Abstract
Rationale:
Sarcomatous intrahepatic cholangiocarcinoma is a rare histological variant of cholangiocarcinoma (ICC). Previous medical literature has not mentioned the prevalence of this kind of disease, but a poorer prognosis than that of ordinary ICC was indicated. The diagnosis of the sarcomatous ICC is established on histopathological and immunohistochemical examinations. In this article, we present a new case of a patient with sarcomatous ICC who had no radiographic sign of intrahepatic tumor preoperatively.
Patient concerns:
A 63-year-old man was noted with cholecystolithiasis and right upper abdominal pain. Liver function was within normal limits, although the gamma-glutamyl transpeptidase level was elevated. Serum carbohydrate antigen 19-9 level was elevated. Radiography showed atrophy of the left lobe of the liver, high-intensity signals on T1 weighted images, and low/high-intensity signals on T2 weighted images in hepatic ducts.
Diagnoses:
The preoperative diagnoses were hepatolithiasis, choledocholithiasis, and cholecystolithiasis.
Interventions:
Exploratory laparotomy, adhesion release, cholecystectomy, choledocholithotomy, and T tube drainage were performed. During the surgery, an ill-defined tumor was detected on the atrophic left lateral lobe of the liver. Hepatic left lateral lobectomy was performed to remove the mass.
Outcomes:
The final diagnosis of sarcomatous ICC was made by histopathology after surgery. No evidence of local recurrence or distant metastasis was noted on imaging during follow-up.
Lessons:
Although rare, sarcomatous ICC does exist in patients presented with cholecystolithiasis and liver atrophy. Surgeons should be aware of the existence of sarcomatous ICC due to the poor prognosis. We recommend that multidisciplinary approaches may be key to improve prognosis, including adjuvant chemotherapy or radiotherapy.
Keywords: liver atrophy, sarcomatoid transformation, sarcomatous intrahepatic cholangiocarcinoma, surgical treatment
1. Introduction
The World Health Organization definition of sarcomatous intrahepatic cholangiocarcinoma (ICC) is ICC with sarcomatous changes, which is an uncommon variant of ICC.[1] The prevalence is unknown, although ICC with sarcomatoid transformation accounts for 4.5% of all ICC.[2] Sarcomatous changes occur more often in hepatocellular carcinoma (HCC) than in cholangiocarcinoma.[3–4] On the macroscopic scale, sarcomatous ICC has both adenocarcinoma and sarcomatous appearance. Immunohistochemical examination of the sarcomatous component reveals both epithelial and mesenchymal molecular expression.[5] These characteristics of sarcomatous ICC make it distinguishable from ICC with sarcomatoid transformation or carcinosarcoma. Clinical manifestations and radiological imaging features of sarcomatous ICC resemble ordinary ICC. Diagnosis without histopathological examination of sarcomatous ICC to differentiate it from ordinary ICC is not yet possible. To our knowledge, 36 cases of sarcomatous ICC have been reported in published English-language literature.[2,5,6–18] Here, we present a new case report and discuss its unusual nature through a review of previous cases.
2. Case presentation
A 63-year-old Chinese man was found to have cholecystolithiasis at a community hospital. He has suffered from intermittent right upper abdominal pain for over 30 years. One month ago, he had another attack of pain in the right upper abdomen, with the visual analogue scoring system score 7. He was diagnosed with hepatolithiasis at a community hospital. The patient was referred and admitted to our hospital on April 10, 2018. He had no complaints involving abdominal distension, nausea, vomiting, diarrhea, fever, or jaundice. The patient had biliary ascariasis during childhood. He has been smoking 20 cigarettes per day for 30 years.
Physical examination showed stable vital signs and revealed no abnormalities in the heart or lungs. The skin and the sclera were not icteric. The abdomen was soft without palpable mass. There were tenderness and rebound pain in the right upper abdomen. The spleen was not enlarged. The liver was not palpable. The results of liver and renal function tests (with the normal range in parentheses) were as follows: alanine aminotransferase 19 U/L (9∼50), aspartate aminotransferase 25 U/L (15∼40), alkaline phosphatase 110 U/L (45∼125), total bilirubin 8.4 μmol/L (5.1∼22.2), albumin 43 g/L (35∼52). Notably, the gamma-glutamyl transpeptidase level was elevated, at 125 U/L (10∼60). The prothrombin time, prothrombin activity, and D-dimer level were within normal limits, at 11.7 s (10.4∼12.6), 109.2% (74.0%∼120.0%), and 0.52 mg/L FEU (0∼0.55), respectively. The activated partial thromboplastin time was decreased, at 22.5 s (22.7∼31.8). The Serum carbohydrate antigen 19-9 level was elevated, at 100.5 U/mL (0∼34.0). Serum carcinoembryonic antigen and alpha-fetoprotein were within normal limits, at 2.17 ng/mL (0∼5.00), and 2.2 ng/mL (0∼20.0), respectively.
Magnetic resonance cholangio-pancreatography showed atrophy of the left hepatic lobe, and the local liver surface was not smooth. There were multiple high-intensity signals on T1 weighted images and low/high-intensity signals on T2 weighted images in left and right hepatic ducts, with dilation of corresponding intrahepatic and extrahepatic bile ducts. The widest dilated bile duct measured 10 mm in diameter. Multiple filling defects of low signal were notable in the distal common bile duct. The cholecyst was constricted with non-thickened walls, and the internal signals were low. Multiple hilar lymph nodes were detected in the hepatic port (Fig. 1). Abdominal ultrasonography showed the bile duct of the hepatic left lateral lobe was widened with a hyperechoic calculus, measuring approximately 2.7 × 0.9 cm. There were also hyperechoic calculi in the bile duct of the hepatic right lobe and cholecyst, measuring approximately 4.6 × 0.7 cm and 2.6 × 0.7 cm, respectively (Fig. 1).
Figure 1.
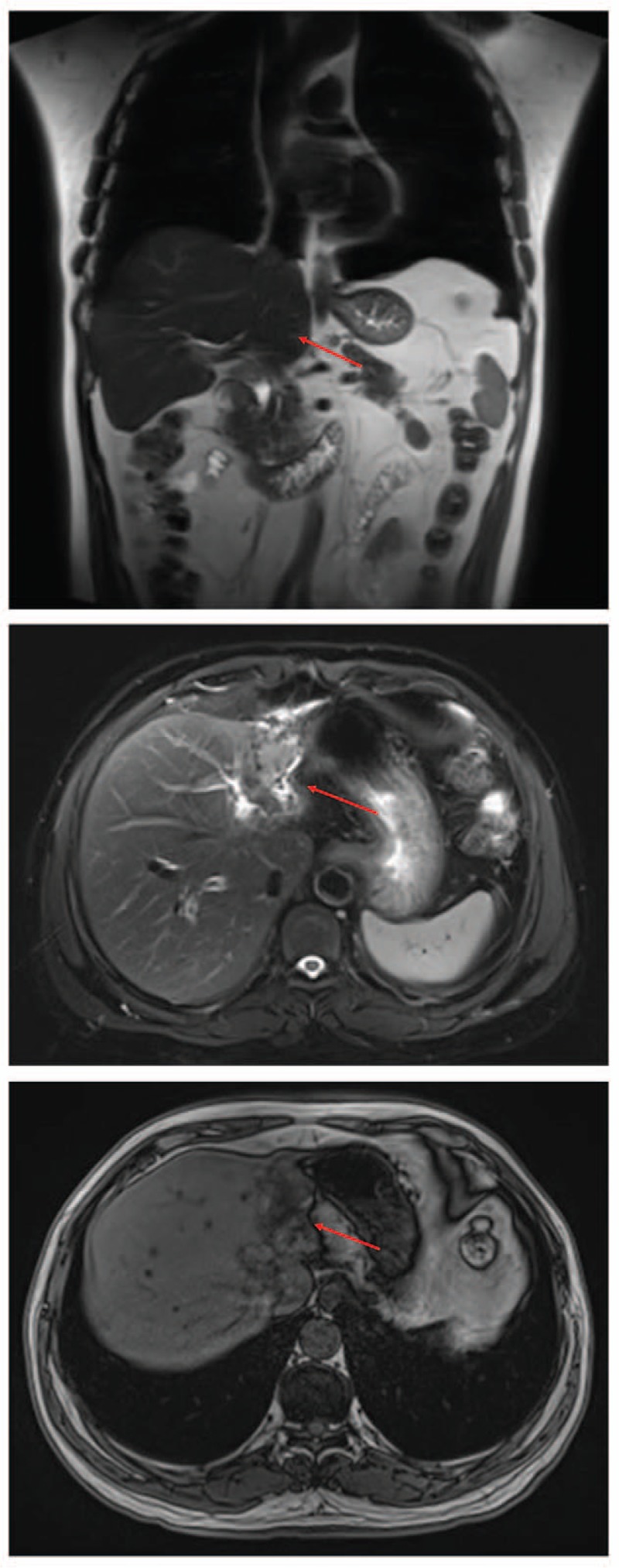
Preoperative magnetic resonance cholangio-pancreatography of our patient, showing atrophy of the left lobe of the liver, and non-smooth local liver surface.
The preoperative diagnoses were hepatolithiasis, choledocholithiasis, and cholecystolithiasis. Exploratory laparotomy, adhesion release, cholecystectomy, choledocholithotomy, and T tube drainage were performed. During the surgery, an ill-defined neoplasm was detected on the atrophic left lateral lobe of the liver. Therefore, hepatic left lateral lobectomy was performed. Two pieces of liver tissue were resected during surgery. The intraoperative frozen section examination reported a malignant tumor, part of which showed intraductal growth, and originated from the atrophic left lateral lobe of the liver.
According to the intraoperative discovering, we considered cholangiocarcinoma and HCC as our main differential diagnosis.
Macroscopically, 1 of the 2 resected pieces of liver tissue measured 5.5 × 3.5 × 2.5 cm, and on cross-section dilated bile ducts were seen, with diameters ranging from 0.5 to 2.4 cm. The bile ducts were filled with intraductal, brittle, papillary, greyish-white nodules, which were rich in medium-textured mucus. The other piece of liver tissue measured 8 × 3 × 2 cm, with notable bile duct dilation, 0.5 ∼ 2.5 cm in diameter. Some of the bile ducts were filled with solid, medium-textured greyish-pink or greyish-yellow nodules. Histological examination of the liver tissue demonstrated that the ill-defined nodules were sarcomatous cholangiocarcinoma with heterogeneous differentiation (chondrosarcoma) (Fig. 2). Focal invasion in the peripheral fibrous tissue was detected. Immunohistochemical examination of the neoplasm showed positive staining for AE1/AE3, STAT6, SOX10, CD34 (vessels), cytokeratin19, Desmin (foci), MUC1, Vimentin, SMA, and S-100. Based on these histopathological and immunohistochemical findings, the ill-defined nodules were diagnosed as sarcomatous ICC.
Figure 2.
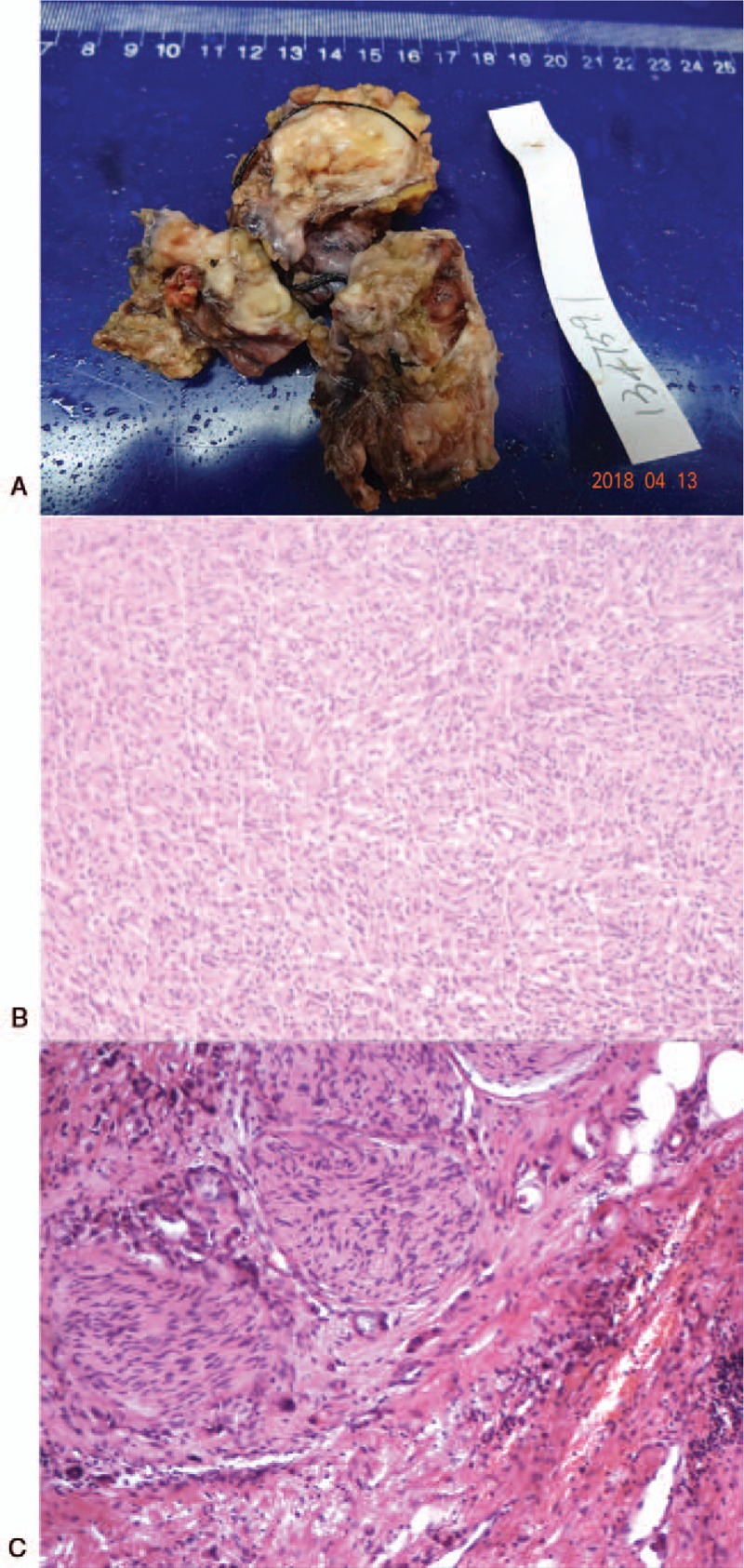
Morphological and histological appearance of the tumor, which consists of Sarcomatous components, with heterogeneous differentiation (chondrosarcoma). (A) The surgical specimens of the liver mass. (B) HE staining of the tumor on left lateral lobe of the liver (60×). (C) HE staining of the tumor on left lateral lobe of the liver (150×). HE = hematoxylin and eosin.
The patient had an uneventful postoperative recovery and left hospital 6 days later. No adjuvant chemotherapy or radiotherapy was performed on this patient, considering the uncertainty of potential benefit of adjuvant therapy. At the 30-day follow-up, a metastatic work-up was performed and showed no signs of local recurrence or distant metastasis. Complaints of right upper abdominal pain and abnormal liver function evident before surgery disappeared. The overall time duration of follow-up is 4 months. And the last known status of the patient was alive without progress. Informed consent was obtained from the patient for publication of this case report and accompanying images.
3. Discussion
Cholangiocarcinoma is a relatively rare adenocarcinoma. Patients with cholangiocarcinoma typically have abdominal pain, jaundice, weight loss, and abnormal liver function tests. Other possible symptoms include generalized itching, fever, and changes in color of stool or urine. On ultrasound of the liver, obstruction and dilatation of the intrahepatic bile ducts are most frequently seen in cholangiocarcinoma patients.[19] Computed tomography may detect a hypodense hepatic lesion with peripheral enhancement, biliary dilatation, and contrast enhancement on delayed images in peripheral ICC.[20] Direct imaging of the bile ducts is highly recommended. Magnetic resonance cholangio-pancreatography is a non-invasive approach to accurately define cholangiocarcinoma, effectively delineate the anatomical extent of the cancerous infiltration, and avoid the risks of invasive procedures.[21] HCC occurs mostly in patients with coexisting signs and symptoms of chronic liver disease, including jaundice, ascites, coagulopathy, anorexia, weight loss, abdominal pain, and fatigue. The diagnosis of HCC is based on risk factors (e.g. liver cirrhosis), blood tests, and imaging. HCC often appears on ultrasound as a small hypoechoic lesion with poorly defined margins and coarse irregular internal echoes. High-intensity signals on T2 weighted images and low-intensity signals on T1 weighted images are typical findings of HCC on magnetic resonance imaging.[22] As for our patient, no sign of tumor occupying the bile ducts was detected on magnetic resonance cholangio-pancreatography. Additionally, our patient had no past medical history of liver cirrhosis or any other morbidities indicating chronic liver disease. An ICC or HCC diagnosis would be made based on the final pathological results.
Sarcomatous ICC is a rare histological variant of ICC. Morphologically, sarcomatous ICC has both adenocarcinoma (ICC component) and sarcomatous components. Histopathologically, the expression of both epithelial (e.g. cytokeratin) and mesenchymal (e.g. vimentin) features on sarcomatous component is characteristic.[5] Clinical manifestations and radiological imaging features of sarcomatous ICC are not sufficient to distinguish it from ordinary ICC due to similarities. Diagnosis of sarcomatous ICC relies on histopathological examination.
To review sarcomatous ICC, we searched PubMed using the keywords “liver”, “sarcomatous”, “sarcomatoid”, and “cholangiocarcinoma”. The citation lists associated with all the studies retrieved were used to identify other potentially relevant publications. Finally, 36 non-repeated cases of sarcomatous ICC reported in 15 published articles were identified. The clinical features of these 36 cases are summarized in Table 1.
Table 1.
Summary of sarcomatous ICC reported in the English-language literature.
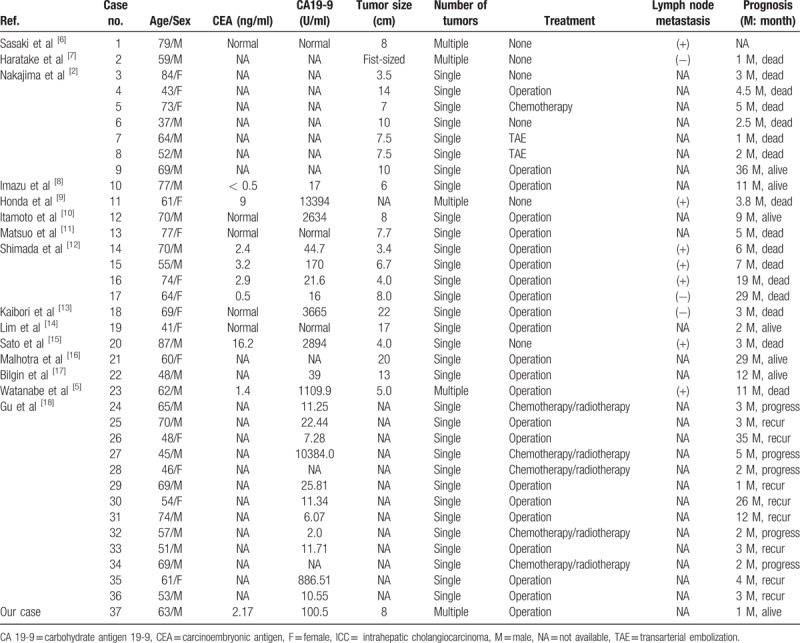
Among these patients, 22 (61.1%) were male and 14 (38.9%) were female. The mean age was 62 (range, 37∼87) years. The mean tumor size was 9.2 (range, 3.4∼22) cm. 16 (44.4%) patients died during follow-up, and 13 (36.1%) patients with progression and recurrence were noted in the cases with available follow-up data. As Bilgin et al[17] demonstrated, radiographic techniques such as computed tomography or magnetic resonance imaging may not be sufficient to make a prospective diagnosis because sarcomatous ICC shares similar features with other intrahepatic tumors, despite the finding that a satellite nodule of the tumor and mixed internal signal intensities on magnetic resonance imaging could be distinguishing features of sarcomatous ICC. The diagnosis of the tumor is established by histopathological and immunohistochemical examinations. Sarcomatous ICC does have some unique histopathological and molecular cytogenetic patterns. Watanabe et al[5] emphasized that all sarcomatous ICC must fulfil the following criteria:
-
1)
coexistence of both adenocarcinoma and sarcomatous components in the tumor morphologically, and
-
2)
the sarcomatous component expresses both mesenchyme and epithelium molecular features.
These characteristics could also be noted in our hematoxylin and eosin-staining figure. Immunochemical staining for our case of sarcomatous ICC was positive for AE1/AE3, STAT6, SOX10, CD34 (vessels), cytokeratin19, Desmin (foci), MUC1, Vimentin, SMA, and S-100, which was consistent with previous literature. The prognosis of sarcomatous ICC seemed to be unpromising. Nakajima et al[2] and Watanabe et al[5] showed that the prognosis with sarcomatous ICC was much poorer than ordinary ICC. According to Watanabe et al[5] and Kaibori et al,[13] patients who underwent operation had significantly higher survival rate than patients who had other or no treatments. Median survival time of sarcomatous ICC patients who had surgical resection was 11 months, comparable to that of ordinary ICC without surgery (8 months). Median survival time of sarcomatous ICC patients without surgery was 3 months, much short than that of ordinary ICC.[5]
Regular follow-up and multidisciplinary approaches may be key to improve prognosis. As Malhotra et al[16] have demonstrated, combination chemotherapy with gemcitabine and cisplatin may be a potential treatment option in patients with advanced sarcomatous ICC. The effect of adjuvant therapy is unclear due to limited number of cases. Future studies of neoadjuvant chemotherapy or radiotherapy before or after surgery to achieve better prognosis of sarcomatous ICC should be considered.
4. Conclusion
Sarcomatous ICC is a rare histological variant of ICC. Adjuvant chemotherapy or radiotherapy before or after surgery could be helpful to improve currently unpromising prognosis of sarcomatous ICC. However, considering the limited number of cases reported, further study is necessary.
Author contributions
Ning Zhang, Yatong Li and MengyunZhao are co-first authors of this article. N. Zhang was the fellow, and Y. Li was the resident and assistant-surgeon who took care of the patient. Both of Y. Li and M. Zhao collected the data and wrote the article.
Xiaoyan Chang was the pathologist of the pathological diagnosis;
Feng Tian and Qiang Qu helped in taking care of the patient;
Xiaodong He was the correspondence author, he led the team and guided all these works.
Data curation: Ning Zhang, Yatong Li, Xiaoyan Chang, Feng Tian, Qiang Qu, Xiaodong He.
Validation: Xiaodong He.
Writing – original draft: Mengyun Zhao.
Writing – review & editing: Yatong Li.
Footnotes
Abbreviations: HCC = hepatocellular carcinoma, ICC = intrahepatic cholangiocarcinoma.
NZ, YL, and MZ are co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 2010;Lyon: International Agency for Research on Cancer, 320–90. [Google Scholar]
- [2].Nakajima T, Tajima Y, Sugano I, et al. Intrahepatic cholangiocarcinoma with sarcomatous change: clinicopathologic and immunohistochemical evaluation of seven cases. Cancer 1993;72:1872–7. [DOI] [PubMed] [Google Scholar]
- [3].Kakizoe S, Kojiro M, Nakashima T. Hepatocellular carcinoma with sarcomatous change: clinicopathologic and immunohistochemical studies of 14 autopsy cases. Cancer 1987;59:310–6. [DOI] [PubMed] [Google Scholar]
- [4].Craig JR, Peters RL, Edmondson HA. Hartmann WH. Tumors of the liver and intrahepatic bile duct. Atlas of Tumor Pathology, Fascicle 26, Series 2. Washington, DC: Armed Forces Institute of Pathology; 1989. 179–80. [Google Scholar]
- [5].Watanabe G, Uchinami H, Yoshioka M, et al. Prognosis analysis of sarcomatous intrahepatic cholangiocarcinoma from a review of the literature. Int J Clin Oncol 2014;19:490–6. [DOI] [PubMed] [Google Scholar]
- [6].Sasaki M, Nakanuma Y, Nagai Y, et al. Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol 1991;13:220–5. [DOI] [PubMed] [Google Scholar]
- [7].Haratake J, Yamada H, Horie A, et al. Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer 1992;69:2444–8. [DOI] [PubMed] [Google Scholar]
- [8].Imazu H, Ochiai M, Funabiki T. Intrahepatic sarcomatous cholangiocarcinoma. J Gastroenterol 1995;30:677–82. [DOI] [PubMed] [Google Scholar]
- [9].Honda M, Enjoji M, Sakai H, et al. Case report: intrahepatic cholangiocarcinoma with rhabdoid transformation. J Gastroenterol Hepatol 1996;11:771–4. [DOI] [PubMed] [Google Scholar]
- [10].Itamoto T, Asahara T, Katayama K, et al. Double cancer—hepatocellular carcinoma and intrahepatic cholangiocarcinoma with a spindle-cell variant. J Hepatobiliary Pancreat Surg 1999;6:422–6. [DOI] [PubMed] [Google Scholar]
- [11].Matsuo S, Shinozaki T, Yamaguchi S, et al. Intrahepatic cholangiocarcinoma with extensive sarcomatous changes: report of a case. Surg Today 1999;29:560–3. [DOI] [PubMed] [Google Scholar]
- [12].Shimada M, Takenaka K, Rikimaru T, et al. Characteristics of sarcomatous cholangiocarcinoma of the liver. Hepatogastroenterology 2000;47:956–61. [PubMed] [Google Scholar]
- [13].Kaibori M, Kawaguchi Y, Yokoigawa N, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol 2003;38:1097–101. [DOI] [PubMed] [Google Scholar]
- [14].Lim BJ, Kim KS, Lim JS, et al. Rhabdoid cholangiocarcinoma: a variant of cholangiocarcinoma with aggressive behavior. Yonsei Med J 2004;45:543–6. [DOI] [PubMed] [Google Scholar]
- [15].Sato K, Murai H, Ueda Y, et al. Intrahepatic sarcomatoid cholangiocarcinoma of round cell variant: a case report and immunohistochemical studies. Virchows Arch 2006;449:585–90. [DOI] [PubMed] [Google Scholar]
- [16].Malhotra S, Wood J, Mansy T, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol 2010;2010:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bilgin M, Toprak H, Bilgin SS, et al. CT and MRI findings of sarcomatoid cholangiocarcinoma. Cancer Imaging 2012;12:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gu KW, Kim YK, Min JH, et al. Imaging features of hepatic sarcomatous carcinoma on computed tomography and gadoxetic acid-enhanced magnetic resonance imaging. Abdom Radiol 2017;42:1424–33. [DOI] [PubMed] [Google Scholar]
- [19].Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics 1999;19:1199–218. [DOI] [PubMed] [Google Scholar]
- [20].Valls C, Gumà A, Puig I, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging 2000;25:490–6. [DOI] [PubMed] [Google Scholar]
- [21].Yeh TS, Jan YY, Tseng JH, et al. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol 2000;95:432–40. [DOI] [PubMed] [Google Scholar]
- [22].Peterson MS, Baron RL. Radiologic diagnosis of hepatocellular carcinoma. Clin Liver Dis 2001;5:123–44. [DOI] [PubMed] [Google Scholar]


