Abstract
Enhanced recovery after surgery (ERAS) is acknowledged to reduce perioperative stress in several surgical diseases. Here, we investigated whether modified ERAS is associated with beneficial effects in the setting of emergency colorectal surgery.
We retrospectively evaluated the medical records of 839 consecutive patients with obstructive colorectal cancer undergoing surgical intervention at 4 institutes. Among them, 356 cases were managed with a multidisciplinary team approach to care (modified ERAS protocols), and the remaining 483 cases were treated based on traditional protocols. According to modified ERAS or traditional care, propensity score (PS) matching was performed to adjust biases in patient selection. The primary outcome was gastrointestinal function recovery. Secondary outcomes included postoperative complications and length of hospital stay.
Modified ERAS was associated with postoperative gastrointestinal function recovery, including time to first flatus (P = .002), first defecation (P = .008), and prolonged ileus (P = .016). According to the Clavien–Dindo classification, fewer total episodes of grade II or higher postoperative complications were observed in patients cared for with modified ERAS than in patients with traditional care (P = .002). Median (interquartile range) postoperative hospital stay in the modified ERAS group was 6 (3–22) days versus 9 (7–27) days in the traditional care group (P < .001). Furthermore, the interval from operation to postoperative chemotherapy (d) was significantly shorter in the modified ERAS group (35.6 ± 11.5 vs 47.6 ± 23.8, P < .001).
The modified ERAS was safe and associated with clinical benefits, including fast recovery of bowel function, reduced postoperative complications, and shorter hospital stay for patients with obstructive colorectal cancer.
Keywords: length of stay, modified enhanced recovery after surgery, obstructive colorectal cancer, postoperative complications
1. Introduction
In the 1990s, enhanced recovery after surgery (ERAS) or fast track surgery strategy was initiated in European countries and the United States to reduce surgical stress and improve outcomes after surgery.[1,2] Various perioperative care approaches were introduced to reduce perioperative stress responses and accelerate postoperative function recovery. Core aspects included no perioperative fasting, optimal nutrition and fluid management, decreased use of tubes, optimizing pain control, and early mobilization.[3,4] Originally, ERAS was launched for elective colorectal surgery and has rapidly gained popularity because of its significant benefits for quick recovery and safety.[5] Recent meta-analyses of evidence-based studies have indicated that a reduction in the length of hospital stay and postoperative complications was achieved following ERAS implementation in the context of elective colorectal surgery, without an increase in readmission rate.[6,7] Therefore, the guidelines for perioperative ERAS care in elective colon surgery[8] have been drafted and continuously updated as new information becomes available.[9]
In clinical practice, acute colonic obstruction is present in over 20% of colorectal cancer,[10] which cannot be preoperatively prepared in the same way and should be managed by emergency surgery, including colonic stenting, proximal diversion, and tumor resection strategies. Patients with obstructive colorectal cancer often suffer high rates of postoperative complications and prolonged hospital stays, which differ from patients who undergo elective surgery, even in the modern surgical era.[11,12] ERAS protocols require no fasting before surgery, but patients with obstructive colorectal cancer cannot eat orally preoperatively, which is in direct contradiction with the requirement of ERAS. In addition to this, the other perioperative ERAS elements, such as no postoperative fasting and avoidance of fluid overload, could be applicable for emergency surgery for acute colonic obstruction.[13] Optimizing the perioperative care for such operations, termed modified ERAS protocols, might potentially improve the surgical outcomes. Recently, modified ERAS protocols were reported safe for obstructive colorectal cancer surgery by groups from Thailand,[14] Switzerland,[15] and Australia[16]; however, their efficacy remains inconclusive, given the pending challenges for modified ERAS protocols in the care of such patients.
ERAS protocols were introduced and launched in China in 2009, and the ERAS strategy was gradually modified and extended to a variety of patient cohorts, including obstructive colorectal cancer, in the involved institutes. This study aimed to compare outcome measures in populations of patients with obstructive colorectal cancer that received modified ERAS or usual care and evaluate how care changed as a result of program implementation.
2. Materials and methods
2.1. Methods
2.1.1. Patient recruitment and characteristics
In the period from 2010 to 2017, ERAS program was implemented and become the standard of care for patients undergoing elective colorectal resection in China, including the institutes involved here: the Department of Surgery, Qingdao Hospital, Qingdao University School of Medicine and the Department of Pediatric General Surgery and Liver Transplantation, Children's Hospital, Chongqing Medical University.
Since that time, ERAS protocols have been modified, and pilot studies have been carried out based on colonic surgery in these hospitals in some patients with obstructive colorectal cancer undergoing the unplanned emergency operation. Choice of ERAS protocols depended on the patient's and surgeon's preferences because some surgeons felt that it might be necessary and safe. All patients consented to the ERAS protocols. In this study, the emergency surgery was defined as any unplanned colorectal resection performed within 24 hours after admission for acute colonic obstruction (the duration was not longer than 1 week).[14,15] The colonic obstructions were confirmed with complete or nearly complete plain abdominal radiograph or computed tomography (CT) scan. The modified ERAS program training was used to ensure that all modified ERAS procedures could be correctly performed during surgery at the institutes. The modified ERAS program implementation was supervised by a multidisciplinary expert team, including surgeons, anesthesiologists and nursing staff from the institutes involved. The patients undergoing emergency colorectal resection were counseled and accepted the modified ERAS program. Written informed consent was obtained from the patients, and the study was approved by the Ethics Committee of Qingdao Hospital, Qingdao University School of Medicine.
Although the patients were managed with the modified ERAS program or conventional perioperative care, the technical aspects of surgery, such as the choice of staplers and other instruments, and the choice of antibiotics, were left to the discretion of each experienced surgical consultant (who experienced >100 cases of elective colorectal surgery) and were not different during this period among the institutes (a total of 500–600 colorectal operations per annum each). We retrospectively selected the patients with obstructive colorectal cancer, which were treated with ERAS care or with traditional, routine postoperative care during the same period (Non-ERAS group), and then, we compared the results between the 2 treatment strategies among the institutes involved. All patients received a one-stage operation. Exclusion criteria were patients with bowel perforation, colonic stents utilization, gastrointestinal hemorrhage, recurrent tumor, neoadjuvant treatments, and mental disease.
2.1.2. Enhanced recovery after surgery protocol
We modified the ERAS program with a focus on several core elements to tailor them for patients with obstructive colorectal cancer. The major differences between the modified ERAS program and conventional care management are summarized in Table 1. Preoperatively, intensive counseling was implemented by surgeons, nurses, and anesthesiologists for psychological comfort and education. All patients received short-term antibiotic prophylaxis 30 minutes before surgery and repeatedly if surgery lasted longer than 3 hours, in agreement with each hospital's guidelines. Moreover, thromboembolism prophylaxis with low molecular weight heparins and anti-emetic prophylaxis was given preoperatively. In patients undergoing left colonic and rectal resection, the evacuating enema was administered the night before and the morning of surgery; otherwise, no bowel preparation was needed. Regular ERAS protocols require a preoperative high-carbohydrate beverage within 2 to 4 hours and/or solids within 6 to 8 hours before surgery to reduce perioperative surgical fasting. For the modified ERAS, it is impossible to orally receive beverage or solids before surgery for every patient with obstructive colorectal cancer.
Table 1.
Changes in perioperative care for patients with obstructive colorectal cancer.
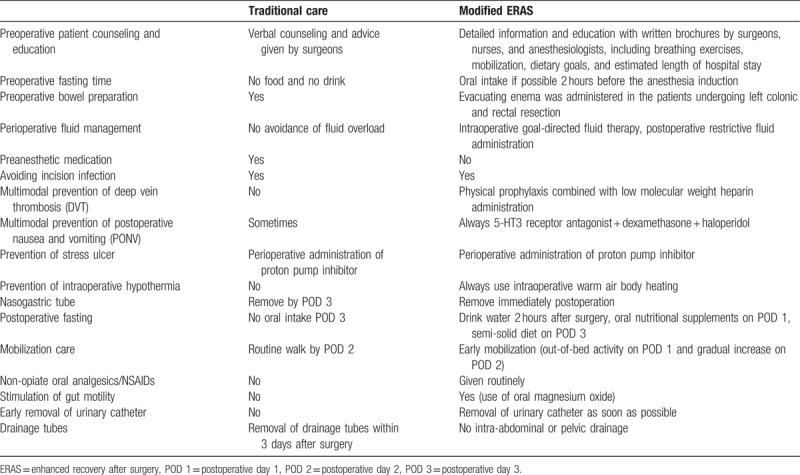
The perioperative fluid management was intraoperatively restricted to avoid any fluid overload. Postoperative pain management involved multimodal opioid-sparing interventions, including perioperative intravenous acetaminophen or bupivacaine, lidocaine, and nonsteroidal anti-inflammatory medication (paracetamol and ibuprofen), which were already routine practices and part of traditional perioperative care. Naso-gastric tubes were usually removed upon awakening to 12 hours after surgery. Oral intake was resumed as soon as possible after extubation and increased gradually from liquids to a solid diet (after approximately 2 days of a liquid diet). When the patients returned to the normal ward, they were encouraged to begin ambulation within 12 hours of surgery and maintain it at least 4 times a day with a daily goal of walking at least 21 ft in the first 3 postsurgical days. Urinary catheters were removed as early as on day 1 (except in patients who underwent low anterior rectal resection, in whom it was removed on the postoperative day 2). In both pathways, patients were discharged from the hospital when they had tolerance of food, good appetite, adequate pain control with oral analgesic, and satisfactory recovery with daily living.
2.1.3. Data collection and clinical assessment
Electronic medical records were thoroughly reviewed for the preoperative baseline variables, intraoperative data including surgical and anesthetic records, and postoperative outcomes. Specifically, the following demographic and perioperative data were recorded in all patients: age, sex, smoking history, body mass index (BMI), comorbidities, CR-POSSUM score, tumor location, stage of colorectal cancer (based on AJCC TNM classification), biochemical profiles (hemoglobin, blood glucose, creatinine, serum electrolytes, albumin, retinol binding protein [RBP], C-reactive protein [CRP] and total lymphocyte count, coagulation profiles, and serum tumor marker level), length of postoperative hospital stay, complications, and re-operation readmission rates. Operative details (tumor location, procedure type, American Society of Anesthesiologists [ASA] grade, type of anesthesia, duration of surgery, operating time, intraoperative blood loss, and transfusion rate) and pathological staging were also recorded. All patients were postoperatively followed for 30 days.
The primary endpoint of the current study was prompt gastrointestinal function recovery. The secondary endpoints included postoperative complications and hospital length of stay (the days from operation to discharge). Gastrointestinal symptoms were collected from the clinical records, including first postoperative flatus or defecation, time to normal diet, abdominal bloating and/or cramps, and nausea or vomiting in the first 5 days following surgery. In the first 5 days, >1 episode of nausea or vomiting was defined as early ileus. Late ileus was defined as nausea or vomiting after the first 5 days.
The complications here only included grade II complications or higher, defined by the Clavien–Dindo classification system, such as septic shock, gastrointestinal bleeding, abdominal abscess, venous thromboembolic disease, late ileus, myocardial infarction, renal failure, and respiratory failure. Major complications were defined as the following situations: the need for repeat laparotomy and interventional radiology procedures or requiring the intensive care unit entry.
2.1.4. Propensity score (PS) matching and statistical analysis
Continuous data were presented as medians and interquartile ranges (IQR) or means ± SDs as appropriate and analyzed with Student t test or the Mann–Whitney U test, respectively. Categorical variables were reported as frequencies (percentages) and were analyzed by a chi-square test or Fisher exact test. To minimize the selection bias in baseline characteristics inherent to an observational study, we compared postoperative endpoints between the 2 groups using the PS matching method, designed to mimic the randomized clinical trial in the context of an observational study. Propensity scores were estimated using demographic and clinical variables (cohort entry date) using a multivariable logistic regression model. The selected variables entered into the propensity model included demographic data information, laboratory values, treatment protocols, lesion location, type of surgery, and ICU admissions. The generalized additive model was used to check linear assumption in PS model. The 2 patient groups were then matched based on the estimated propensity score of each patient with no replacement and a 0.1 caliper width using the PS full-matching method. The balance of baseline covariates after matching was assessed using the standardized difference of the mean, and the overlap degree of PS distribution. The PS matching analyses were performed using SPSS 20.0 (IBM, Armonk, NY) or R software 3.1.2 (The R Foundation for Statistical Computing) and the MatchIt package. The matched modified ERAS patients and controls were further compared using SPSS 20.0. Statistical significance was indicated by P values <.05.
3. Results
3.1. Patient characteristics
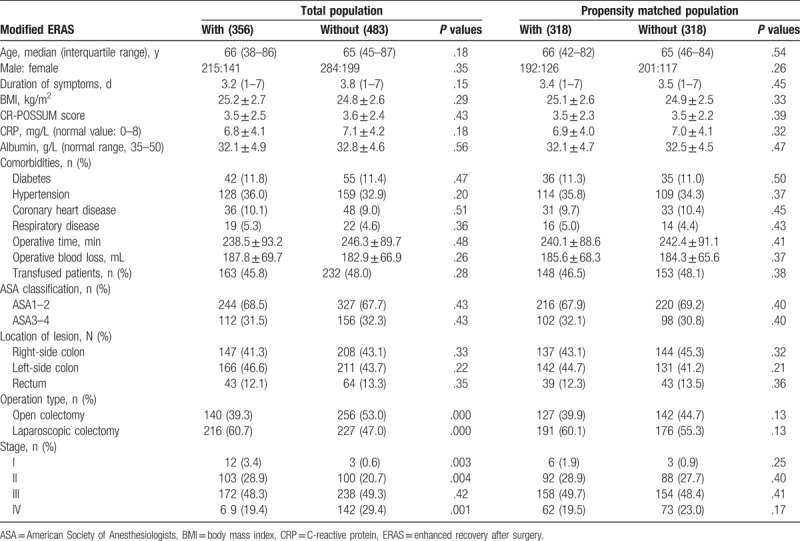
During the study period, a total of 839 consecutive patients with obstructive colorectal cancer were enrolled for analysis. Among them, 356 patients received the modified ERAS program, and 483 patients were cared for with the traditional pathway. The baseline patient characteristics and operative details of modified ERAS patients and traditional care patients are summarized in Table 2. Before PS-matching, there were no significant differences in terms of age, sex, body mass index (BMI), CRP value, American Society of Anesthesiologists (ASA) grade, CR-POSSUM score, duration of symptoms before admission, tumor location, comorbidity characteristics, or operative magnitude between the 2 groups, with the exception of tumor stage and operation type. The operative magnitude was evaluated by measurement of operative time, estimated blood loss, and total units of blood transfused within the 24-hour perioperative period. Under PS-matching, 318 patients cared for with the modified ERAS were matched to 318 patients cared for with the traditional methods. Several variables, including tumor stage and operation type, became comparable after PS-matching (Table 2). For the patients with modified ERAS, an oral liquid within 2 hours after surgery was achieved in 90% of patients, and a soft or solid diet within 24 hours after surgery was achieved in 80%. Ninety-six percent of patients begin out-of-bed activity on postoperative day 1. The overall modified ERAS compliance was approximately 90%. The patients with the traditional care pathway usually begin their oral diet on postoperative day 3 and begin out-of-bed activity at their wish.
Table 2.
Baseline characteristics of eligible patients and surgical parameters.
3.2. Efficacy for gastrointestinal function recovery
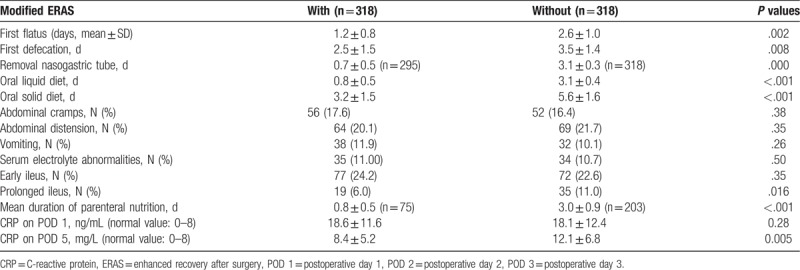
Outcomes with regard to postoperative gastrointestinal function were assessed by first flatus and first defecation. In the propensity-matched cohort, a trend for accelerated recovery of gastrointestinal function (first flatus [P = .002] and first defecation [P = .008]) was observed for patients treated with modified ERAS, as shown in Table 3. The naso-gastric tube was removed significantly earlier in the modified ERAS group than in the conventional group (0.7 ± 0.5 days vs 3.1 ± 0.3 days; P < .001). In our study, the modified ERAS group patients experienced earlier return to the oral liquid diet (P < .001) and oral solid diet (P < .001) than the traditional care group. Gastrointestinal complications were generally mild and recoverable. The incidences of abdominal cramps (P = .38), vomiting (P = .26), and abdominal distention (P = .35) within 5 postoperative days (PODs) in patients cared for with modified ERAS were comparable to those of patients receiving traditional care. In case of discomfort or development of any other complications, the naso-gastric tube was reinserted; the naso-gastric tube reinsert rates and the need for chest x-ray were similar in the 2 groups. The mean durations of parenteral nutrition were 0.8 ± 0.5 and 3.0 ± 0.9 days for the 2 groups, respectively (P < .001). In patients cared for with modified ERAS, CRP was markedly higher on POD 1, and a significant return to near-baseline (P = .005) was observed compared with patients cared with traditional methods. There were significant differences in the incidence of prolonged ileus (P = .016) but no differences for early ileus (P = .35) or serum electrolyte abnormalities (P = .50) between the 2 groups.
Table 3.
Postoperative characteristics of gastrointestinal function recovery in the matched population (Student t test and chi-square test).
3.3. Postoperative complications
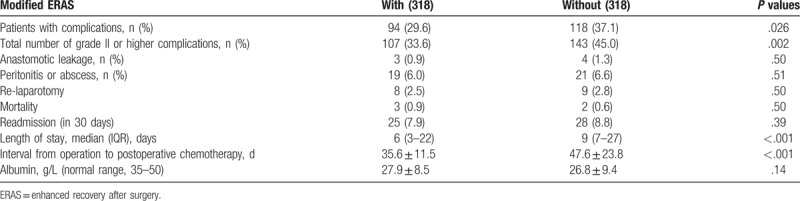
Postoperative complications according to established criteria are summarized in Table 4. Among patients undergoing emergency colorectal resection, incidences of overall postoperative complications (pneumonia, wound, abdominal, and systemic infection) tended to be reduced for the modified ERAS implementation (P = .002). There were no significant differences in the rates of surgical complications such as sepsis, anastomotic leakage, wound infection, and abdominal abscess (data not shown). There was no significant difference in relaparotomy (P = .50) and readmission (P = .39) rates between the 2 groups. The median hospital stay was 6 (3–22) days in the modified ERAS group, significantly shorter than the 9 (7–27) days of the traditional care group (P < .001). No significant difference was found in nutritional variables between the 2 groups on POD 5 (albumin, P = .14).
Table 4.
Postoperative outcome in the matched population (Student t test and chi-square test).
4. Discussion
ERAS had been proved by previous meta-analysis to have benefits in terms of postoperative complications, glucose tolerance, and hospital stay.[17] The current study further verified whether modified ERAS could promote postoperative intestinal function (bowel movements), and so improve outcomes in patients with acute colonic obstruction undergoing emergency surgery. In the present study, to control the confounders of heterogeneity in this multivariate population, we performed propensity score matching and demonstrated the feasibility and effectiveness of modified ERAS protocols. Modified ERAS protocols were associated with prompt postoperative intestinal function recovery, which might result from modulating the systemic immune response and relieving the postoperative intestinal edema formation. All this brings about a shorter length of hospital stay and shorter time before starting adjuvant therapy in patients undergoing emergency colorectal surgery.
The efficiency of smooth recovery is increasingly focused, especially for gastroenterological surgery, because postoperative ileus is the most important reason for the postoperative length of stay.[18] We performed this measurement in the close intestinal function monitoring setting. The clinical intestinal complaints correlated with intestinal function were adequately monitored as far as possible. This study is indicative of the remarkable beneficial effects of modified ERAS postoperative gastrointestinal recovery in patients with modified ERAS, as they had a shorter period to pass the first defecation and resume oral solid diet. These results might be due to the combination of the core ERAS elements, such as early enteral nutrition (EN), judicious fluid therapy, enforced postoperative mobilization, and preferential use of non-opioid analgesia in the modified ERAS pathway.[19] When implementing this program, a multidisciplinary team, including surgeons, anesthesiologists, nurses, physiotherapist, and nutritionists, is indispensable. Unless contraindicated, early enteral nutrition has been encouraged to be initiated as early as possible, with several benefits, including modulating the inflammatory response and metabolism and preserving gut integrity, thereby dampening postoperative ileus (POI). Enteral feeding should stimulate bowel movements, which also contribute to the beneficial effect of early enteral nutrition [20,21]. A common worry for early EN is complications such as diarrhea, abdominal distention, and abdominal cramps, which worsen with increasing caloric intake[22] and which could lead to discontinuance of enteral feeding. In this study, on the first 3 days after surgery, 29 cases in the modified ERAS pathway had slight symptoms. To avoid this condition, the amount of EN should increase slowly and cautiously, or with the administration of medications. In the current study, the slight symptoms resulting from early EN were successfully alleviated, with enteral feeding discontinued for as few as 1 day, and no patients dropped out of the modified ERAS pathway. Recently, enteral nutrition has been proven to preserve the gut flora architecture and prevent gastrointestinal mucosa atrophy.[23] Intraoperative fluid administration with the maintenance of normovolemia is another integral and critical component of modified ERAS protocols, which are associated with certain consequences and complications, postponing the postoperative recovery.[24] Recently, restrictive intraoperative fluid administration has been proposed to favor beneficial postoperative outcomes following major surgery.[25] In this study, the postoperative ileus was significantly reduced with ERAS implementation. Excess crystalloids were proposed to be implicated in coagulation disturbance, which may be associated with the thrombosis event,[21] although this was not found in this study. In addition, tissue oxygenation might decline with fluid supersaturation, which is unfavorable for postoperative recovery.
Moreover, we also showed that modified ERAS in emergent colorectal surgery was associated with a tendency towards a lower incidence of total postoperative complications. Likewise, several studies have recently reported the feasibility of ERAS protocols in emergent surgery for perforated peptic ulcer disease,[26] with beneficial outcomes including length of hospital stay and morbidity. In the present study, modified ERAS protocols led to a median reduction length of hospital stay of 3 days, which is comparable to those reported in previously published meta-analysis for ERAS protocols used in elective colorectal surgery[17] and the setting of emergent colorectal surgery,[13] although there were several small differences from ours. The timely postoperative chemotherapy is very important and a significant determinant of the patient's overall postoperative recovery and performance,[27] which is associated with the overall survival and disease-free survival.[28] Here, we further indicated that modified ERAS protocols might also reduce the convalescence time between surgery and postoperative chemotherapy.
In this clinical setting, although the exact mechanism is difficult to determine, the combination of multimodal perioperative interventions, rather than a single maneuver alone, might contribute to the reduction in hospital stay and postoperative complication in the modified ERAS program for patients undergoing emergency resection for obstructing colorectal cancer. Early reduction of local inflammation by a core modified ERAS element, such as EEN and limited fluid administration, may explain the results reported herein.[29] Recent data also suggest that the recovery of organ function may be explained by an effect of modified ERAS protocols on local inflammation and edema recovery.[30] In this study, CRP was higher immediately after the operation and recovered significantly faster, within 5 PODs after the modified ERAS, which may explain the results found here. In the experimental setting, early enteral feeding reduces the inflammatory response and improves the intestinal blood flow, which could be linked to improvement in intestinal function. Another report also suggested that a better-preserved postoperative immune system may improve postoperative results.[31]
The retrospective design was the main weakness of the current research, and the decision to initiate the modified ERAS protocol was not made randomly. In the pilot stage, we are inclined to not perform the modified ERAS protocol in sicker patients prone to intestinal edema, which might be associated with severe postoperative outcome, and this should be the fact that a performance bias may occur. Retrospectively, the prompt intestinal symptoms were extracted from patient records, which might not be fully accurate, although we have measured this in the intensively monitored institutes. To limit the influence of performance bias, we performed propensity score matching analysis to generate comparison groups of patients. Following the PS matching, this discrepancy was eliminated and comparable to achievements matched with the baseline confounders such as age, ASA grade, CR-POSSUM score, and type of procedure. However, we could not completely avoid variables that may affect this matching. Another limitation of this study was exclusion of the patients with stoma construction. It has been reported that diverting the stoma has a direct impact on length of hospital stay.[32] Therefore, further investigation of ERAS program on emergency colorectal surgery in individuals is still needed. Because the ERAS components cannot be uniformly performed, the optimal outcomes of the ERAS pathway were dependent on the implementation level of ERAS components and adherence to the protocol. Therefore, it will be necessary to conduct larger, adequately powered studies and randomized controlled clinical trials to determine the role of ERAS in patients after emergency gastroenterological surgery.
In conclusion, the modified ERAS protocols are associated with beneficial effects on postoperative complications and length of stay (LOS) in patients undergoing obstructive colorectal cancer surgery. Although we performed a propensity score matching analysis, we acknowledge that these results are based on a homogenous group of patients. These results serve as a pilot study for adequately powered and randomized clinical trials, which may add to the final evaluation of the approach.
Acknowledgments
The authors thank Prof. Xianqing Jin for providing technical assistance and for insightful discussions during the preparation of the manuscript. They also thank Dr Xiaoyong Zhang at the Wistar Institute, USA, for help with the linguistic revision of the manuscript.
Author contributions
YS designed, analyzed, and measured the data. DZ helped design the experiments, performed the statistical analysis, and evaluated the manuscript; CG analyzed and interpreted the data, and wrote the paper.
Software: Yuanyuan Shang.
Writing – original draft: Yuanyuan Shang.
Funding acquisition: Dianliang Zhang.
Writing – review & editing: Dianliang Zhang.
Supervision: Chunbao Guo.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CRP = C-reactive protein, CT = computed tomography, EEN = early enteral nutrition, ERAS = enhanced recovery after surgery, IQR = interquartile ranges, LOS = length of stay, PODs = postoperative days, PS = propensity score, RBP = retinol binding protein.
The research was supported by the National Natural Science Foundation of China (No: 81270448, 81470890).
No potential conflicts of interest relevant to this article are reported.
References
- [1].Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763–4. [DOI] [PubMed] [Google Scholar]
- [2].Kehlet H, Slim K. The future of fast-track surgery. Br J Surg 2012;99:1025–6. [DOI] [PubMed] [Google Scholar]
- [3].ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an International Registry. Ann Surg 2015;261:1153–9. [DOI] [PubMed] [Google Scholar]
- [4].Liu VX, Rosas E, Hwang J, et al. Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg 2017;152:e171032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahmed J, Khan S, Lim M, et al. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis 2012;14:1045–51. [DOI] [PubMed] [Google Scholar]
- [6].Visioni A, Shah R, Gabriel E, et al. Enhanced recovery after surgery for noncolorectal surgery?: a systematic review and meta-analysis of major abdominal surgery. Ann Surg 2018;267:57–65. [DOI] [PubMed] [Google Scholar]
- [7].Zhuang CL, Ye XZ, Zhang XD, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–78. [DOI] [PubMed] [Google Scholar]
- [8].Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS (®)) society recommendations. World J Surg 2013;37:285–305. [DOI] [PubMed] [Google Scholar]
- [9].Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. [DOI] [PubMed] [Google Scholar]
- [10].Ansaloni L, Andersson RE, Bazzoli F, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 2010;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee YM, Law WL, Chu KW, et al. Emergency surgery for obstructing colorectal cancers: a comparison between right-sided and left-sided lesions. J Am Coll Surg 2001;192:719–25. [DOI] [PubMed] [Google Scholar]
- [12].Aslar AK, Ozdemir S, Mahmoudi H, et al. Analysis of 230 cases of emergent surgery for obstructing colon cancer--lessons learned. J Gastrointest Surg 2011;15:110–9. [DOI] [PubMed] [Google Scholar]
- [13].Paduraru M, Ponchietti L, Casas IM, et al. Enhanced recovery after emergency surgery: a systematic review. Bull Emerg Trauma 2017;5:70–8. [PMC free article] [PubMed] [Google Scholar]
- [14].Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol 2014;20:13950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roulin D, Blanc C, Muradbegovic M, et al. Enhanced recovery pathway for urgent colectomy. World J Surg 2014;38:2153–9. [DOI] [PubMed] [Google Scholar]
- [16].Wisely JC, Barclay KL. Effects of an enhanced recovery after surgery programme on emergency surgical patients. ANZ J Surg 2016;86:883–8. [DOI] [PubMed] [Google Scholar]
- [17].Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292–8. [DOI] [PubMed] [Google Scholar]
- [18].Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg 2014;259:649–55. [DOI] [PubMed] [Google Scholar]
- [19].Short HL, Taylor N, Piper K, et al. Appropriateness of a pediatric-specific enhanced recovery protocol using a modified Delphi process and multidisciplinary expert panel. J Pediatr Surg 2018;53:592–8. [DOI] [PubMed] [Google Scholar]
- [20].Powell JJ, Murchison JT, Fearon KC, et al. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg 2000;87:1375–81. [DOI] [PubMed] [Google Scholar]
- [21].van Barneveld KW, Smeets BJ, Heesakkers FF, et al. Beneficial effects of early enteral nutrition after major rectal surgery: a possible role for conditionally essential amino acids? Results of a randomized clinical trial. Crit Care Med 2016;44:e353–61. [DOI] [PubMed] [Google Scholar]
- [22].Ralls MW, Demehri FR, Feng Y, et al. Enteral nutrient deprivation in patients leads to a loss of intestinal epithelial barrier function. Surgery 2015;157:732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Braga M, Gianotti L, Gentilini O, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001;29:242–8. [DOI] [PubMed] [Google Scholar]
- [24].Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011;149:830–40. [DOI] [PubMed] [Google Scholar]
- [25].Sun Y, Chai F, Pan C, et al. Effect of perioperative goal-directed hemodynamic therapy on postoperative recovery following major abdominal surgery-a systematic review and meta-analysis of randomized controlled trials. Crit Care 2017;21:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gonenc M, Dural AC, Celik F, et al. Enhanced postoperative recovery pathways in emergency surgery: a randomised controlled clinical trial. Am J Surg 2014;207:807–14. [DOI] [PubMed] [Google Scholar]
- [27].Strouch MJ, Zhou G, Fleshman JW, et al. Time to initiation of postoperative chemotherapy: an outcome measure for patients undergoing laparoscopic resection for rectal cancer. Dis Colon Rectum 2013;56:945–51. [DOI] [PubMed] [Google Scholar]
- [28].Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–42. [DOI] [PubMed] [Google Scholar]
- [29].Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: a clinical, randomized study. Ann Surg 2006;244:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang G, Jiang Z, Zhao K, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg 2012;16:1379–88. [DOI] [PubMed] [Google Scholar]
- [31].Chen L, Sun L, Lang Y, et al. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016;16:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu Y, Zheng H, Guo T, et al. Temporary diverting stoma improves recovery of anastomotic leakage after anterior resection for rectal cancer. Sci Rep 2017;7:15930. [DOI] [PMC free article] [PubMed] [Google Scholar]


