Abstract
The management of gynaecological cancers in elderly women and high-risk patients is an even more relevant issue, because the increase in longevity and comorbidities. The assumption of frailty based on age alone may lead to inadequate and inappropriate treatment and frailty assessment is recommended. The aim of this study was to assess if Vulnerable Elders Survey-13 (VES-13), as indicator of frailty, can predict the toxicity of chemotherapy in gynaecological cancers.
VES-13 was administered to patients aged ≥ 70 years with ovarian, endometrial and cervical cancers who underwent chemotherapy from 2010 to 2016.
Eighty-four patients aged ≥ 70 years (mean age 74.6) were included, 36 patients (42.9%) resulted vulnerable (score ≥ 3). Thrombocytopenia and anaemia were more prevalent in the vulnerable subjects (81.3% versus 18.7%, P = .0005, and 81.8% versus 18.2%, P = .005, respectively), while neutropenia was similar between the 2 groups. Vulnerable women had higher risk of non-haematological toxicities. Most of the patients (77.4%) completed chemotherapy, but dose reductions and discontinuations were more common in the vulnerable group (66.7% versus 33.3%, P = .07 and 68.4% versus 31.6%, P = .01, respectively).
To our knowledge, this is the first study to evaluate VES-13 exclusively in elderly women with gynaecological cancers. VES-13 may be useful to stratify this category of patients according to vulnerability in order to identify women at risk of toxicity and to prevent complications induced by chemotherapy.
Keywords: chemotherapy toxicity, elderly, frailty, gynaecological cancers, VES-13
1. Introduction
Because the significant increase in longevity, management of gynaecological cancers in elderly women is an issue that is going to be even more relevant. Elderly patients affected by gynaecological cancers will rise further and gynaecologic oncologists have to focus on these patients and their specific needs.[1]
Ovarian cancer is common in the elderly and its incidence increases with age to reach a peak during the 7th decade of life. Also endometrial cancer, the most common gynaecologic cancer, primarily affects elderly women, with a mean age at diagnosis of 68 years. Furthermore, in this subpopulation endometrial cancer has specific features which make it more aggressive leading to a poor prognosis.[1] Cervical cancer has a bimodal age distribution, with peaks at ages 30 to 39 years and at ages 60 to 69 years; nearly 20% of women with cervical cancer are diagnosed when they are over age 65.[2]
Geriatric population has frequently acquired comorbidities and is less likely to receive standard antineoplastic treatments and is underrepresented in clinical trials.[3–8]
An emergent concept is frailty, a state of vulnerability to poor resolution of homeostasis following a stress and it is a consequence of cumulative decline in multiple physiological systems.[9,10] Chronological age alone is a poor predictor of frailty. Comprehensive geriatric assessment (CGA) is the most used method to identify frailty, but it is time-consuming to administer and it may not be suitable for use in clinical practice. Some authors have proposed screening tools that can be used to rapidly identify vulnerable patients. Recent studies have shown that some of the geriatric parameters, included in the complete CGA and in some screening tests, can have a predictive value for chemotherapy toxicity, and specific scores have been identified in order to assist the clinical decision process of physicians.[11–14]
Vulnerable Elders Survey-13 (VES-13) is a simple function-based frailty screening tool that can be administered within 5 minutes. VES-13 was developed in 2001 to identify older people at risk of health deterioration.[15] The construction of VES-13 was based on analyses of survey data from a sample of elders in the Medicare Current Beneficiary Survey.[16]
The aim of this observational prospective study is to assess if frailty, determined by VES-13, can predict the risk of toxicity in patients with gynaecological cancers undergoing chemotherapy.
2. Methods
2.1. Patients and treatment
The analysis involved patients aged ≥ 70 years (elderly) with ovarian, endometrial and cervical cancers, treated at the Gynaecological Oncologic Unit of the Academic Division of Gynaecology and Obstetrics, Mauriziano Hospital, Torino, from January 2010 to May 2016. All of the patients included in the study must have received chemotherapy.
Institutional review board approved the study protocol, and all participating patients provided informed consent.
Clinical and demographic data included medical comorbidities, amount of medications, type of cancer (ovarian cancer, endometrial cancer or cervical cancer), age at diagnosis, histology and International Federation of Gynecology and Obstetrics (FIGO) stage, type of treatment and recurrence.
In the analysis of the chemotherapy treatment and its toxicity, a record was kept of the type of chemotherapy (single-agent or combination), the line of chemotherapy, the number of cycles, the amount of dose reductions and the reason for each reduction, the number of dose delays and the reason for each delay, treatment discontinuations, the use of colony stimulating factors (CSF), the need of blood transfusions and the patient's response. The toxicity was assessed using the Common Terminology Criteria for Adverse Event (CTCAE) v. 4.0, and grades 3 to 4 haematological and non-haematological toxicities were included in the analysis.
Finally, the last follow up and patient's clinical condition were reported.
2.2. VES-13
VES-13 (Table 1) was administered to all of the women at the initial evaluation. VES-13 consisted of 4 groups of questions: age, self-perceived health, difficulties to perform 6 specific activities (crouching or kneeling, carrying heavy objects, extending arms above shoulder level, handling small objects, walking for 500 meters, doing heavy housework) and difficulties to perform daily living tasks due to health concerns (shopping for personal items, managing money, walking across the room, doing light housework, bathing, and showering). The score ranged from 0 to 10 and a score ≥ 3 was considered to show impairment.[15,16]
Table 1.
Elements included in VES-13[16].
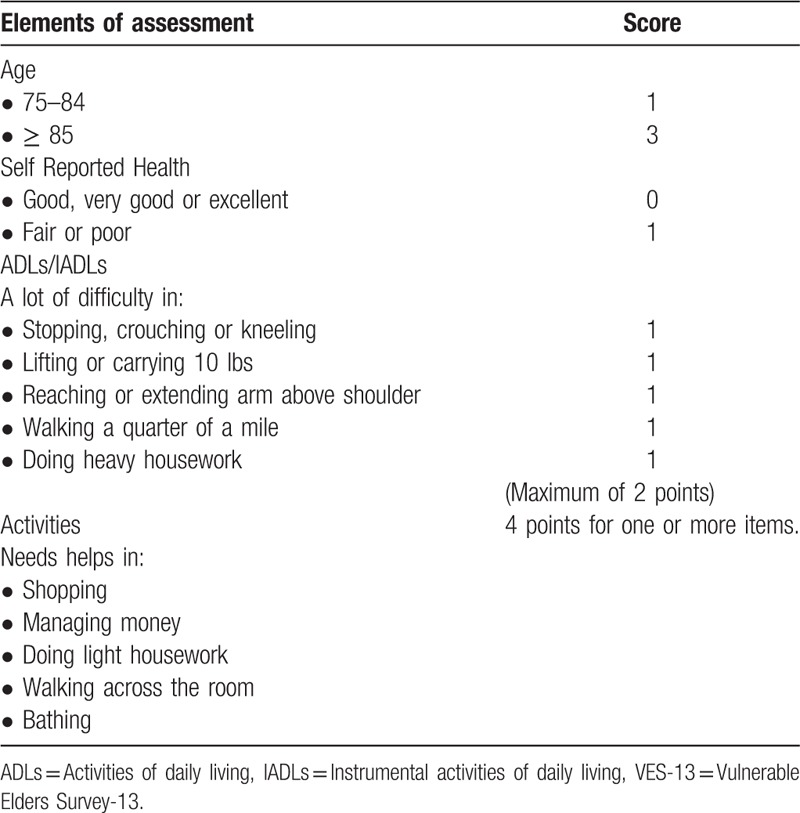
At the end of the treatment, chemotherapy toxicities and compliance to treatment were compared with results of VES-13.
2.3. Statistical analysis
The characteristics of the studied subjects were summarised in the sample as a whole, and in the groups defined by VES-13 scores. The categorical variables were compared using the chi-squared test or Fisher test, and the continuous variables were compared using T Student test. Statistical significance was set at P = .05. The analyses were performed using statistical package SPSS Ver. 17 for Windows (Chicago, IL).
3. Results
The study included 84 patients affected by gynaecological malignancies (ovarian, endometrial, and cervical cancer). All of the patients underwent chemotherapy. VES-13 was administered to all 84 women at the initial evaluation and it identified 36 patients (42.9%) as vulnerable (score ≥ 3). Three patients (3.6%) were more than 85 years old at the beginning of the treatment and therefore were vulnerable even based on age.
3.1. Clinical and demographic characteristics
Mean age at the time of chemotherapy was 75.4 and median age 75 (range 70– 89).
Patients were taking a mean number of 3.3 daily medications and a median number of 3 (range 0–10). However, 20 patients (23.8%) were taking 5 or more daily medications, the threshold value to define poly-pharmacy. Incidence of poly-pharmacy resulted greater in vulnerable subjects (75% in vulnerable versus 25% in non-vulnerable, P = .0003).
The mean number of comorbidity was 2.1 and the median number 2 (range 0–7). The most prevalent comorbidities were: hypertension (73.4%), diabetes mellitus (19%), cardiovascular diseases (17.9%), peripheral vascular diseases (16.7%), cerebrovascular diseases (13.1%), and respiratory problems (9.5%). The mean number of comorbidities was greater in the group of vulnerable patients (3.14 in vulnerable versus 1.77 in non-vulnerable, P = .0001).
3.2. Cancer characteristics
The most frequent malignancy was ovarian cancer (64 patients, 76.2%), followed by endometrial cancer (13 patients, 15.5%) and cervical cancer (7 patients, 8.3%).
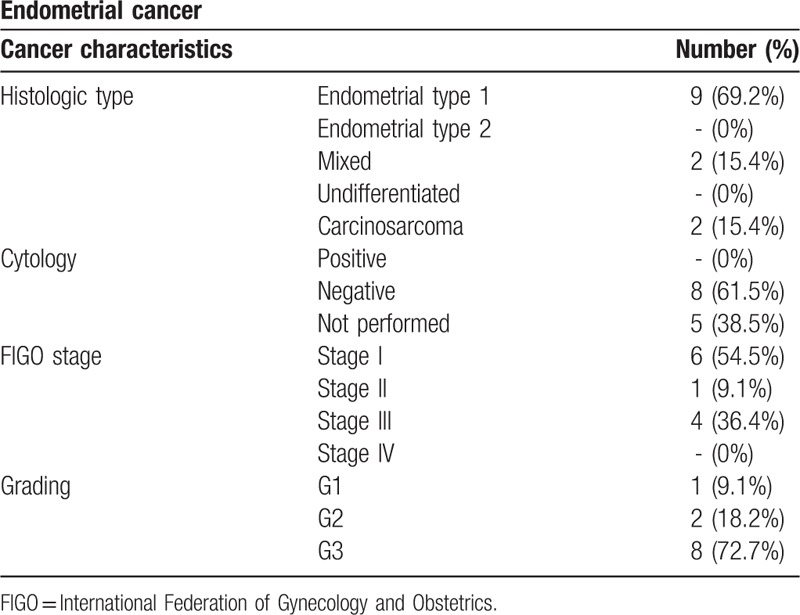
Tables 2 and 3 display histologic type, cytology, grading, and FIGO stage of ovarian and endometrial cancers. All patients with diagnosis of cervical cancer, included in the study, showed at histopathologic analysis a squamous cells cancer.
Table 2.
Ovarian cancer characteristics.
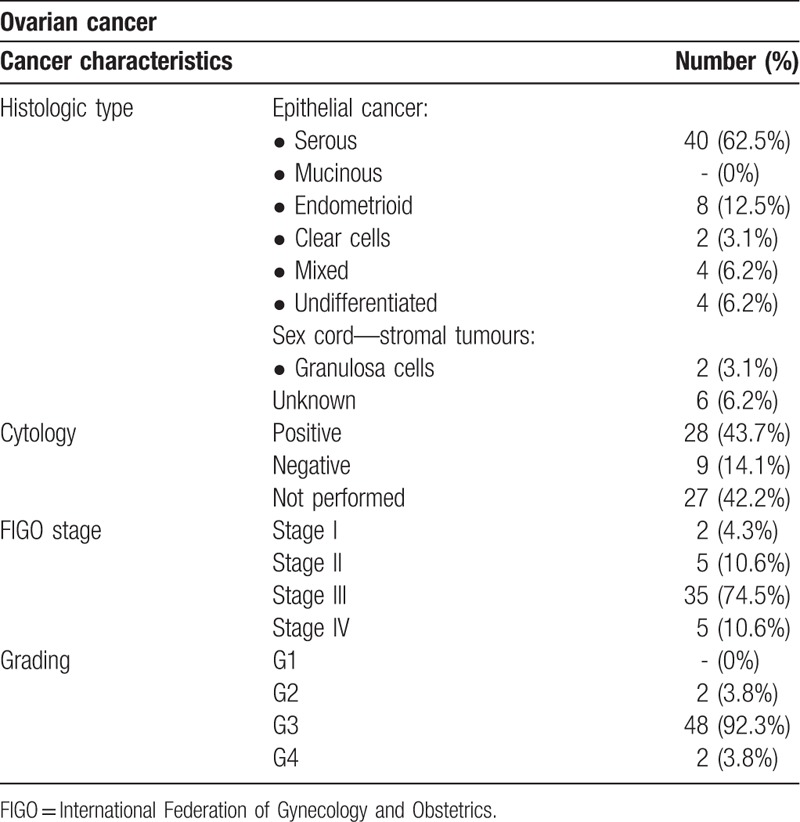
Table 3.
Endometrial cancer characteristics.
3.3. Chemotherapy treatment and toxicities
Twenty-one patients received neoadjuvant chemotherapy (25%), 36 received adjuvant/first-line chemotherapy (42.8%) and 17 a second line (20.2%); a smaller part of patients (11.9%) received third and fourth lines. The mean number of cycles was 6. Sixty-two patients (73.8%) underwent combination chemotherapy and, of these, 46 women (74.2%) received an association of paclitaxel and carboplatin; other combination chemotherapies were carboplatin and pegylated liposomial doxorubicin, gemcitabine and vinorelbin, paclitaxel—ifosfamide—cisplatin (TIP) and paclitaxel—epirubicin—cisplatin (TEP). Single-agent therapy was administered to 17 women (20.2%); chemotherapy agents administered were carboplatin or pegylated liposomial doxorubicin. As shown in Table 4, combination chemotherapy was prevalent in non-vulnerable women rather than in non-vulnerable ones (62.9% versus 37.1%, P = .07, respectively); vulnerable women mostly received a single-agent therapy (59.1% in vulnerable versus 40.9% in non-vulnerable, P = .07). Analysis of patients response to treatment revealed a statistically significant difference (P = .001) between the 2 cohorts of patients: complete response was mostly experienced in non-vulnerable subjects (83.3% versus 16.7%, respectively, P = .001), whereas progression of the disease was prevalent in the vulnerable ones (64.3% in vulnerable versus 35.7%, in non-vulnerable, P = .001). Recurrence of disease occurred in both groups without significant differences.
Table 4.
Chemotherapy treatment, toxicity, and compliance by VES-13.
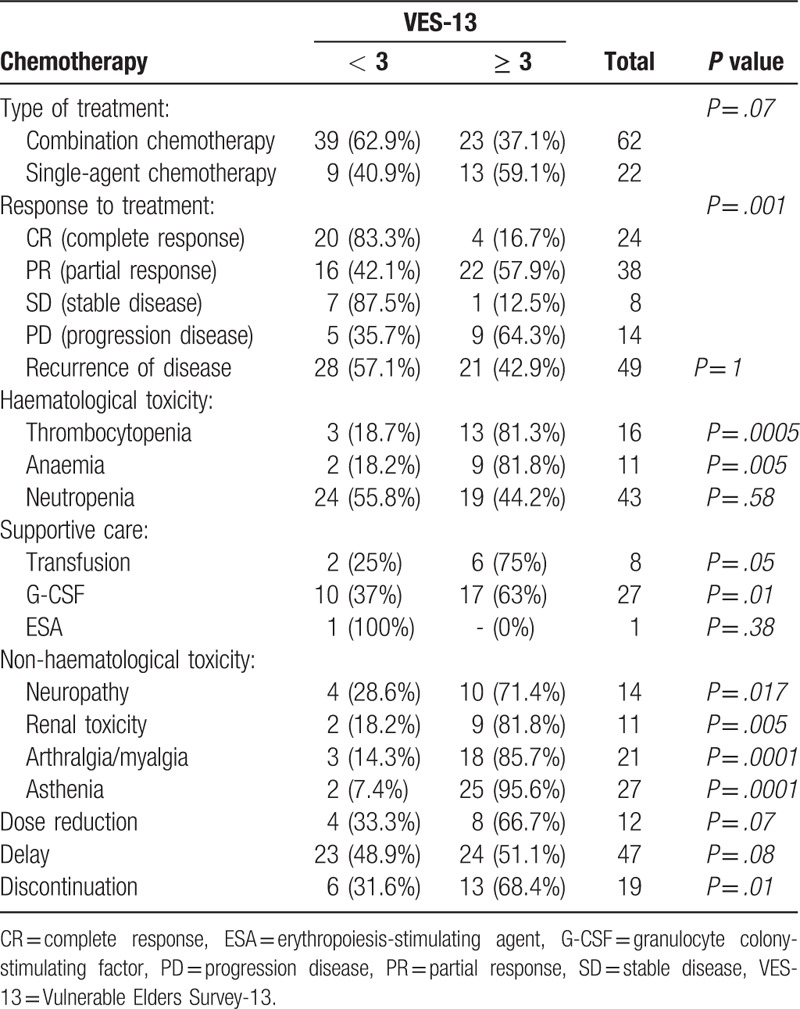
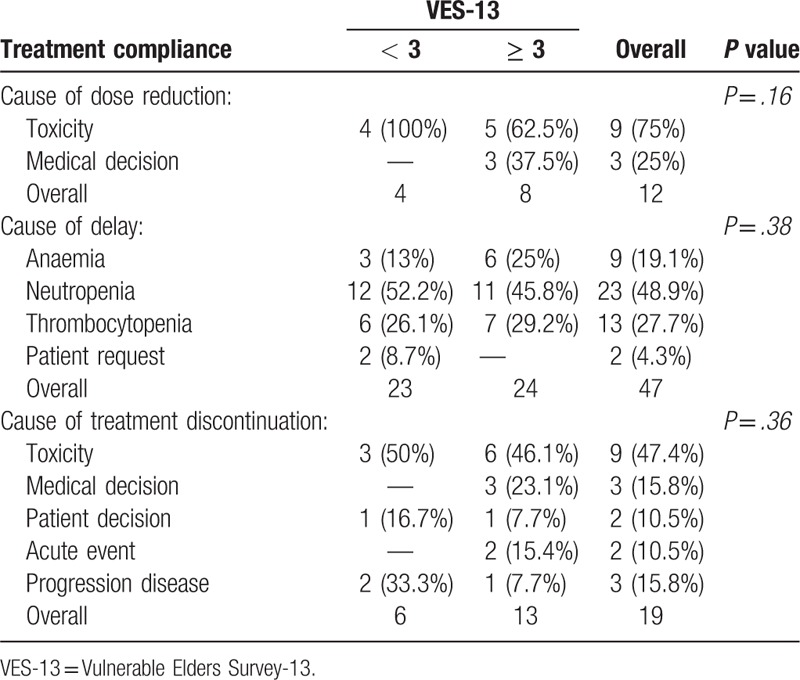
Chemotherapy-related toxicities are reported in Table 4. Within haematological toxicities, thrombocytopenia and anaemia were more frequent in vulnerable subjects rather than in non-vulnerable 1 (81.3% versus 18.7%, P = .0005, and 81.8% versus 18.2%, P = .005, respectively). Incidence of neutropenia, the most frequent haematological toxicity (51.2% of all patients), was comparable between the 2 groups. Non-haematological toxicities (neuropathy, renal toxicity, arthralgia or myalgia and asthenia) were more prevalent in vulnerable patients. Supportive care was necessary in some patients and in particular granulocyte colony-stimulating factor (G-CSF) (32.1%), erythropoiesis-stimulating agent (ESA) (1.2%), and blood transfusions (9.5%). G-CSF administrations and blood transfusions were more prevalent in vulnerable subjects rather than in non-vulnerable ones (63% versus 37%, P = .10, and 75% versus 25%, P = .05, respectively); ESA was used in only 1 non-vulnerable patient. A total of 12 patients (14.3%) required a dose reduction during therapy, 47 (55.9%) had 1 or more dose delays and 19 (22.6%) discontinued treatment. Dose reductions were prevalent in vulnerable subjects even if the difference between the 2 groups was not statistically significant (66.7% in vulnerable versus 33.3% in non-vulnerable, P = .07), whereas delays were similar between vulnerable and non-vulnerable subjects (51.1% in vulnerable versus 48.9% in non vulnerable, P = .08). Treatment discontinuations mostly occurred in vulnerable patients (68.4% in vulnerable versus 31.6% in non-vulnerable, P = .01). Leading indications for dose reductions, delays and treatment discontinuations, according to VES-13, are reported in Table 5.
Table 5.
Treatment compliance.
4. Discussion
The aim of this study was to assess the role of VES-13 in identifying elderly women with gynaecological cancers at risk of toxicity related to chemotherapy.
In this sample, VES-13 identified 32% of individuals as vulnerable. The vulnerable group had 4.2-fold greater risk of functional decline and death over the next 2 years in comparison to elders with a lower score (VES-13 < 3).[16]
Although VES-13 was not originally designed to answer specific oncological questions, it has been studied as a screening tool in oncology in several studies and 2 of them concluded that VES-13 could be a useful preliminary screening tool with a sensitivity of 73% and 87% respectively.[17–21] The 42.9% of the patients in our series were classified as being vulnerable, a similar value to that obtained in other studies, even if concerning other types of cancers (from 32% to 53%).[16,20,22–24] In literature is still unclear a new possible role of VES-13 in the management of older patients with cancer: it could be high capable of identifying elderly patients at risk of toxicity related to treatment. This new possible role was described in few previous studies, but none of these analysed exclusively elderly women with gynaecological cancer.[22,25]
Vulnerability condition influenced the chemotherapy treatment, both in terms of toxicity and compliance. The most appropriate choice of chemotherapy is a combination of agents and it shouldn’t be excluded only due to chronological age. However, for vulnerable patients, several strategies have been evaluated to improve feasibility and tolerability while maintaining dose intensity, such as single-agent therapy or weekly schedules. In our study response to treatment showed differences between the 2 groups. The higher prevalence of progression disease in vulnerable patients could be explained by the fact that these women often fail to complete all chemotherapy cycles or they complete them with several delays and dose reductions with a suboptimal result.[26,27]
Aging is associated with the decrease of bone marrow reserve and an increased risk of myelosuppressive-associated complications from chemotherapy.[28,29] In our cohort of patients, thrombocytopenia and anaemia were more prevalent in vulnerable women, in line with the results of the study of Luciani et al.[22] This difference didn’t occur for neutropenia, but the use of supportive care (G-CSF) has been prevalent in vulnerable subjects as a preventive treatment. G-CSFs s are effective in lowering neutropenia incidence and are also associated with improved survival in elderly patients.[30] The study of Poonawalla et al demonstrated that ESAs are effective in reducing the need of a blood transfusion, however other studies showed that administration of ESA is associated with an increased risk of venous thromboembolic events and mortality.[30,31] In our study, only 1 patient used this growth factor. According to Luciani's study, non-haematological toxicities were prevalent in patients identified as vulnerable by VES-13.[22]
The elderly population is more likely to experience reduction of delivered dose intensity and delays in chemotherapy administration. The delay in chemotherapy administration is associated with a worst overall survival in elderly oncologic patients and haematological toxicity is a common reason for the delay, as shown in our study. Furthermore, in our study, discontinuations were prevalent in vulnerable women, mostly due to an excessive toxicity. Thus, appropriate starting dose reductions and preventive measures should be taken in elderly patients, mostly the vulnerable ones, in order to complete the treatment without delay.[26]
In clinical practice, a widely-used screening tool is G8, a questionnaire composed of 7 questions and the age of patient. In a comparison between G8 and VES13, the first demonstrated a higher sensitivity (76.5% versus 68.7%), but at the expense of a lower specificity (64.4% versus 74.3%).[32] However other 2 studies concluded that VES-13 has a sensitivity of 73% and 87% respectively.[20,21] These variations may result from differences in administration. In other studies, VES-13 was administered predominantly by the nursing staff, while in the Luciani et al study it was administered by a physician. Similarly, in our study VES-13 was administered by a physician.
Literature shows that VES-13 is 1 of the most investigated instruments in geriatric oncology and it is widely used for screening purposes in order to identify patients who need to undergo a full geriatric evaluation. National Comprehensive Cancer Network (NCCN) guidelines encourage the use of a full CGA when it is required after the pre-assessment.[14] However, CGA is time-consuming and it is often not suitable for clinical practice; a simply and rapid tool such as VES-13 can help oncologists optimize assessment times. In our study 48 patients (57.1%) resulted non-vulnerable, which means that a full CGA could not be required by more half of the patients. Moreover, it could be considered a good candidate for further evaluation as a predictor of risk of chemotherapy toxicity.
Even if there are some limitations, such as the limited number of patients, the not randomized design and the inclusion of different type of gynaecological cancers with different rates of recurrence and survival, this study is the first to evaluate VES-13 exclusively in elderly women with gynaecological cancers. VES-13 could be considered an effective tool to stratify elderly patients with gynaecological cancer according to vulnerability in order to estimate and to prevent complications induced by chemotherapy. Further evaluations and randomized studies are recommended with the aim of validating VES-13 as a tool to choose the most appropriate treatment and to guide gynaecologic oncologists decision-making.
Author contributions
Conceptualization: Annamaria Ferrero, Elisa Tripodi, Luca Fuso, Guido Menato.
Data curation: Annamaria Ferrero, Michela Villa, Elisa Tripodi, Luca Fuso.
Formal analysis: Annamaria Ferrero, Michela Villa, Luca Fuso.
Methodology: Annamaria Ferrero, Michela Villa, Elisa Tripodi, Luca Fuso.
Project administration: Annamaria Ferrero, Guido Menato.
Software: Luca Fuso.
Supervision: Annamaria Ferrero, Elisa Tripodi, Luca Fuso, Guido Menato.
Validation: Annamaria Ferrero, Guido Menato.
Writing – original draft: Michela Villa, Elisa Tripodi.
Writing – review & editing: Annamaria Ferrero, Michela Villa, Elisa Tripodi, Guido Menato.
Footnotes
Abbreviations: CGA = comprehensive geriatric assessment, CSF = colony stimulating factors, ESA = erythropoiesis-stimulating agent, FIGO = International Federation of Gynecology and Obstetrics, G-CSF = granulocyte colony-stimulating factor, VES-13 = Vulnerable Elders Survey-13.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2008 incidence and mortality web-based report. Atlanta: U.S: Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2012. Available at https://nccd.cdc.gov/uscs/ Accessed 22, March 2016. [Google Scholar]
- [3].Fourcadier E, Trétarre B, Gras-Aygon C, et al. Under-treatment of elderly patients with ovarian cancer: a population based study. BMC Cancer 2015;15:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright JD, Lewin SN, Barrena Medel NI, et al. Endometrial cancer in the oldest old: tumor characteristics, patterns of care, and outcome. Gynecol Oncol 2011;122:69–74. [DOI] [PubMed] [Google Scholar]
- [5].Sharma C, Deutsch I, Horowitz DP, et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer 2012;118:3618–26. [DOI] [PubMed] [Google Scholar]
- [6].Hilpert F, Wimberger P, du Bois A, et al. Treatment of elderly ovarian cancer patients in the context of controlled clinical trials: a joint analysis of the AGO Germany experience. Onkologie 2012;35:76–81. [DOI] [PubMed] [Google Scholar]
- [7].Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US food and drug administration. J Clin Oncol 2004;22:4626–31. [DOI] [PubMed] [Google Scholar]
- [8].Gilles Freyer. Ovarian cancer in elderly patients. Springer International Publishing Switzerland 2016;23–61. [Google Scholar]
- [9].Clegg A, Young J. The frailty syndrome. Clin Med (Lond) 2011;11:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Balducci L, Colloca G, Cesari M, et al. Assessment and treatment of elderly patients with cancer. Surg Oncol 2010;19:117–23. [DOI] [PubMed] [Google Scholar]
- [12].Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer 2012;118:3377–86. [DOI] [PubMed] [Google Scholar]
- [13].Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hurria A. Older Adult Oncology, version 2.2017. National Comprehensive Cancer Network (NCCN) Guidelines in Oncology. Available at https://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed 18, November 2017. [Google Scholar]
- [15].Saliba D, Orlando M, Wenger NS, et al. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci 2000;55:M750–6. [DOI] [PubMed] [Google Scholar]
- [16].Saliba S, Elliott M, Rubenstein LA, et al. The vulnerable elders survey (VES-13): a tool for identifying vulnerable elders in the community. J Am Geriatr Soc 2001;49:1691–9. [DOI] [PubMed] [Google Scholar]
- [17].Molina-Garrido MJ, Guillen-Ponce C. Comparison of two frailty screening tools in older women with early breast cancer. Crit Rev Oncol Hematol 2011;79:51–64. [DOI] [PubMed] [Google Scholar]
- [18].Pottel L, Boterberg T, Pottel H, et al. Determination of an adequate screening tool for identification of vulnerable elderly head and neck cancer patients treated with radio(chemo)therapy. J Ger Oncol 2012;3:24–32. [Google Scholar]
- [19].Falci C, Brunello A, Monfardini S. Detecting functional impairment in older patients with cancer: is vulnerable elders survey-13 the right prescreening tool? An open question. J Clin Oncol 2010;28:e665–6. [DOI] [PubMed] [Google Scholar]
- [20].Luciani A, Ascione G, Bertuzzi C, et al. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol 2010;28:2046–50. [DOI] [PubMed] [Google Scholar]
- [21].Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer 2007;109:802–10. [DOI] [PubMed] [Google Scholar]
- [22].Luciani A, Biganzoli L, Colloca G, et al. Estimating the risk of chemotherapy toxicity in older patients with cancer: the role of the vulnerable elders survey-13 (VES-13). J Geriatr Oncol 2015;6:272–9. [DOI] [PubMed] [Google Scholar]
- [23].Kenig J, Richter P, Zychiewicz B, et al. Vulnerable Elderly Survey 13 as a screening method for frailty in Polish elderly surgical patient—prospective study. Pol Przegl Chir 2014;86:126–31. [DOI] [PubMed] [Google Scholar]
- [24].Carneiro F, Sousa N, Azevedo LF, et al. Vulnerability in elderly patients with gastrointestinal cancer—translation, cultural adaptation and validation of the European Portuguese version of the vulnerable elders survey (VES-13). BMC Cancer 2015;15:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stokoe JMPJ, Ring Sinha R A. G8 and VES-13 scores predict chemotherapy toxicity in older patients with cancer. J Geriatr Oncol 2012;3:S81. [Google Scholar]
- [26].Joseph N, Clark RM, Dizon DS, et al. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol Oncol 2015;137:401–5. [DOI] [PubMed] [Google Scholar]
- [27].Von Gruenigen VE, Huang HQ, Beumer JH, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer— an NRG oncology/gynecologic oncology group study. Gynecol Oncol 2017;144:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balducci L, Repetto L. Increased risk of myelotoxicity in elderly patients with non-Hodgkin lymphoma. Cancer 2004;100:6–11. [DOI] [PubMed] [Google Scholar]
- [29].Begg CB, Carbone PP. Clinical trials and drug toxicity in the elderly. The experience of the eastern cooperative oncology group. Cancer 1983;52:1986–92. [DOI] [PubMed] [Google Scholar]
- [30].Poonawalla IB, Piller LB, Lairson DR, et al. Impact of hematopoietic growth factors on blood transfusion needs, incidence of neutropenia, and overall survival among elderly advanced ovarian cancer patients treated with chemotherapy. Int J Gynecol Cancer 2016;26:95–103. [DOI] [PubMed] [Google Scholar]
- [31].Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–14. [DOI] [PubMed] [Google Scholar]
- [32].Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE prospective multicenter cohort study. PLoS One 2014;9:e115060. [DOI] [PMC free article] [PubMed] [Google Scholar]


