Abstract
Background:
Although different clinical and experimental parameters have been used to estimate disease activity in systemic lupus erythematosus (SLE) patients, the relationship between red blood cell distribution width-to-platelet ratio (RPR) and disease activity in SLE has not been previously illuminated. Therefore, the aim of this study was to investigate the association between RPR levels and disease activity in SLE.
Methods:
This study enrolled 105 SLE patients and 105 healthy subjects. We divided the patients into 2 groups using the SLE Disease Activity Index (SLEDAI) 2000. Group 1 included patients with SLEDAI score ≤9 (mild disease activity group) and group 2 with SLEDAI >9 (severe disease activity group). Correlations between RPR and disease activity were then analyzed. A subgroup follow-up analysis of 93 patients was conducted to explore the effect of SLE-related glucocorticoid therapy.
Results:
The PLR and RPR values of SLE patients were significantly higher compared with the controls (both P < .001), whereas mean platelet volume was decreased (P < .05). The RPR level was found to be positively correlated with SLEDAI (r = 0.368, P < .001) and erythrocyte sedimentation rate (r = 0.313, P = .027). According to the receiver-operating characteristic (ROC) curve, the optimal cut-off value for predicting SLE using RPR was 0.073, and the area under ROC curve was 0.817. RPR level was correlated with clinical disease activity in SLE, and its value was normalized after treatment.
Conclusion:
RPR may be a useful measurement for the assessment of disease activity in SLE patients.
Keywords: autoimmune disease, biological marker, systemic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease with an unclear pathogenesis and complicated clinical symptoms involving multiple organ dysfunction such as the loss of immunological tolerance. The incidence of SLE varies between countries, from 20 to 70 per 100,000.[1] It occurs in both males and females, but it affects predominantly women (especially 20–40 years old, ie, women of childbearing age). The symptoms associated with each sex are different. SLE symptoms vary widely and unpredictably, so diagnosis can thus be elusive, with some people having unexplained symptoms of SLE for years.
Unrestricted hyperactivation of the immune system results in the overproduction of autoantibodies, immune complexes, and inflammatory cytokines, which interact to produce eventual disease onset. Immune-system aberrations, and also heritable, hormonal, and environmental factors, contribute to the expression of this chronic inflammatory process and organ damage.[2,3] This disease of protean manifestations has periodic remissions and relapses, and varies from acutely progressive to chronic forms, resulting in a prolonged and repeated disease course.[4]
The accurate evaluation of disease activity is of great importance to assessment of disease progression and prognosis for SLE patients. Many different inflammation indices have been used to appraise inflammatory status in lupus and as a marker of disease activity, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Antidouble-stranded DNA (anti-dsDNA) antibody and serum complement are also used to determine some of the clinical manifestations of lupus, especially nephritis.[5] The platelet-to-lymphocyte ratio (PLR), red blood cell distribution width (RDW), and similar parameters (eg, neutrophil-to-lymphocyte ratio [NLR] and mean platelet volume [MPV]), which can be easily obtained using peripheral blood parameters, have been regarded as novel, accurate inflammatory biomarkers in many diseases.[6–10]
In recent years, many studies have shown that NLR and PLR may be useful for the evaluation of autoimmune diseases activity, such as rheumatoid arthritis (RA) and SLE.[11–14] NLR has been deemed to be a useful index for the differential diagnosis or prognostic prediction of chronic inflammatory diseases. Specifically, Li et al[15] regarded NLR as an independent factor associated with SLE and a promising marker reflecting renal involvement. MPV is 1 biomarker of platelet turnover, whereas platelet activation is a marker of inflammation. Previous studies have reported that MPV is correlated with the inflammatory process and disease activity in RA and ankylosing spondylitis, but the relationship between MPV and SLE remains controversial.[16] Red blood cell distribution width-to-platelet ratio (RPR) has been presented as a novel and rapid laboratory index to predict mortality in various diseases.[17,18] For example, Chen et al[19] concluded RPR to be a noninvasive and economical predictor of fibrosis and cirrhosis in chronic hepatitis B, compared with liver biopsy. Similarly, Cetinkaya et al[17] used the RPR value to estimate the severity of acute pancreatitis (AP), and to improve survival in AP patients.
Nevertheless, to the best of our knowledge, the relationship between RPR value and disease activity in SLE patients has not been elucidated. Therefore, this study aimed to determine whether RPR can be used as a predictor of disease activity and severity in SLE patients, and to investigate its possible association with other inflammatory markers in patients with SLE.
2. Methods
2.1. Participants
All patients enrolled in this study were identified from electronic medical records at The First Affiliated Hospital of Guangxi Medical University (Nanning, China). SLE patients who were admitted to the hospital between January, 2012 and December, 2017 were included in this study. No particular treatment had been received by any of the patients at the time of laboratory tests. The diagnosis of SLE was made based on criteria established by the American College of Rheumatology (ACR).[20] The control group was composed of 105 healthy individuals who visited the hospital for a routine check-up. Patients were excluded from the study if they had 1 of following comorbidities: other autoimmune disease, such as RA, mixed connective tissue disease (MTCD), or myasthenia gravis (MG); malignant diseases; inflammatory diseases, including acute infection or chronic inflammation status; coronary artery disease, hypertension, or cerebrovascular disease; renal or liver disease; hematological disease or any blood transfusion in the previous past 4 months. Finally, 105 newly diagnosed SLE patients were included in this study. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
2.2. Data extraction
Clinical characteristics and laboratory datum were collected from patient medical records; these included age, sex, and also levels for white blood cell (WBC) count, hemoglobin, platelets, neutrophils, lymphocytes, RDW, MPV, ESR, CRP, immunoglobulins, albumin, urine protein, complement and anti-dsDNA antibody. PLR was calculated using the ratio of platelet over lymphocytes, and RPR was calculated by the ratio of RDW over platelet. Additionally, Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score was used to assess disease activity of based on clinical symptoms and laboratory results. We divided the patients into 2 groups according to the SLEDAI-2K system.[21] Group 1 included patients with a SLEDAI score ≤9 (patients with mild disease) and group 2 with SLEDAI >9 (patients with severe disease activity). To explore the effect of SLE-related treatment on PLR and RPR, follow-up of 93 patients receiving glucocorticoid therapy among the general sample population of 105 SLE patients were analyzed separately. This study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee. Clinical findings herein were not used for the subsequent management of patients.
2.3. Statistical analysis
All the continuous variables were evaluated for normal distribution by the Kolmogorov-Smirnov test. Parametric or nonparametric tests were used to analyze data according to normal or abnormal distribution, respectively. Continuous data are shown as mean ± standard deviation (SD) or median (25th, 75th percentiles), and were compared using Student t test or Mann-Whitney U test, as appropriate. The Spearman correlation coefficient was calculated to examine the association between 2 continuous variables. Receiver-operating characteristic (ROC) curve analysis was then performed to determine the sensitivity and specificity of possible inflammatory markers in predicting SLE. The statistical analysis was conducted using SPSS 17.0 (Chicago, IL). P values <.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of sample population
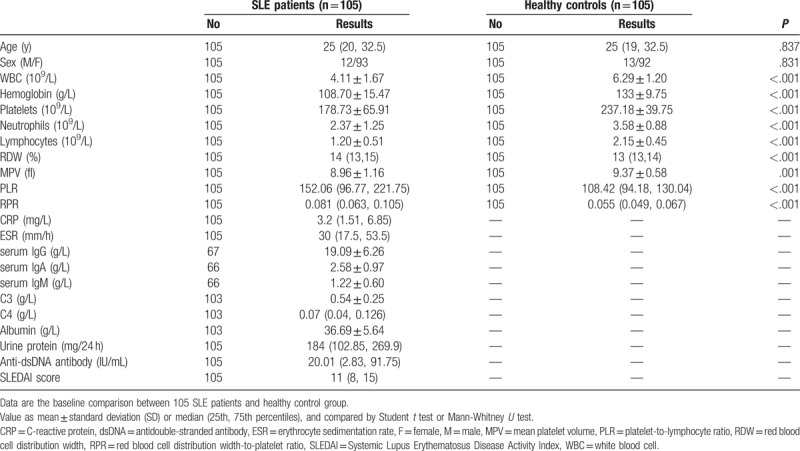
Patient group had a median age of 25 years (range 16–45), with a sex distribution of 93 women (88.6%) and 12 men (11.4%). The median age was 27 years in the control group (range 16–46), with a sex distribution of 92 women (87.6%) and 13 men (12.4%). No statistically significant differences were observed in age and sex between patient and control groups (P = .837 and P = .831, respectively) (Table 1).
Table 1.
Basic characteristics and laboratory results of patients and control group.
3.2. PLR and RPR were increased in SLE patients while MPV was decreased
The levels of RPR and PLR were increased in SLE patients, whereas MPV was decreased as compared with healthy controls. There was a statistically significant difference in RPR, PLR, and MPV between the patient and control groups (both P < .001). We found that SLE patients had lower neutrophil count, lymphocyte count, and hemoglobin than the healthy controls. WBC and platelet counts were decreased, but were within normal limits (Table 1, Fig. 1).
Figure 1.
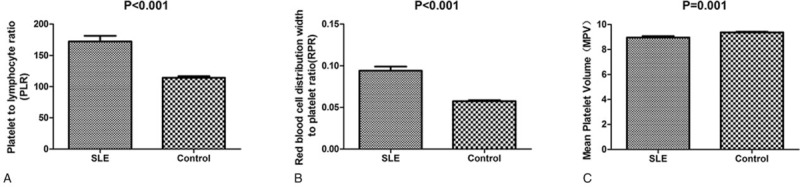
Comparison of PLR, RPR, and MPV in SLE patients and healthy controls. (A) PLR level was significantly increased in SLE patients. (B) RPR level was significantly increased in SLE patients. (C) MPV was decreased in SLE patients (all P < .05). MPV = mean platelet volume, PLR = platelet-to-lymphocyte ratio, RPR = red blood cell distribution width-to-platelet ratio, SLE = systemic lupus erythematosus.
3.3. RPR was associated with SLE clinical disease activity
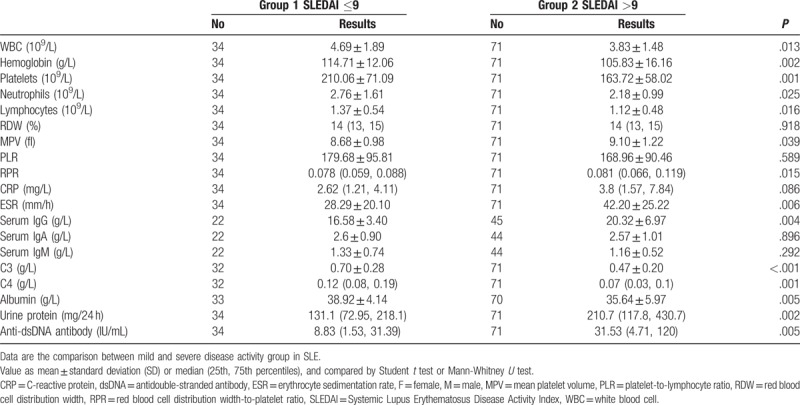
In this study, patients in group 1 (SLEDAI score ≤9) had an RPR of 0.078 (range 0.059–0.088), whereas patients in group 2 (SLEDAI >9) had a higher RPR of 0.081 (0.066–0.119). The differences in RPR between the 2 groups were statistically significant (P = .015). Other clinical indicators according to SLEDAI score subgroups were shown in Table 2. Patients with higher SLEDAI score had higher anti-dsDNA antibody, urine protein, serum IgG and ESR, whereas complement C3, C4, and albumin were decreased significantly.
Table 2.
Laboratory data of patients according to SLEDAI scores.
3.4. Effect of glucocorticoid treatment on PLR, RPR, and MPV
Glucocorticoids are widely used to treat patients with autoimmune diseases such as SLE,[22] as it is an effective anti-inflammatory and immunosuppressive agent. To investigate the effects of glucocorticoid treatment, we analyzed changes in the laboratory parameters of SLE patients before and after treatment. As shown in Fig. 2, we found that PLR and RPR were decreased after treatment, accompanied by a decrease of both anti-dsDNA and SLEDAI. All differences were statistically significant, except the effect of treatment on MPV.
Figure 2.
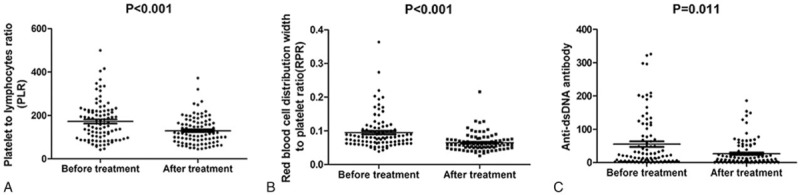
Comparison of PLR, RPR, and anti-dsDNA before and after treatment. All 3 indicators of SLE were reduced after treatment (P < .05). dsDNA = antidouble-stranded antibody, PLR = platelet-to-lymphocyte ratio, RPR = red blood cell distribution width-to-platelet ratio, SLE = systemic lupus erythematosus.
3.5. Correlations of RPR with clinical parameters of SLE patients
The SLEDAI scores were positively correlated with RPR, MPV, ESR, urine protein, and anti-dsDNA, whereas this score displayed a negative relationship with WBC, hemoglobin, PLT, albumin, and complement C3 and C4 (P < .05 for all). RPR was positively correlated with SLEDAI (r = 0.368, P < .001), ESR (r = 0.313, P = .027), anti-dsDNA (r = 0.275, P = .036), and urine protein (r = 0.25, P = .01), whereas it was negatively correlated with complement C3 and C4, and albumin (P < .05 for all). Interestingly, RPR, PLR, and MPV were each found to be correlated with the other 2 measures (P < .001) (Table 3, Fig. 3).
Table 3.
Correlation of SLEDAI score and RPR with laboratory indices in SLE.
Figure 3.
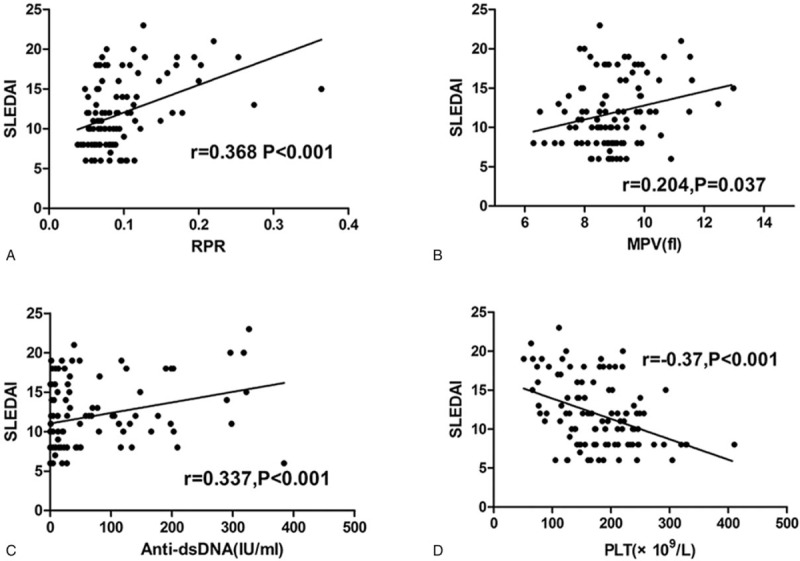
Correlation between SLEDAI and RPR, MPV, anti-dsDNA, and PLT in SLE patients. Spearman correlation analysis was performed to examine the association between SLEDAI and RPR, and other laboratory parameters. (A) RPR level was positively correlated with SLEDAI score. (B) MPV was positively correlated with SLEDAI score. (C) Anti-dsDNA was positively correlated with SLEDAI score. (D) PLT was negatively correlated with SLEDAI score (all P < .05). dsDNA = antidouble-stranded antibody, MPV = mean platelet volume, RPR = red blood cell distribution width-to-platelet ratio, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index.
3.6. Receiver-operating characteristic analysis of RPR in prediction of SLEDAI score
Receiver-operating characteristic analysis was performed to differentiate between SLE and healthy controls. The ROC curve was drawn based on the sensitivity and the specificity for different threshold. For predicting SLE, the optimal threshold for RPR was 0.073, which had a sensitivity of 0.60 and a specificity of 0.905 (area under ROC curve [AUC] 0.817, 95% confidence interval [CI] 0.760–0.874) (Fig. 4). Compared with other 2 indicators, RPR yielded a higher AUC than PLR and MPV in differentiating SLE patients from healthy controls. The AUC for PLR and MPV was less than 0.7 (data not shown).
Figure 4.
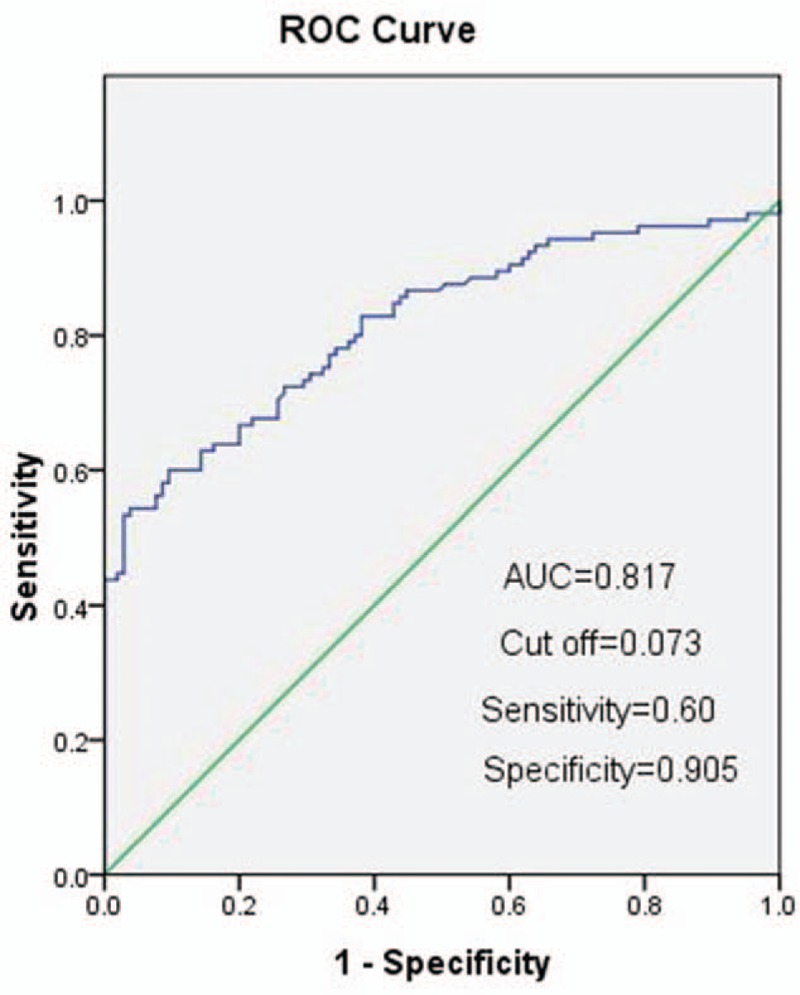
Receiver-operating characteristic (ROC) curves of RPR for the differentiation of SLE patients from healthy controls. RPR yielded a higher AUC than either PLR and MPV. MPV = mean platelet volume, RPR = red blood cell distribution width-to-platelet ratio, SLE = systemic lupus erythematosus.
4. Discussion
For the first time, the relationship between RPR, other inflammatory factors, auto-antibodies, and SLEDAI in SLE patients was evaluated. Controlling for the potential effects of medication, we compared the RPR of healthy controls and naïve SLE patients who had never received any SLE-related treatment. According to our findings, RPR was significantly increased in SLE patients when compared with healthy individuals, and elevated RPR levels were correlated with increasing SLEDAI score. RPR levels were also found to be correlated with ESR, anti-dsDNA, and complement C3 and C4, and albumin concentrations. Furthermore, RPR yielded a higher AUC than PLR and MPV, and the ROC curve analysis indicated that RPR had a promising predictive value for SLE. Therefore, we concluded that RPR could be a new inflammatory marker that could be useful to rapidly estimate severity in patients with SLE.
It is well known that peripheral blood circulating WBC reflects changes in systemic inflammation, especially with respect typical of lymphocytes and neutrophils counts. WBC and its subgroup classifications have been identified as biomarkers in various inflammatory diseases, like NLR, as the ratio of neutrophils over lymphocytes.[23,24] This measure was widely used in the evaluation of clinical inflammatory progress of diseases, including autoimmune and nonautoimmune diseases. Because its stability is rarely influenced by physiological, pathological, and physical factors, and it is cost-effective and easy to calculate. Specifically, NLR is used for prognostic monitoring in cancer and systemic inflammatory diseases, such as diabetes, cardiovascular diseases, and colorectal cancer.[6,25,26] Previous studies have reported a relationship between NLR and SLE,[12,15,27] and a direct relationship between NLR and the clinical characteristics of SLE. This basic measure has been inferred as a useful predictive marker in the development of SLE, especially in lupus nephritis.
Similar to NLR, PLR is another inflammatory index that is widely used in routine blood tests, and its value reflects changes in inflammation and cytokine concentration. Although the number of lymphocytes and platelets generally decreases in SLE patients, PLR only fluctuates with changes in disease activity. Qin et al found that PLR was positively related to disease activity in SLE and was higher in lupus nephritis as compared with those lupus patients without nephritis. Nevertheless, our study demonstrated that PLR was significantly higher in patients with SLE, but a correlation between PLR and SLEDAI was not found. The reason for this lack of correlation may be that our subjects were all newly diagnosed SLE patients, and the impact of renal function cannot be evaluated. Different study populations also might lead to the inconsistent findings. The relationship between PLR and lupus nephritis needs to be further explored.
Mean platelet volume is also an indicator of systemic inflammation and a marker of disease activity in different diseases.[28,29] It was found that MPV is considerably higher in RA and associated with other inflammatory factors. Safak et al[10] have reported that low levels of MPV are associated with disease activity in SLE patients with arthritis. On the contrary, Yavuz et al[30] revealed that elevated MPV can be used as a predictor of disease activity in juvenile SLE patients, whereas Qin et al[14] confirmed that MPV was also increased in adult SLE patients. Our research cohort included a wide range of ages, both the juveniles and adults. In our study, MPV was decreased in SLE patients, and positively related to SLEDAI score. This result was different from previous studies, probably because the MPV value was affected by platelet count and clinical factors. We did not adjust for additional factors that may have caused bias. In addition, Noris et al[31] reviewed MPV measurement in clinical practice, and suggested that MPV should have no role in making diagnosis or other clinical applications. More prospective studies are needed to validate these findings.
Life expectancy is lower among people with SLE,[32] and it significantly increases the risk of cardiovascular disease, the most common cause of death worldwide. The severe impact of SLE is well-known, so monitoring of the patient condition is very important in long-term treatment. Assessment of disease activity is a critical step in the estimation of disease severity. CRP and ESR are the most widely used indicators of disease activity in inflammatory diseases, but these 2 indicators are easily affected by other infectious events. Under these circumstances, CRP and ESR are used to combine other indicators for the determination of inflammatory states in SLE.
Traditionally, serum complements are often used to diagnose and assess the activity of patients with SLE. Previous study has revealed that low C3 and C4 levels in SLE patients are associated with disease activity,[33] a finding exactly consistent with our results. In the present study, we found that RPR levels in patients with decreased complement C3 and C4 were relatively elevated, suggesting that RPR may also be an indicator of SLE disease activity.
Anti-dsDNA antibodies are additional conventional parameters used in the estimation of SLE disease activity. Anti-dsDNA titer been shown to have a clear relationship with clinical state and progression of disease.[34] Our study further demonstrated that anti-dsDNA antibody was significantly associated with SLEDAI score. Based on the data we obtained, RPR was positively correlated with SLEDAI score, ESR, dsDNA, and urine protein, from which we suggested that RPR may be a potential marker in predicting disease status in SLE patients. Whether RPR is related to nephritis requires further study and verification. Our results showed that elevated RPR and PLR in SLE patients were decreased and normalized after treatment, but there was no effect on MPV. As these 3 indicators can reflect the disease state of SLE patients to a certain extent, we conducted a ROC curve analysis to explore their predictive value in monitoring and evaluation. Analysis of RPR values yielded the maximum AUC compared with the other 2 markers. Nevertheless, the sensitivity of RPR was slightly lower, suggesting that RPR should be combined with other clinical indicator to better validate the clinical value in SLE when used in practice.
There are some underling limitations in this retrospective study. Firstly, the present study was performed at a single center and all data were obtained from the hospital electronic medical records; therefore, patient selection bias was unavoidable. Secondly, this study was designed as a retrospective study lacking longitudinal observation. Thirdly, the sample size is relatively small, which may lead to an underestimate or overestimate of the relationship between RPR and SLE disease activity in patients. Finally, the complexity of the disease itself and its variability may lead to inaccurate conclusions. Therefore, further prospective studies are required to clarify this issue and confirm whether RPR is a predictor of SLE disease activity.
5. Conclusions
In summary, the present study is the first to evaluate the RPR value in newly diagnosed SLE patients and explore its clinical significance. Results of analysis showed that RPR was independently related to SLE without nephritis. RPR testing is inexpensive, widely available and easies to measure than traditional indicators, such as ESR, CRP, and anti-dsDNA levels. Finally, RPR was positively correlated with SLEDAI and other inflammatory markers so may be of great diagnostic and prognostic value in the evaluation of SLE patients.
Acknowledgments
Many thanks to teacher Xuejie Chen for his help in data processing and language indication.
Author contributions
Data curation: Siyan Xie.
Formal analysis: Siyan Xie.
Investigation: Siyan Xie.
Supervision: xuejie Chen.
Validation: xuejie Chen.
Writing – original draft: Siyan Xie.
Writing – review & editing: xuejie Chen.
Footnotes
Abbreviations: anti-dsDNA = antidouble-stranded antibody, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, MPV = mean platelet volume, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, RDW = red blood cell distribution width, ROC = receiver-operating characteristic, RPR = red blood cell distribution width-to-platelet ratio, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index.
The authors have no conflicts of interest to disclose.
References
- [1].Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus 2006;15:308–18. [DOI] [PubMed] [Google Scholar]
- [2].Quartier P, A-M P. Systemic lupus erythematosus. Lancet 2004;23:111. [Google Scholar]
- [3].Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell 2007;130:25–35. [DOI] [PubMed] [Google Scholar]
- [4].Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Models Mechanisms 2014;7:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zivković V, Stanković A, Cvetković T, et al. Anti-dsDNA, anti-nucleosome and anti-C1q antibodies as disease activity markers in patients with systemic lupus erythematosus. Srpski Arhiv Za Celokupno Lekarstvo 2014;142:431. [PubMed] [Google Scholar]
- [6].Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102:653–7. [DOI] [PubMed] [Google Scholar]
- [7].Ayça B, Akin F, Çelik Ö, et al. Platelet to lymphocyte ratio as a prognostic marker in primary percutaneous coronary intervention. Platelets 2014;26:638. [DOI] [PubMed] [Google Scholar]
- [8].Clarke K, Sagunarthy R, Kansal S. RDW as an additional marker in inflammatory bowel disease/undifferentiated colitis. Digest Dis Sci 2008;53:2521–3. [DOI] [PubMed] [Google Scholar]
- [9].Hu ZD, Sun Y, Guo J, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjogren's syndrome. Clin Biochem 2014;47:287–90. [DOI] [PubMed] [Google Scholar]
- [10].Safak S, Uslu AU, Serdal K, et al. Association between mean platelet volume levels and inflammation in SLE patients presented with arthritis. Afr Health Sci 2015;14:919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fu H, Qin B, Hu Z, et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin Lab 2015;61:269. [DOI] [PubMed] [Google Scholar]
- [12].Oehadian A, Suryadinata H, Dewi S, et al. The role of neutrophyl lymphocyte count ratio as an inflammatory marker in systemic lupus erythematosus. Acta Med Indones 2013;45:170–4. [PubMed] [Google Scholar]
- [13].Uslu AU, Küçük A, Şahin A, et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis 2015;18:731–5. [DOI] [PubMed] [Google Scholar]
- [14].Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol 2016;26:372–6. [DOI] [PubMed] [Google Scholar]
- [15].Li L, Xia Y, Chen C, et al. Neutrophil-lymphocyte ratio in systemic lupus erythematosus disease: a retrospective study. Int J Clin Exp Med 2015;8:11026. [PMC free article] [PubMed] [Google Scholar]
- [16].Kisacik B, Tufan A, Kalyoncu U, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine 2008;75:291–4. [DOI] [PubMed] [Google Scholar]
- [17].Cetinkaya E, Senol K, Saylam B, et al. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol 2014;20:14450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taefi A, Huang CC, Kolli K, et al. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int 2015;9:1–7. [DOI] [PubMed] [Google Scholar]
- [19].Chen B, Bo Y, Jian Z, et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. Plos One 2013;8:e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 2010;40:1725. [DOI] [PubMed] [Google Scholar]
- [21].Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- [22].Guiducci C, Gong M, Xu Z, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 2010;465:937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5–14. [PubMed] [Google Scholar]
- [24].Ahsen A, Ulu MS, Yuksel S, et al. As a new inflammatory marker for familial mediterranean fever: neutrophil-to-lymphocyte ratio. Inflammation 2013;36:1357–62. [DOI] [PubMed] [Google Scholar]
- [25].Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014;134:2403–13. [DOI] [PubMed] [Google Scholar]
- [26].Huang W, Huang J, Liu Q, et al. Neutrophil–lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol 2015;82:229–33. [DOI] [PubMed] [Google Scholar]
- [27].Wu Y, Chen Y, Yang X, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 2016;36:94–9. [DOI] [PubMed] [Google Scholar]
- [28].Yazici S, Yazici M, Erer B, et al. The platelet indices in patients with rheumatoid arthritis: mean platelet volume reflects disease activity. Platelets 2010;21:122–5. [DOI] [PubMed] [Google Scholar]
- [29].Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011;17:47–58. [DOI] [PubMed] [Google Scholar]
- [30].Yavuz S, Ece A. Mean platelet volume as an indicator of disease activity in juvenile SLE. Clin Rheumatol 2014;33:637–41. [DOI] [PubMed] [Google Scholar]
- [31].Noris P, Melazzini F, Balduini CL. New roles for mean platelet volume measurement in the clinical practice? Platelets 2016;27:607–12. [DOI] [PubMed] [Google Scholar]
- [32].Murphy G, Isenberg D. Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology 2013;52:2108–15. [DOI] [PubMed] [Google Scholar]
- [33].Chen M, Daha MR, Kallenberg CGM. The complement system in systemic autoimmune disease. J Autoimmun 2010;34:J276–86. [DOI] [PubMed] [Google Scholar]
- [34].Swaak AJ, Aarden LA, Lw SVE, et al. Anti-dsDNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum 2010;22:226–35. [DOI] [PubMed] [Google Scholar]


