Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among the women of reproductive age. We conducted a nationwide population-based retrospective cohort study to analyze the association between PCOS and the subsequent development of gynecological cancers, namely endometrial, breast, and ovarian cancer.
For this population-based cohort study, we used the Taiwan National Health Insurance Research Database, which contains information on approximately 24.7 million insured individuals. The cohort included women who had received a diagnosis of PCOS between 1998 and 2013. An age-matched systematic random-sampling method with a ratio of 1:4 was used for patient selection for the non-PCOS reference cohort. Multivariate Cox proportional hazard regression analysis was used to determine the effects of PCOS on the risks of gynecologic and breast cancer. The data are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
The PCOS cohort consisted of 8155 patients with PCOS, and the comparison cohort consisted of 32,620 matched patients without PCOS. The incidence of endometrial cancer was 226 and 15 per 100,000 person-years in the PCOS and comparison groups, respectively. A statistically significant higher risk of endometrial cancer was found in the PCOS cohort (adjusted HR [aHR] = 17.7, 95% CI = 4.9–64.2) than in the comparison cohort. However, no association was observed between PCOS and ovarian (aHR = 1.64, 95% CI: 0.63–4.27) or breast cancer (aHR = 0.98, 95% CI: 0.58–1.65).
The results of this large population-based cohort study supported the premise that women with PCOS might have an increased risk of endometrial cancer, but no association between PCOS and the risks of ovarian and breast cancer was found.
Keywords: breast cancer, endometrial cancer, ovarian cancer, polycystic ovarian syndrome, population
1. Introduction
Polycystic ovarian syndrome (PCOS) is a common endocrine disease, with a prevalence of 6.5% in the female population in Spain.[1] Women with PCOS may present with hyperandrogenism, menstrual irregularity, and a polycystic morphology of the ovary.[2] The prevalence of insulin resistance and insulin secretory defects, menstrual dysfunction, and androgen excess is high in patients with PCOS.[3,4] The irregular metabolic and hormonal status of women with PCOS may increase their risk of some cancer types.
Cancers related to chronic hormone stimulation in women include endometrial, ovarian, and breast cancer. In women with PCOS, chronic estrogen stimulation can often cause endometrial hyperplasia or endometrial cancer.[5–10] Ovarian cancer was reported to be associated with PCOS in one study.[11] Some studies have reported a link between PCOS and breast cancer but others have not.[12,13] Previous studies have been limited by significant methodological biases such as the selection criterion, length of follow-up, and inclusion of a heterogeneous population. In this study, we addressed the concern of a heterogeneous population. Large cohort studies are justified for examining the entire spectrum of cancer in women with PCOS, especially associations between PCOS and hormone-related endometrial, breast, and ovarian cancer.
We investigated the risk of the aforementioned 3 cancers in a population-based cohort of Taiwanese women with PCOS identified in the National Health Insurance Research Database (NHIRD) between 1998 and 2013 and followed for cancer in the Taiwan Cancer Registry through 2013.
2. Methods
2.1. Data source
The Taiwanese government launched the National Health Insurance (NHI) program in 1995, and it covers >99% of Taiwan's population.[14] The National Health Research Institute released the NHIRD for research purposes. The Longitudinal Health Insurance Database 2000 (LHID2000) contains the details of 1 million patients randomly selected from the NHIRD. The NHIRD also includes the Registry of Catastrophic Illness Patient Database to protect vulnerable beneficiaries by exempting these patients from copayments for the corresponding medical services. This study was approved by the institutional review board (IRB) of China Medical University and the Hospital Research Ethics Committee (IRB permit number: CMUH-104-REC2-115).
2.2. Participants
Women aged 15 to 49 years who had received a new diagnosis of PCOS (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 256.4) between 1998 and 2013 were identified for the potential PCOS cohort. Valid diagnoses were based on blood tests for luteinizing hormone, follicle-stimulating hormone, and testosterone (NHI codes: 09078B2, 09126B, 09126C, 09078B1, 09125B, 09125C, 09064B2, 09121B, and 09121C) or ultrasonography (NHI code: 19003C) or both. We excluded patients who had received diagnoses of breast cancer (ICD-9-CM code 174 and 175), endometrial cancer (ICD-9-CM code 182), or ovarian cancer (ICD-9-CM code 183) and those who had withdrawn from the insurance program before the index date. We also excluded patients who had received a diagnosis of cancer either before the diagnosis of PCOS or within one year after receiving the diagnosis of PCOS and also in the comparison group before the index date.
The comparison group of this study was randomly selected from women without PCOS and was 4-fold size matched with the PCOS group by sex, age, and index year. Figure 1 illustrates the selection procedure.
Figure 1.

Flowchart of the process for establishing the PCOS cohort and comparison cohort using theNHIRD. NHIRD = National Health Insurance Research Database, PCOS = polycystic ovarian syndrome.
2.3. Outcome, relevant variables, and comorbidities
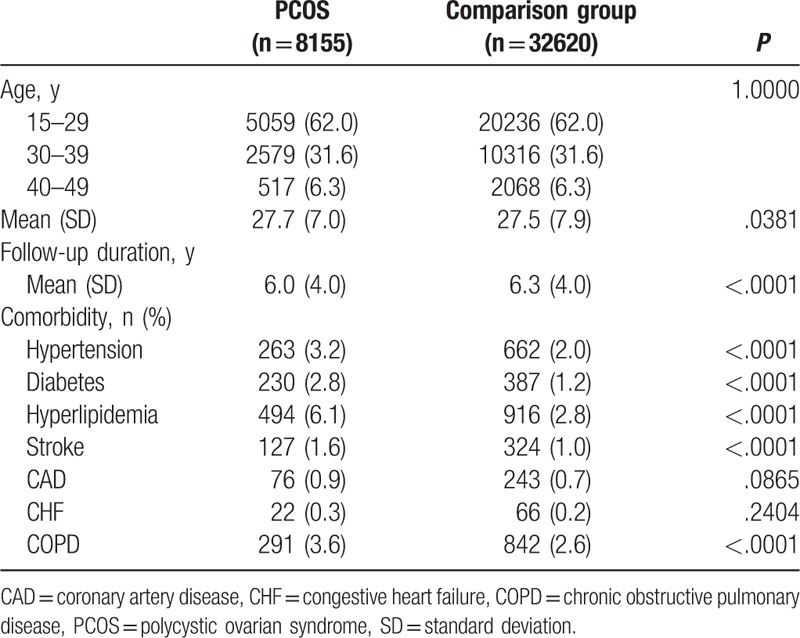
Outcomes assessed in this study were breast, endometrial, and ovarian cancer. The study endpoint was the date on which a patient received the diagnosis of one of these cancers, withdrew from the insurance, or the end of 2013. Person-years were the sum of the follow-up time for each individual, and the follow-up time was the period from the index date to the endpoint. We assessed hypertension (HTN; ICD-9-CM codes 401–405), diabetes mellitus (DM; ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430–438), coronary artery disease (CAD; ICD-9-CM codes 410–413, 414.01–414.05, 414.8, and 414.9), congestive heart failure (CHF; ICD-9-CM codes 398.91, 402.01, 402.11, 402.91, and 428), and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 491, 492, and 496) as comorbidities in this study.
2.4. Statistical analysis
To analyze the demographics of the PCOS and comparison groups, we used the χ2 tests for categorical variables and t tests for continuous variables. The incidence of an outcome was calculated using person-years. We compared the incidence of breast, endometrial, and ovarian cancer between the groups by using the incidence rate ratio (IRR) and hazard ratio (HR) and used univariate and multivariate Poisson regression models to estimate the IRR and 95% confidence interval (CI) of the 2 groups. The HR and 95% CI of the two groups were estimated using univariate and multivariate Cox proportional hazard regression models. The variables used in the multivariate model were age and all comorbidities. We used the Kaplan–Meier method to describe the incidence of an outcome for the 2 groups and examined the difference between the groups by using the log-rank test. We considered the 3 cancers to be the endpoints of this study and computed subhazard ratios (SHRs) by performing risk regression. The SHR for one of the cancers and 95% CIs were estimated by considering the other 2 cancers as competing risks. Statistical analyses of the data were performed using SAS statistical software (Version 9.4 for Windows; SAS Institute, Inc., Cary, NC). A P value of <.05 was considered statistically significant.
3. Results
3.1. Demographics and clinical characteristics
In this study, 8155 and 32,620 patients were included in the PCOS and comparison groups, respectively (Table 1). The 2 groups exhibited no differences in age grouping and mean [standard deviation, (SD)] age (27.7 [7.0] and 27.5 [7.9] in the PCOS and comparison group, respectively). The mean (SD) follow-up time for the PCOS and comparison groups was 6.0 (4.0) and 6.3 (4.0) years, respectively. The proportion of CAD and CHF did not differ between the groups, but the PCOS group had a higher proportion of other comorbidities, namely HTN, DM, hyperlipidemia, stroke, and COPD, than did the comparison group.
Table 1.
Baseline characteristcs in women with and without PCOS.
3.2. Incidence of endometrial cancer in patients with PCOS
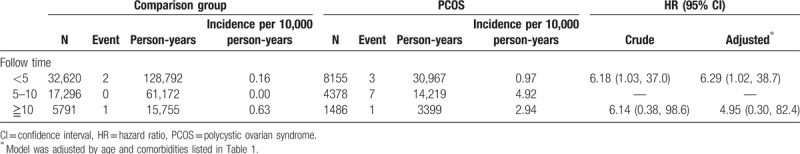
During the mean follow-up period of 6.0 years, 11 patients (11/8155) in the PCOS group and 3 patients (3/32,620) in the comparison group developed endometrial cancer (Table 2). The incidence of endometrial cancer was 22.6 and 1.5 per 100,000 person-years in the PCOS and comparison groups, respectively. The adjusted IRR of endometrial cancer for the PCOS and comparison groups was 17.1 (95% CI = 15.7–18.6). Compared with the patients without PCOS, the patients with PCOS had a significantly increased adjusted HR for endometrial cancer (17.7, 95% CI = 4.89–64.2; Table 2). When breast and ovarian cancer were considered as competing risks, the patients with PCOS also had a significantly increased adjusted SHR for endometrial cancer compared with the patients without PCOS (17.7, 95% CI = 4.81–65.3; Table 3). We also assessed the risk of endometrial cancer between the groups after stratification by the follow-up period (Table 4). Compared with the patients without PCOS, the patients with PCOS had a significantly higher risk of endometrial cancer in the first 5 years of follow-up (adjusted HR = 6.29, 95% CI = 1.02–8.7). The incidence of endometrial cancer in the PCOS group was 49.2 per 100,000 person-years between 5 and 10 years of follow-up; however, no event occurred in the comparison group during these years. After >10 years of follow-up, no statistically significant difference in the incidence of endometrial cancer was observed between the groups.
Table 2.
Risk of with endometrial cancer, ovarian cancer, and breast cancer in women with PCOS compared with the comparison group.
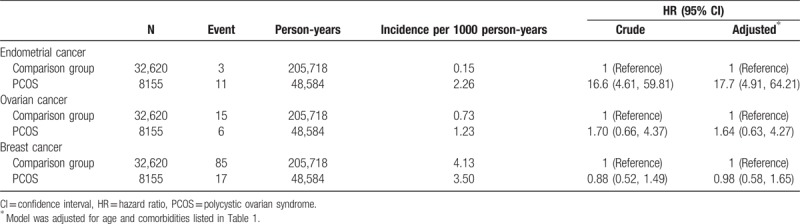
Table 3.
Subhazard ratios of endometrial cancer, ovarian cancer, and breast cancer based on the competing-risk regression.

Table 4.
Risk of endometrial cancer in women with PCOS compared with the comparison group stratified by follow-up year.
The Kaplan–Meier curves indicated that the endometrial cancer-free survival rate was significantly higher in the comparison group than in the PCOS group (P < .0001 in the log-rank test; Fig. 2).
Figure 2.
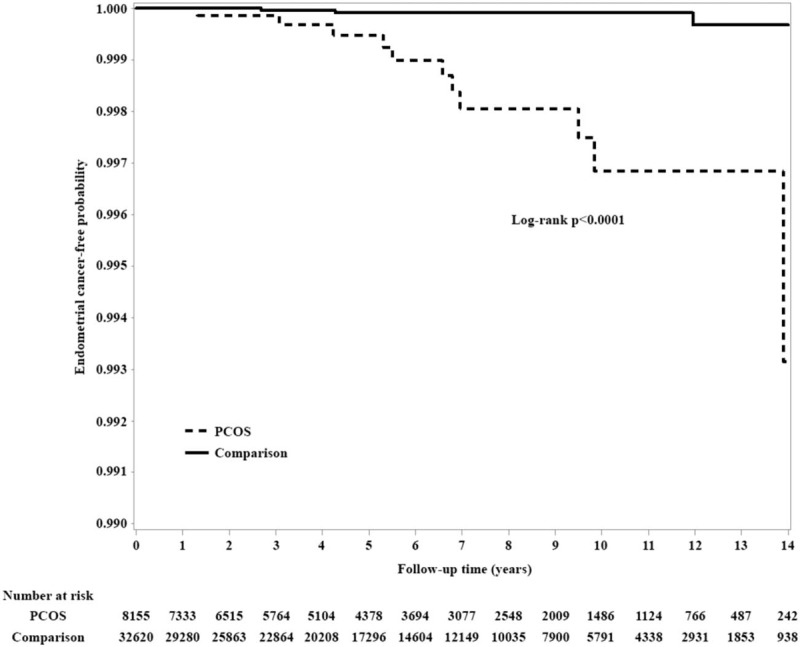
Comparison of Kaplan–Meier survival estimates for endometrial cancer in the 2 groups. PCOS = polycystic ovarian syndrome.
3.3. Incidence of ovarian cancer in the patients with PCOS
During the mean follow-up period of 6.0 years, 6 patients (6/8155) in the PCOS group and 15 patients (15/32,620) in the comparison group developed ovarian cancer (Table 2). The incidence of ovarian cancer was 12.3 and 7.3 per 100, 000 person-years in the PCOS and comparison groups, respectively. The adjusted IRR of ovarian cancer in the PCOS and comparison groups was 1.63 (95% CI = 1.50–1.78). Compared with the patients without PCOS, the patients with PCOS did not have a significantly increased adjusted HR for ovarian cancer (1.64, 95% CI = 0.63–4.27; Table 2). When breast and endometrial cancer were considered as competing risks, no statistically significant difference in the incidence of ovarian cancer was observed between the groups (adjusted SHR = 1.64, 95% CI = 0.60–4.45; Table 3).
3.4. Incidence of breast cancer in the patients with PCOS
During the mean follow-up period of 6 years, 17 patients (17/8155) in the PCOS group and 85 patients (85/32,620) in the comparison group developed breast cancer (Table 2). The incidence of breast cancer was 35.0 and 41.3 per 100 000 person-years in the PCOS and comparison groups, respectively. The adjusted IRR of breast cancer in the PCOS and comparison groups was 0.94 (95% CI = 0.86–1.03). Compared with the patients without PCOS, the patients with PCOS did not have a significantly decreased adjusted HR for breast cancer (0.98, 95% CI = 0.58–1.25; Table 2), and no statistically significant difference in the incidence of breast cancer was observed between the groups in the competing risk regression model (adjusted SHR = 0.97, 95% CI = 0.58–1.65; Table 3).
4. Discussion
In this large cohort study, an overall 17-fold higher risk of endometrial cancer was found in more than 8155 Taiwanese women with PCOS than in the woman without PCOS. The incidence of endometrial cancer was 22.6 and 1.5 per 100,000 person-years in the PCOS and comparison groups, respectively. However, no association was observed between PCOS and either breast or ovarian cancer.
The results of our study showed that PCOS was associated with endometrial cancer; this finding is consistent with that of a previous meta-analysis of 5 case–control studies that reported a significantly increased odds ratio (OR) of 2.79 for endometrial cancer.[11,15,16] Five case–control studies have reported an association between PCOS and endometrial cancer without accounting for body mass index (BMI).[5–8,17] This result is consistent with our finding. All our patients with PCOS were aged <50 years, indicating that the increased risk of this malignancy may be more likely in younger women with PCOS.
A recent Danish study did not find a higher prevalence of endometrial cancer in women with PCOS or hyperandrogenism than in controls.[18] Several studies that evaluated polycystic ovarian morphology have reported no association with endometrial cancer.[19,20]
One population-based case–control study of ovarian cancer[21] indicated that women with PCOS had a 2.5-fold higher risk of ovarian cancer than did controls (95% CI = 1.1–5.9). However, a recent meta-analysis demonstrated that the risk of ovarian cancer was not significantly increased in women with PCOS (OR = 1.4; 95% CI = 0.9–2.2).[11] In a Danish registry cohort study, researchers determined a nonsignificant increase in the risk of ovarian cancer in women with PCOS compared with the general Danish female population (standardized incidence ratio = 1.8; 95% CI = 0.8–3.2).[5] No association between PCOS and ovarian cancer was evident in cohort studies conducted in Taiwan and England.[17,22] Oral contraceptive use can reduce the risk of ovarian cancer.[23] Thus, patients with PCOS are typically treated with oral contraceptives according to the guidelines that suggest that OCs may counteract the potential cancer-promoting effects of PCOS.
In a long-term follow-up of 786 women who had received a diagnosis of PCOS, breast cancer was found to be the most common cause of death.[24] However, most other studies have identified no association between PCOS and breast cancer.[5,11,17]
The major reason PCOS increases the risk of endometrial cancer is the prolonged exposure of the endometrium to unopposed estrogen caused by anovulation.[25] This prolonged exposure can cause endometrial hyperplasia and may lead to endometrial cancer. Other risk factors for endometrial cancer are obesity, unopposed long-term estrogen use, nulliparity, infertility, DM, and HTN. Most of these factors also contribute to the development of PCOS.[26]
The strengths of our study are the large sample size of >24 million patients that enabled an accurate evaluation of the association between specific diseases (e.g., PCOS and cancers) and age. Diagnoses of PCOS were made by specialists. Additionally, our study design included an unbiased participant selection process. Because participation in the NHI is mandatory and all residents of Taiwan can access health care with low copayments, there is low referral bias and high follow-up compliance.
Certain limitations to our findings should be considered. First, the NHIRD does not provide detailed information on patients concerning smoking, alcohol consumption, or family history of malignant diseases—these are all major risk factors for cancer development. Moreover, data concerning risk factors for endometrial cancer, such as BMI, metabolic syndrome, and fertility, were not included in the NHIRD. Thus, we were unable to control for these potentially confounding factors. Second, the follow-up period could have been too short, considering that most cases of uterine cancer occur after the age of 60 years. Third, the diagnoses of PCOS were identified using ICD-9-CM codes from the database; thus, its prevalence may have been underestimated because only patients seeking medical evaluation could be identified, which would most likely result in an underestimation of the association between PCOS and gynecological cancers. Fourth, the stage, histology, and outcome of uterine cancers found within the PCOS group could not be obtained in the NHIRD, which somewhat obscures the clinical utility of our results. Additional studies may be necessary to explore these clinical issues. Fifth, although the data we obtained on PCOS and cancer diagnoses were highly reliable, the diagnoses in NHI claims are primarily for administrative billing and do not undergo verification for scientific purposes. Sixth, few cases of endometrial cancer were noted (14/10,000), which may reduce the sensitivity and positive predictive value. Seventh, the PCOS validation process used only the ICD-9-CM diagnosis codes, laboratory test results, or ultrasound results; no independent reviewer or physician provided confirmation. However, several studies used these same criteria to define PCOS in the NHIRD.[17,27]
In conclusion, our nationwide population-based retrospective cohort study provided evidence of an increased risk of endometrial cancer in the patients with PCOS. Chronic estrogen stimulation of the endometrium may be the underlying pathophysiology. DM, HTN, and hyperlipidemia are also risk factors for endometrial cancer. The clinical utility of this information remains to be determined in light of the low incidence of the cancers.
Acknowledgment
The authors thank Dr. Jon-Son Kuo and Wallace Academic Editing for English editing.
Author contributions
Study concept and design: DCD. Acquisition, analysis, and interpretation of data: DCD, WSC, JHW, SZL. Drafting of the manuscript: DCD, WSC. Critical revision of the manuscript for important intellectual content: DCD, WSC, JHW, SZL. Statistical analysis: WSC. Obtained funding: DCD, WSC. Administrative, technical, or material support: DCD, JHW, SZL. Study supervision: DCD.
Conceptualization: Dah-Ching Ding.
Data curation: Dah-Ching Ding, Weishan Chen.
Formal analysis: Weishan Chen, Jen-Hung Wang.
Funding acquisition: Dah-Ching Ding, Weishan Chen, Jen-Hung Wang, Shinn-Zong Lin.
Investigation: Dah-Ching Ding, Weishan Chen, Jen-Hung Wang.
Methodology: Weishan Chen.
Project administration: Dah-Ching Ding.
Resources: Dah-Ching Ding, Shinn-Zong Lin.
Software: Weishan Chen.
Supervision: Dah-Ching Ding, Shinn-Zong Lin.
Validation: Dah-Ching Ding.
Writing – original draft: Dah-Ching Ding, Weishan Chen.
Writing – review & editing: Dah-Ching Ding, Weishan Chen.
Footnotes
Abbreviations: BMI = body mass index, CAD = coronary artery disease, CHF = congestive heart failure, CI = confidence interval, Clinical Modification, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HR = hazard ration, HTN = hypertension, ICD-9-CM: International Classification of Diseases, IRB = Institutional Review Board, IRR = incidence rate ratio, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, Ninth Revision, OR = odds ratio, PCOS = polycystic ovarian syndrome, RCIPD = Registry of Catastrophic Illness Patient Database, SD = standard deviation, SHRs = subhazard ratios.
Funding source: This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital, Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
The authors report no conflicts of interest.
References
- [1].Asunción M, Calvo RM, San Millán JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected caucasian women from Spain. J Clin Endocrinol Metab 2000;85:2434–8. [DOI] [PubMed] [Google Scholar]
- [2].Azziz R, Carmina E, Dewailly D, et al. Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab 2006;91:4237–45. [DOI] [PubMed] [Google Scholar]
- [3].Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. [DOI] [PubMed] [Google Scholar]
- [4].Ehrmann DA, Sturis J, Byrne MM, et al. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 1995;96:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gottschau M, Kjaer SK, Jensen A, et al. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol 2015;136:99–103. [DOI] [PubMed] [Google Scholar]
- [6].Escobedo LG, Lee NC, Peterson HB, et al. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol 1991;77:124–8. [PubMed] [Google Scholar]
- [7].Niwa K, Imai A, Hashimoto M, et al. A case-control study of uterine endometrial cancer of pre- and post-menopausal women. Oncol Rep 2000;7:89–93. [PubMed] [Google Scholar]
- [8].Iatrakis G, Zervoudis S, Saviolakis A, et al. Women younger than 50 years with endometrial cancer. Eur J Gynaecol Oncol 2006;27:399–400. [PubMed] [Google Scholar]
- [9].Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol 1983;61:403–7. [PubMed] [Google Scholar]
- [10].Brinton LA, Moghissi KS, Westhoff CL, et al. Cancer risk among infertile women with androgen excess or menstrual disorders (including polycystic ovary syndrome). Fertil Steril 2010;94:1787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2014;20:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Terry KL, Willett WC, Rich-Edwards JW, et al. A prospective study of infertility due to ovulatory disorders, ovulation induction, and incidence of breast cancer. Arch Intern Med 2006;166:2484–9. [DOI] [PubMed] [Google Scholar]
- [13].Miles L. The new WCRF/AICR report—food, nutrition, physical activity and the prevention of cancer: a global perspective. Nutr Bull 2008;33:26–32. [Google Scholar]
- [14].Chien HC, Kao Yang YH, Bai JPF. Trastuzumab-related cardiotoxic effects in Taiwanese women: a nationwide cohort study. JAMA Oncol 2016;2:1317–25. [DOI] [PubMed] [Google Scholar]
- [15].Harris HR, Terry KL. Polycystic ovary syndrome and risk of endometrial, ovarian, and breast cancer: a systematic review. Fertil Res Pract 2016;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chittenden BG, Fullerton G, Maheshwari A, et al. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009;19:398–405. [DOI] [PubMed] [Google Scholar]
- [17].Shen CC, Yang AC, Hung JH, et al. A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist 2015;20:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holm NSL, Glintborg D, Andersen MS, et al. The prevalence of endometrial hyperplasia and endometrial cancer in women with polycystic ovary syndrome or hyperandrogenism. Acta Obstet Gynecol Scand 2012;91:1173–6. [DOI] [PubMed] [Google Scholar]
- [19].Zucchetto A, Serraino D, Polesel J, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev 2009;18:316–21. [DOI] [PubMed] [Google Scholar]
- [20].Fearnley EJ, Marquart L, Spurdle AB, et al. Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control 2010;21:2303–8. [DOI] [PubMed] [Google Scholar]
- [21].Schildkraut JM, Schwingl PJ, Bastos E, et al. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 1996;88:554–9. [DOI] [PubMed] [Google Scholar]
- [22].Harris HR, Titus LJ, Cramer DW, et al. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer 2017;140:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beral V, Doll R, Hermon C, et al. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008;371:303–14. [DOI] [PubMed] [Google Scholar]
- [24].Pierpoint T, McKeigue PM, Isaacs AJ, et al. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 1998;51:581–6. [DOI] [PubMed] [Google Scholar]
- [25].Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids 2013;78:782–5. [DOI] [PubMed] [Google Scholar]
- [26].Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009;13:90–2. [PMC free article] [PubMed] [Google Scholar]
- [27].Pan ML, Chen LR, Tsao HM, et al. Relationship between polycystic ovarian syndrome and subsequent gestational diabetes mellitus: a nationwide population-based study. PLoS One 2015;10:e0140544. [DOI] [PMC free article] [PubMed] [Google Scholar]


