotonin, and noradrenaline) activity in central nervous system centers (nucleus accumbens, prefrontal cortex), and suppression of GABAergic transmission in regulatory sleep-related areas of the brain (locus coeruleus, preoptic areas, posterior hypothalamus).[16–20] Clinically, studies of chronic modafinil administration have demonstrated improvements in attention, executive functioning, general alertness, sleep architecture and activities of daily living in both normal and OSA patient populations.[21–23] In addition, there have been studies examining its role in improving subjective parameters of postoperative recovery after sedation and general anesthesia.[24,25] In this proof-of-concept study, we hypothesized that preoperative single dose modafinil administration would improve functional recovery after general anesthesia by reducing time to readiness for discharge from the post-anesthesia care unit (PACU) and time to extubation in patients with OSA.
2. Methods
2.1. Study design and eligible participants
This clinical study was approved by the Penn State College of Medicine Institutional Review Board (Study No. 2957) and written informed consent was obtained from all subjects participating in the trial. Food and Drug Administration Investigational New Drug exemption (PIND 127491) was obtained before patient enrollment for off-label use of modafinil. The trial was registered before patient enrollment at clinicaltrials.gov (NCT02494102, Principal Investigator: Zyad J. Carr, M.D., Date of Registration: July 10, 2015). Patients were pre-screened approximately 1 to 2 weeks before informed consent and were initially approached with a phone call. Pre-screened participants were then recruited and all signed informed consent in the pre-anesthesia clinic of the Penn State Health Milton S. Hershey Medical Center or 2 to 3 hours before their elective surgical procedure. In total, 540 patients were screened, 105 enrolled and 89 completed the study (Fig. 1).
Figure 1.
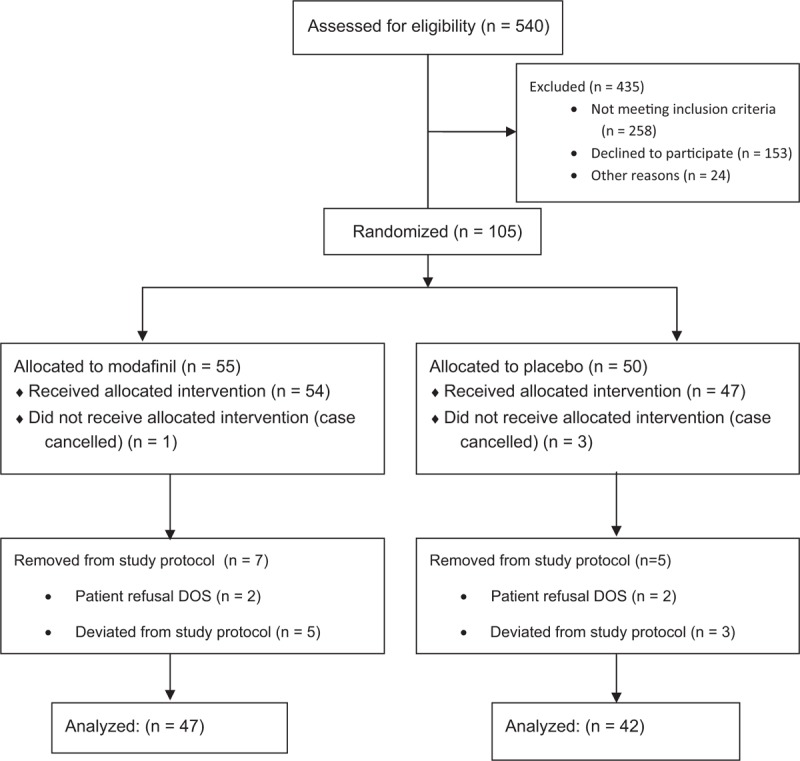
CONSORT diagram of patient screening, recruitment and outcome of participants through the clinical trial.
Inclusion criteria included: age greater than or equal to 18 years, mild, moderate, or severe OSA with documented sleep study diagnosis and scheduled for an elective surgical procedure to be performed under general anesthesia. Exclusion criteria included medical conditions that, in the physician's judgement, would interfere with safe administration of the study drug. Patients were excluded if the following conditions were present; angina, poorly controlled hypertension, severe valvular heart disease, recent myocardial infarction (within 6 months), severe renal or liver disease, poorly controlled diabetes, or elevated liver enzymes greater than twice normal). In addition, patients with neurological disorders or major psychiatric disorders, concurrent modafinil, methylphenidate, ethynyl estradiol, or triazolam administration or current dependence on recreational drugs were excluded. We attempted to select for patients at the least risk for modafinil's rare cardiac, neurological and respiratory adverse effects and to homogenize the study population. Due to these limiting exclusion criteria, overall recruitment success was low in this clinical investigation.
2.2. Procedures
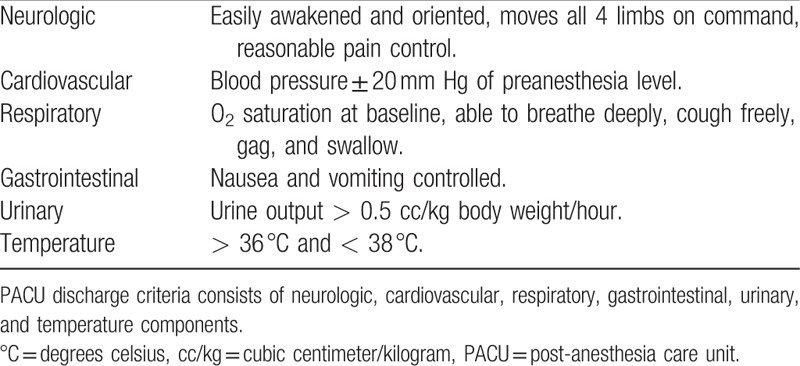
After obtaining informed consent, patients were randomized in a 1 to 1 ratio to receive a single dose of modafinil (ProVigil, 200 mg) or placebo 30 to 45 minutes before their elective surgery to ensure adequate gastrointestinal absorption. The investigational pharmacy prepared and dispensed the drug according to a computer-generated randomization list. Patients, research team members and all clinical providers were blinded to the patient's assignment. After receiving the study medication or placebo, patients proceeded for their elective surgical procedure. No preoperative midazolam was administered by the anesthesia care team. Induction of general anesthesia was at the discretion of the attending anesthesiologist. The general anesthetic plan was standardized as follows: All patients received 1 to 2 mg/kg of propofol for induction of anesthesia, general anesthesia was maintained with either desflurane or sevoflurane, minimum alveolar concentration (MAC) was maintained at 0.8 to 1.2 for the duration of the procedure. To prevent the loss of study drug, no gastric suctioning was performed for 45 minutes after ingestion of the study medication. A bispectral index (BIS) monitor (BIS, Medtronic, Minneapolis, MN) was placed within 15 minutes after the induction of general anesthesia to record electroencephalography (EEG) information for the duration of the anesthetic procedure. At the end of the surgical procedure, the clinician was instructed to terminate the flow of volatile anesthetic, and administer oxygen and/or air flows at 10 liters per minute to standardize the measurement of the secondary outcome (termination of volatile anesthetic to extubation time). After extubation, patients were transported to the PACU and the attending nurse was instructed to document the time that the patient met nursing discharge based on the modified Aldrete Scale used in our institution (Table 1). Postoperative patients that were continuous positive airway pressure (CPAP) compliant were placed on CPAP as per usual practice at our institution.
Table 1.
PACU recovery discharge criteria.
2.3. Measurements
The primary endpoint was PACU length of stay (PACULOS) and was defined as the time period from extubation to readiness to discharge from Phase I of PACU. We utilized this measure as a useful clinical metric of improved recovery from general anesthesia and utilized “readiness to discharge” as documented by the PACU nurse to reduce measurement errors related to delays in transport from Phase I to Phase II. The secondary outcome was the time period from the termination of the volatile anesthetic to extubation time as documented by the clinical anesthesia provider to assess rapidity of emergence from general anesthesia. In addition, the Postoperative Quality Recovery Scale (PQRS) was performed on the first 38 patients randomized to the study to comprehensively assess physiologic, cognitive, and functional postoperative recovery.[26] Pre- and postoperative PQRS batteries were performed in the preoperative clinic and in the PACU at 1 hour post-admission, respectively. Three intraoperative variables were compared between groups: intraoperative blood pressure, BIS scores and narcotic consumption. To standardize time periods in the setting of a wide range of anesthesia times for surgical procedures, we examined 2 separate time periods; from the induction of general anesthesia (measured as the time of administration of the intravenous sedative-hypnotic) to 60 minutes and 30 minutes before extubation. Blood pressure and BIS measurements were averaged over each time period per subject and compared between intervention and placebo groups. Intraoperative narcotic consumption was converted into morphine equivalent units (MEU) to determine the average per minute of narcotic consumption between groups.[27] In addition to PACULOS, we measured 5 additional postoperative variables. The PACU pain score was recorded from the initial PACU admission vital signs by the PACU nurse and compared between groups. We compared mean arterial blood pressure, respiratory rate, and pulse oximetry values, all measured every ten minutes for the first 60 minutes of the PACU stay. PACU narcotic consumption was converted into MEUs and the average per minute was analyzed over the first 60 minutes. After 2 weeks, participants were contacted by phone and underwent a brief semi-structured survey to assess for anesthesia-related complications and subjective recovery after their general anesthesia.
2.4. Statistical analysis
Factoring in an anticipated 10% subject attrition over the course of the study, the target sample size for this proof-of-concept trial was 120 subjects. This sample size provided 90% power to detect a difference of 45 minutes, assuming a standard deviation of 70 minutes, in the primary endpoint of PACULOS between the modafinil and placebo groups using a 2-sided Wilcoxon rank-sum test having a significance level of 0.05. The average PACU length of stay was calculated utilizing a random sample of 100 patients with OSA undergoing general anesthesia for elective surgery at our institution. For continuous outcomes that were non-normally distributed (e.g., PACULOS, anesthesia time, pain measurements), the Wilcoxon rank-sum test was used to compare the treatment groups and the descriptive data are reported as median (Q1, Q3), where Q1 is the first quartile and Q3 is the third quartile. For continuous outcomes that were normally distributed (e.g., age, body mass index [BMI]), the 2-sample t test was used to compare the treatment groups and the descriptive data are reported as mean ± SD, where SD is the standard deviation. The Pearson chi-square test, or the Fisher exact test if the expected cell counts were small, was used to compare binary variables (e.g., presence of hypertension, presence of gastroesophageal reflux disease [GERD]) between the 2 treatment groups. For ordinal variables, such as ASA physical status class, the exact Mantel–Haenszel chi-square test was used to compare the 2 treatment groups. All hypothesis tests were 2-sided and a P < .05 was considered significant. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
One hundred five patients were randomized, and data from 89 patients (47 patient in modafinil group and 42 patients in placebo group) were analyzed. The clinical trial was stopped for futility on interim statistical analysis. Proportions by surgical procedure were as follows (n(% total)): abdominal surgery (modafinil:30 (60%), placebo: 27 (64%)), orthopedic surgery (modafinil: 5 (10%) placebo: 2 (5%)), gynecology (modafinil: 7 (15%), placebo: 4 (10%), otolaryngology (modafinil: 5 (11%), placebo: 8 (19%)), ophthalmology (modafinil: 0 (0%) placebo: 1 (2%)). There were no clinically significant differences between groups based on patient age, sex, ASA, physical status class, or medical history (Table 2). The BMI in the modafinil group was greater (44.9 ± 11.6) than in the placebo group (38.2 ± 11.1, P = .007). Intraoperative and postoperative variables were also similar between groups (Table 3). Length of surgery, time to extubation from termination of volatile agent, mean arterial pressure, BIS, and intraoperative MEU were not significant. PACULOS was similar between groups (P = .28). Postoperative MEU and pain score upon PACU admission did not demonstrate a statistically significant difference. PACU pulse oximetry values were similar between groups but there was a statistically significant difference in PACU MAP (Placebo: 101.1 ± 12.8 mm Hg versus Modafinil: 95.3 ± 11.7 mm Hg, P = .03) and respiratory rate between groups (Placebo: 16.6 ± 2.5 breaths/min versus Modafinil: 18.0 ± 2.7 breaths/min P = .01). The PQRS was performed on the first 38 patients in this study (Table 4). In addition, intra-operative surgically related variables were not significant between groups (Table 5). Post-anesthesia recovery was found to be similar between groups (P = .44). Two-week subjective recovery and adverse effects were assessed utilizing a semi-structured telephone interview. We found no evidence that modafinil treatment improves subjective recovery from general anesthesia. Furthermore, we found no evidence that modafinil treatment was associated with higher adverse events (nausea, headache) in the immediate 24 hours post treatment.
Table 2.
Demographical data. There was a statistically significant higher BMI in the modafinil group compared to the control group.
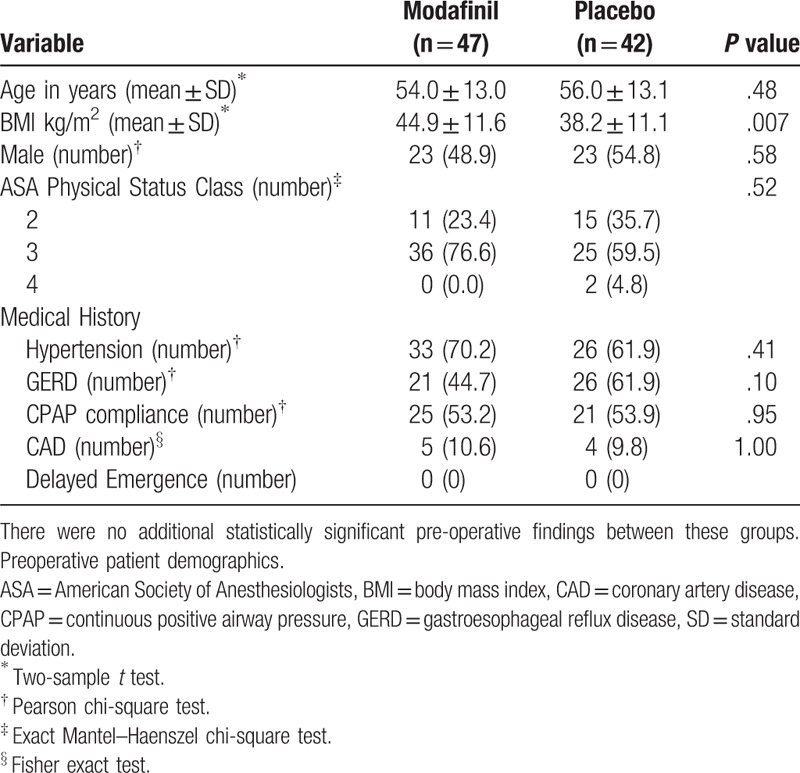
Table 3.
Intraoperative and Postoperative Outcomes. There were no statistically significant differences in assessed intraoperative variables.
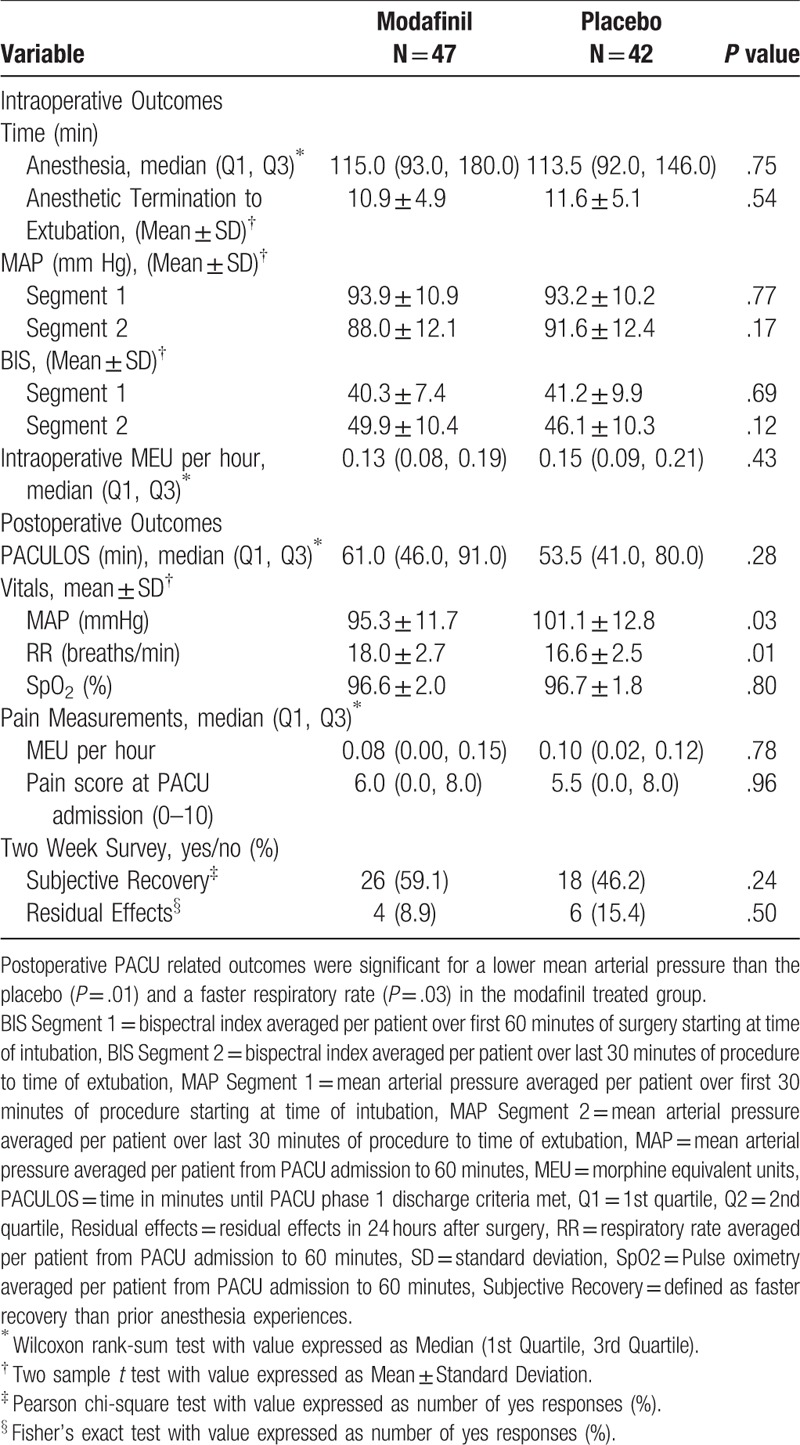
Table 4.
Component analysis of the PQRS findings did not demonstrate any difference in quality of postoperative recovery between groups (N = 38).
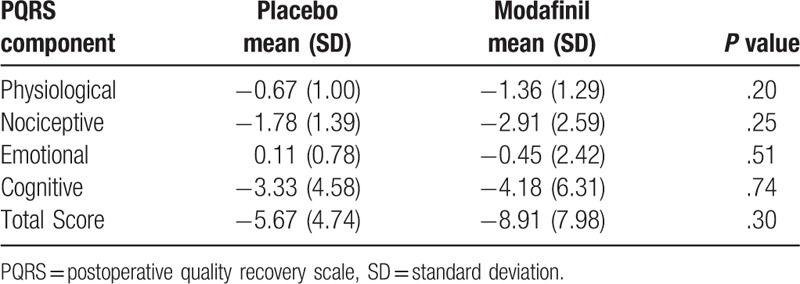
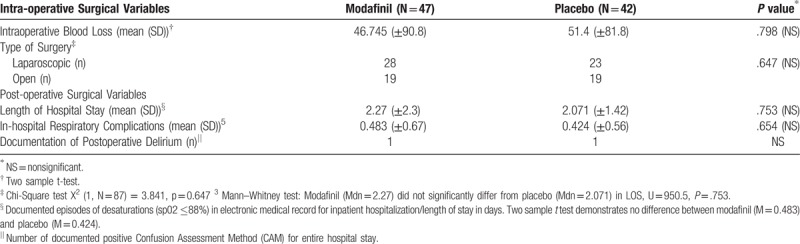
Table 5.
Intra-operative surgical variables analysis between groups did not demonstrate significant differences between groups in estimated blood loss, laparoscopic rates, length of stay, delirium, and postoperative oxygen desaturations.
4. Discussion
In this proof-of-concept study, we tested the efficacy of an atypical psychomotor stimulant, modafinil, to improve quantitative and qualitative measures of cognitive recovery after general anesthesia in patients with OSA. We were unable to find any significant difference in clinically relevant metrics of anesthesia-related emergence and recovery. In addition, we did not demonstrate any significant adverse effects that could be directly attributable to the effects of perioperative administration of modafinil. The value of psychomotor stimulants to improve cognitive recovery after general anesthesia remains unclear. There is evidence that methylphenidate accelerates emergence from isoflurane and propofol anesthesia in animal studies.[28,29] As modafinil is a psychomotor stimulant with similar properties to methylphenidate, we opted to utilize the termination of volatile anesthesia to extubation time as a measure of the rapidity of emergence but were unable to demonstrate a significant difference between groups. Though there was a wide range in anesthesia times, we believe that it was unlikely to affect modafinil's efficacy given its long half-life (t1/2: 15 h). Similarly, performance on the PQRS was nonsignificant between groups. A trend towards more postoperative nausea was observed in the modafinil group. In addition, postoperative PQRS cognitive recovery was not statistically improved (Table 4). We presumed that intraoperative and postoperative narcotic consumption would be increased with the addition of a psychomotor stimulant but did not find a difference between groups. Interestingly, PACU respiratory rate was increased in the modafinil treatment group. This finding was independent of postoperative pain and may indicate a subtle increase in arousal in patients who received modafinil. Finally, though our trial was terminated for futility, our primary outcome, PACU length of stay, was not different between groups. Strengths of this study include its randomized placebo-controlled study design, standardized general anesthetic protocol and observational endpoints. However, there are several limitations to the findings of our study. Patients in the modafinil group had higher BMI in comparison to controls. There is a positive relationship between BMI and severity of OSA as quantified by apnea-hypopnea index and higher frequency of multi-level obstruction.[30,31] In addition, higher BMIs are associated with longer operation and emergence times.[32] Given this difference, it is possible that patients in the modafinil group trended towards having more severe OSA, mitigating any subtle beneficial properties of modafinil treatment. It is unknown if the administered dose, the recommended dose for the treatment of daytime somnolence for OSA, was adequate for functional recovery after anesthesia. However, in clinical trials of daytime somnolence with OSA, insignificant differences in performance measures of wakefulness were observed between 200 and 400 milligrams per day dosing regimens.[33] In addition, clinical heterogeneity in induction, length of anesthesia time, extubation thresholds, and type of surgery were potential factors in masking any beneficial treatment effect. In conclusion, we did not find any clinically relevant evidence that the addition of a single preoperative dose of an atypical psychomotor stimulant improved functional recovery from general anesthesia in an at-risk population. We believe that further studies are needed before abandoning the use of psychomotor stimulants to ameliorate recovery from anesthesia. Further research should consider the use of typical psychomotor stimulants such as methylphenidate, timing of dosing, continued perioperative dosing and examining other at-risk subpopulations such as geriatric populations and patients with documented delayed emergence from general anesthesia. In conclusion, further studies examining psychomotor stimulants will be needed before recommending their inclusion as part of a balanced general anesthetic technique in patients with OSA.
Author contributions
Conceptualization: Zyad James Carr, Brian Vells, Allen A Kunselman, Sonia J Vaida, Kunal Karamchandani.
Data curation: Zyad James Carr, Brian Vells, Brendan R Wood, Joshua D Lowery, Ann M Rogers, Allen A Kunselman, Sonia J Vaida, Kunal Karamchandani.
Formal analysis: Zyad James Carr, Brendan R Wood, Allen A Kunselman.
Investigation: Zyad James Carr, Brian Vells, Brendan R Wood, Joshua D Lowery.
Methodology: Zyad James Carr, Brian Vells, Joshua D Lowery, Allen A Kunselman, Kunal Karamchandani.
Project administration: Zyad James Carr, Brendan R Wood.
Supervision: Zyad James Carr.
Validation: Zyad James Carr, Joshua D Lowery.
Writing – original draft: Zyad James Carr, Brian Vells, Ann M Rogers, Sonia J Vaida, Kunal Karamchandani.
Writing – review & editing: Zyad James Carr, Brendan R Wood, Joshua D Lowery, Ann M Rogers, Sonia J Vaida, Kunal Karamchandani.
Zyad James Carr orcid: 0000-0002-6970-3340
Footnotes
Abbreviations: BIS = bispectral index, BMI = body mass index, CPAP = continuous positive airway pressure, EEG = electroencephalography, MAC = minimum alveolar concentration, MEU = morphine equivalent units, OSA = obstructive sleep apnea, PACU = post-anesthesia care unit, PQRS = postoperative quality recovery scale.
ZJC, BV, BRW, JDL, AMR, AAK, KK, SJV. These authors helped design the study, conduct the study, collect the data, analyze the data, and prepare the manuscript.
This study was supported by a pilot study grant (00002957) from the Department of Anesthesiology and Perioperative Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey PA.
Clinical Trial Number: NCT02494102 (https://clinicaltrials.gov/ct2/show/NCT02494102).
The authors have no conflicts of interest to disclose.
References
- [1].Chung F, Liao P, Elsaid H, et al. Factors associated with postoperative exacerbation of sleep-disordered breathing. Anesthesiology 2014;120:299–311. [DOI] [PubMed] [Google Scholar]
- [2].Liao P, Yegneswaran B, Vairavanathan S, et al. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth 2009;56:819–28. [DOI] [PubMed] [Google Scholar]
- [3].Benumof JL. Obesity, sleep apnea, the airway and anesthesia. Curr Opin Anaesthesiol 2004;17:21–30. [DOI] [PubMed] [Google Scholar]
- [4].Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med 2012;8:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Opperer M, Cozowicz C, Bugada D, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the society of anesthesia and sleep medicine task force on preoperative preparation of patients with sleep-disordered breathing. Anesth Analg 2016;122:1321–34. [DOI] [PubMed] [Google Scholar]
- [6].Fernandez-Bustamante A, Bartels K, Clavijo C, et al. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth Analg 2017;125:593–602. [DOI] [PubMed] [Google Scholar]
- [7].Fouladpour N, Jesudoss R, Bolden N, et al. Perioperative complications in obstructive sleep apnea patients undergoing surgery: a review of the legal literature. Anesth Analg 2016;122:145–51. [DOI] [PubMed] [Google Scholar]
- [8].Pack AI, Black JE, Schwartz JR, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med 2001;164:1675–81. [DOI] [PubMed] [Google Scholar]
- [9].Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med 2005;353:476–86. [DOI] [PubMed] [Google Scholar]
- [10].Schwartz JR, Hirshkowitz M, Erman MK, et al. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea: a 12-week, open-label study. Chest 2003;124:2192–9. [DOI] [PubMed] [Google Scholar]
- [11].Moldofsky H, Broughton RJ, Hill JD. A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy. Sleep medicine 2000;1:109–16. [DOI] [PubMed] [Google Scholar]
- [12].Keating GM, Raffin MJ. Modafinil: a review of its use in excessive sleepiness associated with obstructive sleep apnoea/hypopnoea syndrome and shift work sleep disorder. CNS drugs 2005;19:785–803. [DOI] [PubMed] [Google Scholar]
- [13].Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol Oxf Engl 2000;14:53–60. [DOI] [PubMed] [Google Scholar]
- [14].Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol 2000;23:149–56. [DOI] [PubMed] [Google Scholar]
- [15].Roth T, Schwartz JR, Hirshkowitz M, et al. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007;3:595–602. [PMC free article] [PubMed] [Google Scholar]
- [16].Ferraro L, Antonelli T, O’Connor WT, et al. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport 1997;8:2883–7. [DOI] [PubMed] [Google Scholar]
- [17].Ferraro L, Antonelli T, O’Connor WT, et al. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett 1998;253:135–8. [DOI] [PubMed] [Google Scholar]
- [18].Ferraro L, Fuxe K, Agnati L, et al. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse NY 2005;55:230–41. [DOI] [PubMed] [Google Scholar]
- [19].Tanganelli S, Ferraro L, Bianchi C, et al. 6-hydroxy-dopamine treatment counteracts the reduction of cortical GABA release produced by the vigilance promoting drug modafinil in the awake freely moving guinea-pig. Neurosci Lett 1994;171:201–4. [DOI] [PubMed] [Google Scholar]
- [20].Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2008;33:1477–502. [DOI] [PubMed] [Google Scholar]
- [21].Battleday RM, Brem AK. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol 2015;25:1865–81. [DOI] [PubMed] [Google Scholar]
- [22].Arnulf I, Homeyer P, Garma L, et al. Modafinil in obstructive sleep apnea-hypopnea syndrome: a pilot study in 6 patients. Respir Int Rev Thorac Dis 1997;64:159–61. [DOI] [PubMed] [Google Scholar]
- [23].Erman MK, Rosenberg R. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: effects on patient functioning and health-related quality of life. Prim Care Companion J Clin Psychiatry 2007;9:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Larijani GE, Goldberg ME, Hojat M, et al. Modafinil improves recovery after general anesthesia. Anesth Analg 2004;98:976–81. [DOI] [PubMed] [Google Scholar]
- [25].Galvin E, Boesjes H, Hol J, et al. Modafinil reduces patient-reported tiredness after sedation/analgesia but does not improve patient psychomotor skills. Acta Anaesthesiol Scand 2010;54:154–61. [DOI] [PubMed] [Google Scholar]
- [26].Royse CF, Newman S, Chung F, et al. Development and feasibility of a scale to assess postoperative recovery: the post-operative quality recovery scale. Anesthesiology 2010;113:892–905. [DOI] [PubMed] [Google Scholar]
- [27].Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage 2009;38:426–39. [DOI] [PubMed] [Google Scholar]
- [28].Chemali JJ, Van Dort CJ, Brown EN, et al. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology 2012;116:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Solt K, Cotten JF, Cimenser A, et al. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology 2011;115:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zeng F, Wang X, Hu W, et al. Association of adiponectin level and obstructive sleep apnea prevalence in obese subjects. Medicine 2017;96:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Phua CQ, Yeo WX, Su C, et al. Multi-level obstruction in obstructive sleep apnoea: prevalence, severity and predictive factors. J Laryngol Otol 2017;131:982–6. [DOI] [PubMed] [Google Scholar]
- [32].Sanford JA, Kadry B, Brodsky JB, et al. Bariatric surgery operating room time—size matters. Obes Surg 2015;25:1078–85. [DOI] [PubMed] [Google Scholar]
- [33].Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep 2005;28:464–71. [DOI] [PubMed] [Google Scholar]


