Abstract
Glial tumors constitute the majority of primary intracranial brain tumors. The expression of specific markers of lymphangiogenesis in gliomas still remains unclear.
A total of 40 surgical specimens from 20 patients with recurrent gliomas were included in the study. The expression of D2-40, vascular endothelial growth factor (VEGF)-C, VEGF-D, and VEGF receptor-3 (VEGR-3) was detected by immunohistochemistry (IHC). The clinicopathologic data (p53 and Ki67) were also collected and analyzed.
At relapse malignant transformation rate was 65% (13/20 cases). D2-40, VEGF-C, VEGF-D, and VEGFR-3 were expressed in 20%, 30%, 60%, and 20% of primary and 45%, 30%, 75%, and 35% of recurrent glioma tumors (P < .01, P = 1.00, P = .03, P = .03). In 13 cases with increased malignancy grade, the expression of Ki67 and p53 were higher at relapse compared with the primary tumors (P = .001, P = .045). Multivariate survival analysis showed VEGF-D was an independent prognostic factor for malignant transformation (HR = 0.376, P = .045).
Glioma is easy to relapse with tumor progression. VEGF-D was an independent prognostic factor for malignant transformation.
Keywords: D2-40, glioma, Ki67, lymphangiogenesis, p53, vascular endothelial growth factor-C, vascular endothelial growth factor-D, vascular endothelial growth factor receptor-3
1. Introduction
Glial tumors constitute the majority (65%) of primary intracranial brain tumors. Despite advances in surgical, medical, and radiation therapies, the patients with high-grade gliomas showed poor prognosis.[1] In those patients, extensive vasogenic edema is associated with short survival.[2] Drugs against mediator of cerebral edema can treat edema caused by tumor or brain radiation necrosis. Clinical experience suggested that bevacizumab, a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF), provided clinical benefit by decreasing peritumoral edema.[3] Bevacizumab can inhibit angiogenesis and lymphangiogenesis. However, the expression of specific markers of lymphangiogenesis in glioma remains unclear.
The D2-40 has been shown to be a very sensitive and specific marker for lymphatic endothelium in most tissues.[4] VEGF-C and VEGF-D bind vascular endothelial growth factor receptor (VEGFR)-3, and they are key regulators in the formation and maintenance of lymphatic vessels in various solid tumors.[5,6] In physiologic adult tissues, VEGFR-3 is not expressed in the endothelium of blood vessels but highly expressed in the lymphatic endothelium.
In our present study, the clinicopathologic characteristics of primary and recurrent glioma were analyzed, and the distribution and expression level of D2-40, VEGF-C, VEGF-D, and VEGFR-3 in primary and recurrent gliomas were examined by immunohistochemistry (IHC).
2. Materials and methods
2.1. Study specimens
A total of 40 surgical specimens (20 primary and 20 recurrent specimens) from 20 recurrent glioma patients (8 males and 12 females) were included. The patients included in this study were enrolled from the Second Hospital of Shandong University between June 2011 and February 2016. The inclusion criteria were listed as follows: glioma was located in the noneloquent areas of the cerebral hemisphere, postoperative magnetic resonance imaging (MRI) scans were available to confirm total tumor resection, also MRI/computed tomography scans were used to determine the recurrence of glioma. Tumor type and grade were determined by Department of Pathology, according to the World Health Organization classification.[7,8] From those patients, 12 surgical specimens of adjacent normal brain tissue were available and were used as control. Tissues were collected during craniotomy and fixed in 10% buffered formalin. Patients of grades III and IV received standard radiotherapy, as follows: 30 to 33 fractions, 1.8 to 2.0 Gy/fraction, 5 days/wk, to a total dose 60 Gy using 6 to 10 MV X-rays in vitro. The patients were also given daily oral temozolomide (TMZ) 75 mg/m2 during radiotherapy. Four weeks after radiotherapy, all of the patients received 6 cycles of TMZ. Each cycle lasted 5 days with 28 days interval between each cycle. About 150 mg/m2 of TMZ was given for the first cycle for 5 days, followed by 200 mg/m2 of drug for the rest of the cycles if no significant drug-related toxicities were observed. The patient's clinicopathologic data (p53 and Ki67, scored as a continuous variable, rounded to the closest 5th percentile) were also collected and analyzed.
This study was approved by the Human Research Ethics Committees from the Second Hospital of Shandong University. Written informed consent was obtained from all participants.
2.2. Immunohistochemistry
Five-micrometer-thick sections of human tumor tissues were deparaffinized and rehydrated. For D2-40, VEGF-C, VEGF-D, and VEGFR-3 staining, heat-induced epitope retrieval was performed by boiling the tissue section in Tris-EDTA buffer (pH 9.0) for 15 minutes, in microwave oven. After cooling to room temperature, the section was exposed to methanol (containing 3% H2O2) for 10 minutes to block endogenous peroxidase. D2-40 was detected with an anti-D2-40 monoclonal antibody (1:200 dilution, ab77854, Abcam, Cambridge, UK), VEGF-C was detected with an anti-VEGF-C monoclonal antibody (1:200 dilution, ab191274, Abcam), VEGF-D was detected with an anti-VEGF-D monoclonal antibody (1:200 dilution, ab155288, Abcam), and VEGFR-3 was detected with an anti-VEGFR-3 antibody (1:100 dilution, ab27278, Abcam) for 2 hours at room temperature in a humidified chamber. The slide was then washed with phosphate-buffered saline (PBS) and incubated with the secondary detection antibody polyperoxidase-labeled anti-rabbit/mouse IgG (ZSGB-BIO, Beijing, China) for 60 minutes in accordance to the manufacturer's instructions. 3,3-Diamino benzidine was used as a chromogen. The section was counterstained with hematoxylin and mounted in gelatin. Negative and positive controls for each immunohistochemical marker were included with every experiment. A lymphangioma tissue sample served as a positive control and replacement of the primary antibody by 0.01 mol/L PBS was used as a negative control.
2.3. Histologic assessment
Immunohistochemical staining of 10 randomly selected fields in each tumor specimen was evaluated under light microscope (200×). Evaluation of immunostaining was performed independently by 2 pathologists who were blind to the clinical data. In case of discordance between pathologists, a 3rd pathologist reviewed the result to achieve a consensus. Immunostaining was semiquantitatively recorded by estimating the proportion of positive cells and classified as follows: negative = 0%, “1+” = 1% to 25%, “2+” = 26% to 50%, and “3+” ≥ 50% positively stained cells.
2.4. Statistical analysis
Statistical analysis was performed with SPSS 19.0 and GraphPad.cameyo. Nonparametric Spearman correlation test was used to analyze the relationship between the pathologic grades and expression levels of D2-40, VEGF-C, VEGF-D, VEGFR-3, p53, or Ki67. Nonparametric Spearman correlation test was also used to assess relationship between the first disease-free survival time and the expressions of different markers. The expression levels of markers at diagnosis and relapse were analyzed by Wilcoxon matched-pairs signed rank. Multivariate prognostic analyze of glioma was performed using the Cox proportional hazards regression model. P-value of .05 or less was considered statistically significant.
3. Results
3.1. Clinicopathologic characteristics of patients
The clinicopathologic characteristics of 20 glioma patients are detailed in Table 1. The median age of patients at diagnosis was 43.5 years old (range: 40–48.75 years old), and the mean tumor-free survival was 1.65 ± 1.34 years. The most common clinical presentation was headache (n = 14), limb paralysis (n = 3), and epilepsy (n = 3). The mean tumor size was 4.3 ± 1.7 cm. There were 1 case of grade I, 6 cases of grade II, 9 cases of grade III, and 4 cases of grade IV at diagnosis but 2 cases of grade II, 2 cases of grade III, and 16 cases of grade IV at relapse. At relapse malignant transformation rate was 65% (13/20 cases).
Table 1.
The clinical and pathologic characteristics of glioma patients.
3.2. Expression of D2-40, VEGF-C, VEGF-D, and VEGFR-3 in control, primary, and recurrent glioma tissues
There was no expression of D2-40, VEGF-C, VEGF-D, and VEGFR-3 in the adjacent normal brain tissue. D2-40-positive staining was observed in the cytoplasm and detected in 13 of 40 glioma specimens (Fig. 1A). VEGF-C-positive staining was mostly observed in the cytoplasm or/and weakly in the nucleus in 12 of 40 glioma specimens (Fig. 1B). VEGF-D expression was observed in the cytoplasm or/and in the nucleus in 27 of 40 glioma specimens (Fig. 1C). VEGFR-3-positive tumor cells were observed in the cytoplasm and nucleus in 11 of 40 glioma specimens (Fig. 1D). IHC showed that D2-40, VEGF-C, VEGF-D, and VEGFR-3 was expressed in 20%, 30%, 60%, and 20% of primary and 45%, 30%, 75%, and 35% of recurrent glioma tumors (P < .01, P = 1.00, P = .03, P = .03). The first disease-free survival time was not related to the expressions of D2-40 (P = .78), VEGF-C (P = .64), VEGF-D (P = .11), VEGFR-3 (P = .31), Ki67 (P = .69), and p53 (P = .35). There were 6 cases with negative staining for all lymphangiogenesis markers (all of D2-40, VEGF-C, VEGF-D, and VEGFR-3). There was no significant difference of the first disease-free survival time between them and others (P = 1.665).
Figure 1.
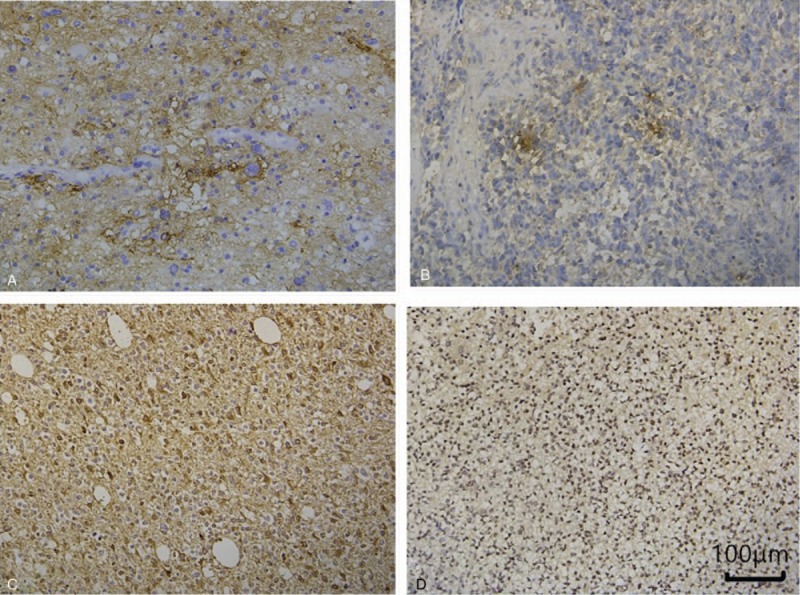
D2-40, vascular endothelial growth factor (VEGF)-C, VEGF-D, and vascular endothelial growth factor receptor (VEGFR)-3 expression in glioma tissues. A representative section from: (A) D2-40-positive glioma specimens IV (+). (B) VEGF-C-positive glioma specimens IV (++). (C) VEGF-D-positive glioma specimens IV (2+). (D) VEGFR-3-positive glioma specimens IV (+).
Moreover, the pathologic grade was significantly related to the positive stainings for D2-40 (P = .0060), VEGF-D (P = .017), and VEGFR-3(P = .032), and to the scores of Ki67 (P < .001) and p53 (P = .003), while was not significantly related to VEGF-C expression (P = .059).
In 13 cases with increased malignancy, the expression levels of Ki67, p53, D2-40, VEGF-C, VEGF-D, and VEGFR-3 were increased at relapse (Fig. 2). Ki67 and p53 scores were significantly higher at relapse compared with primary tumor (P = .001, 10% [5–27.5%] vs 40% [35–55%] for Ki67; P = .045, 10% [0–25%] vs 25% [17.5–37.5%] for p53). There was significant difference in the D2-40 and VEGF-D expression between recurrent and primary tumors (P = .031, P = .047), but there is no significant difference in the VEGF-C and VEGFR-3 expression between recurrent and primary tumors (P = .094 and P = .25). A multivariate survival analysis, including expression of D2-40, VEGF-C, VEGF-D, VEGFR-3, Ki67, p53, primary tumor grade, and relapse tumor grade, showed that VEGF-D was an independent prognostic factor for malignant transformation (HR = 0.376, P = .045).
Figure 2.
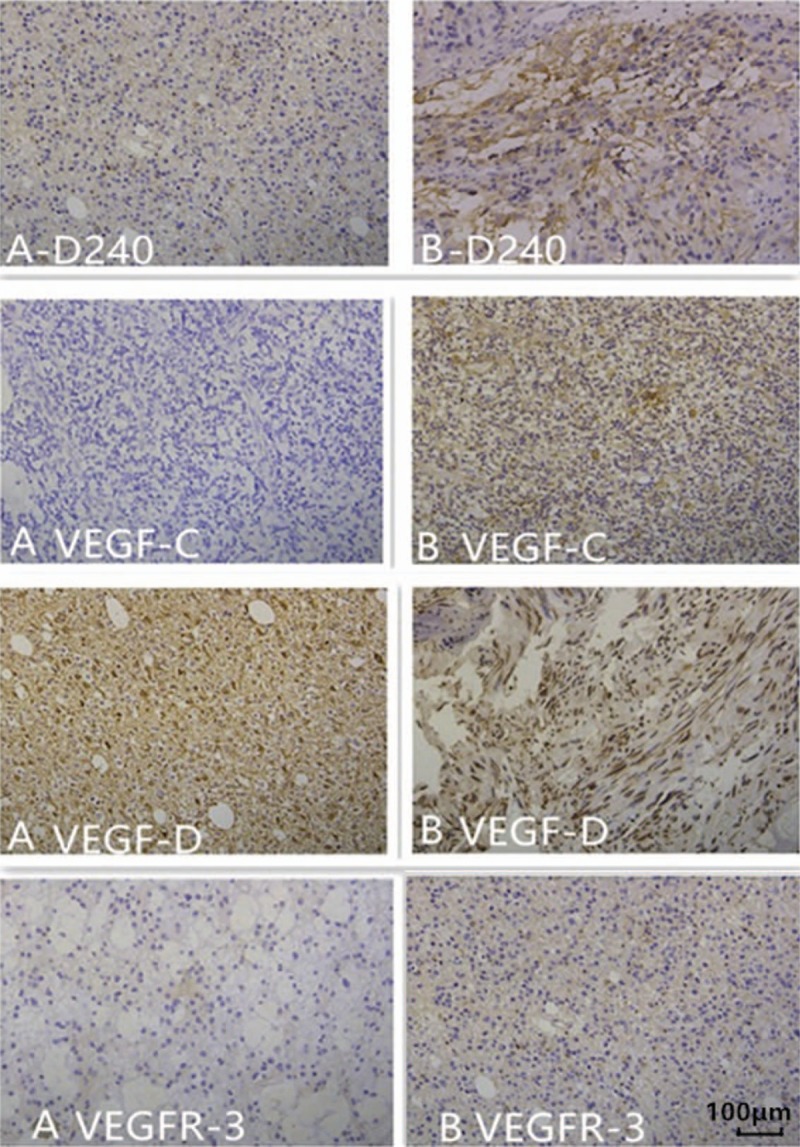
D2-40, vascular endothelial growth factor (VEGF)-C, VEGF-D, and VEGFR-3 expression in the primary (A) and recurrent specimens (B) from the same patient. (A-D240) A representative section from specimens of grade II (−). (B-D240) A representative section from specimens of grade IV (2+). (A VEGF-C) A representative section from specimens of grade III (−). (B VEGF-C) A representative section from specimens of grade IV (+). (A VEGF-D) A representative section from specimens of grade III (+). (B VEGF-D) A representative section from specimens of grade IV (2+). (A VEGFR-3) A representative section from specimens of grade III (−). (B VEGFR-3) A representative section from specimens of grade IV (+).
4. Discussion
Glial tumors account for approximately 1.5% of all cancers. As a rule, high-grade gliomas almost always grow back even after complete surgical excision, so they are called recurrent cancer of the brain. And gliomas at relapse usually transform to higher grade glioma. In our study, at relapse malignant transformation rate was 65%. There is a great need for biomarkers associated with prognosis. Overexpression of VEGF is associated with several CNS tumors. Multivariate survival analysis showed VEGF-D was an independent prognostic factor for malignant progression. Debinski et al found the activating protein-1 family of transcription factors play a potentially critical role in the progression of gliomas by eliciting uncontrolled upregulation of VEGF-D.[9] The VEGF-D system appears to be a very attractive target for new molecular diagnostics and rational molecular anticancer therapies.[10] Weickhardt et al also found patients with low expression of VEGF-D benefited from bevacizumab treatment, patients with higher VEGF-D expression received less benefit.[11] Clinical utilization of VEGF-D needs further evaluation.
In our study, we want to detect the status of the expression of lymphangiogenesis markers in primary and recurrent glioma tumors. It was reported endothelial cells in brain tumors may either acquire lymphangiogenic mechanisms or be derived from precursor cells carrying lymphatic abilities.[12] Case report by Frank et al indicated a lymphatic pathway of metastasis, demonstrated by histopathologic analysis.[13] We found the expression levels of Ki67, p53, D2-40, VEGF-D, and VEGFR-3 were increased at relapse. But the disease-free survival time was not related to the expressions of them. The anatomical relationship between malignant tumor and its vascular and lymphatic bed is an important factor influencing metastasis. Brain tumors are different from tumors of other parts of the body at metastasis and relapse. There may be unique barriers in brain, such as the dura mater and thickened basement membrane of blood vessels, they prevent lymphatic and hematogenous spread of intracranial tumors. The tumor cells in CNS lack extracellular matrix proteins for tumor dissemination. In addition, intracranial tumor has earlier and more serious symptoms and shorter median survival time before extracranial metastasis detected. The main goal of the current surgical treatment of GBM is maximal safe resection, removing as much as possible the tumor while preserving the neurologic function. The partial resection of glioma preserving functional cortex may affect the first disease-free survival time.
Staining for p53 is often performed to detect single-invading astrocytoma cells. Ki67, a marker of cell proliferation, represents an excellent biomarker to predict the aggressiveness of gliomas and patients’ outcomes.[14,15] Despite involved in tumor angiogenesis, VEGF-C and VEGF-D are thought to stimulate the growth of lymphatic vessels because the expression of their specific receptor, VEGFR-3, has been demonstrated to be expressed in the lymphatic vessels. VEGFR-3, which is not expressed in normal brain, has been proposed as a marker for lymphatic endothelial cells,[16] and VEGFR-3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels.[17] VEGF-C has been shown to be expressed in the lymphatic vessels in the tumor microenvironment.[18] It is important in tumor biology elsewhere in the body, and the levels of VEGF-C correlate with increased lymph node metastasis and poor prognosis.[19–22] Glioblastomas, the most frequent high-grade gliomas, are aggressive, rapidly growing, destructive lesions, while low-grade gliomas are less aggressive in their behavior. In our study, the pathologic grade was significantly related to the expression of biomarkers (D2-40, VEGF-C, VEGF-D, and VEGFR-3, excepting VEGF-C), and to the score of p53 and Ki67. The expression of most markers (D2-40, VEGF-D, p53, and Ki67) was higher in patients with increased malignancy at relapse compared with the primary tumors. It is interesting, but not surprising that various genes not expressed in normal tissue might be expressed particularly in the higher grade tumors. These results should be more persuasive in a large number of patients. This study has limitations: pathologic markers such as IDH mutation, 1p/19q codeletion, and O6-methylguanine-DNA methyltransferase (MGMT) are not included in our study. They are important in the diagnosis and treatment of brain glioma. The patients selected in our study all underwent primary and recurrent gliomas surgery, bias in patient selection is a limitation.
5. Conclusion
Our results suggest that glioma is easy to relapse with tumor progression. VEGF-D was an independent prognostic factor for malignant transformation.
Author contributions
Conceptualization: Jun Jiang, Shun Wang, Chuncheng Qu.
Data curation: Jun Jiang, Shun Wang.
Formal analysis: Jun Jiang, Shun Wang.
Funding acquisition: Jun Jiang.
Investigation: Jun Jiang, Shun Wang, Yuan Chen, Chuncheng Qu, Yuguang Liu.
Methodology: Yuan Chen.
Project administration: Jun Jiang, Shun Wang.
Resources: Jun Jiang.
Software: Jun Jiang.
Supervision: Shun Wang, Chengwei Wang, Chuncheng Qu, Yuguang Liu.
Validation: Jun Jiang.
Visualization: Jun Jiang.
Writing – original draft: Jun Jiang, Shun Wang.
Writing – review & editing: Jun Jiang, Shun Wang, Chengwei Wang, Chuncheng Qu, Yuguang Liu.
Footnotes
Abbreviations: CNS = central nervous system, VEGF = vascular endothelial growth factor, VEGFR = vascular endothelial growth factor receptor.
This work was supported by Youth Fund of the 2nd Hospital of Shandong University.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) Classification of Tumours of the Central Nervous System: newly codified entities. Brain Pathol 2007;17:304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carlson MR, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res 2007;13:2592–8. [DOI] [PubMed] [Google Scholar]
- [3].Wang Y, Wang E, Pan L, et al. A new strategy of CyberKnife treatment system based radiosurgery followed by early use of adjuvant bevacizumab treatment for brain metastasis with extensive cerebral edema. J Neurooncol 2014;119:369–76. [DOI] [PubMed] [Google Scholar]
- [4].Evangelou E, Kyzas PA, Trikalinos TA. Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Mod Pathol 2005;18:1490–7. [DOI] [PubMed] [Google Scholar]
- [5].Yu M, Tang Z, Alousi S, et al. Expression patterns of lymphangiogenic and angiogenic factors in a model of breast ductal carcinoma in situ. Am J Surg 2007;194:594–9. [DOI] [PubMed] [Google Scholar]
- [6].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- [7].Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;1318:803–20. [DOI] [PubMed] [Google Scholar]
- [8].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Debinski W, Gibo D, Mintz A. Epigenetics in high-grade astrocytomas: opportunities for prevention and detection of brain tumors. Ann N Y Acad Sci 2003;983:232–42. [DOI] [PubMed] [Google Scholar]
- [10].Debinski W, Slagle-Webb B, Achen MG, et al. VEGF-D is an X-linked/AP-1 regulated putative onco-angiogen in human glioblastoma multiforme. Mol Med 2001;7:598–608. [PMC free article] [PubMed] [Google Scholar]
- [11].Weickhardt AJ, Williams DS, Lee CK, et al. Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br J Cancer 2015;113:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grau SJ, Trillsch F, von Lüttichau I, et al. Lymphatic phenotype of tumour vessels in malignant gliomas. Neuropathol Appl Neurobiol 2008;34:675–9. [DOI] [PubMed] [Google Scholar]
- [13].Frank S, Kuhn SA, Brodhun M, et al. Metastatic glioblastoma cells use common pathways via blood and lymphatic vessels. Neurol Neurochir Pol 2009;43:183–90. [PubMed] [Google Scholar]
- [14].Paulus W. GFAP, Ki67 and IDH1: perhaps the golden triad of glioma immunohistochemistry. Acta Neuropathol 2009;118:603–4. [DOI] [PubMed] [Google Scholar]
- [15].Yuan Y, Xiang W, Yanhui L, et al. Ki-67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure 2013;22:877–81. [DOI] [PubMed] [Google Scholar]
- [16].Niki T, Iba S, Yamada T, et al. Expression of vascular endothelial growth factor receptor 3 in blood and lymphatic vessels of lung adenocarcinoma. J Pathol 2001;193:450–7. [DOI] [PubMed] [Google Scholar]
- [17].He Y, Rajantie I, Pajusola K, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res 2005;65:4739–46. [DOI] [PubMed] [Google Scholar]
- [18].Clarijs R, Schalkwijk L, Hofmann UB, et al. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res 2002;62:7059–65. [PubMed] [Google Scholar]
- [19].Jia YT, Li ZX, He YT, et al. Expression of vascular endothelial growth factor-C and the relationship between lymphangiogenesis and lymphatic metastasis in colorectal cancer. World J Gastroenterol 2004;10:3261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trojan L, Rensch F, Voss M, et al. The role of the lymphatic system and its specific growth factor, vascular endothelial growth factor C, for lymphogenic metastasis in prostate cancer. BJU Int 2006;98:903–6. [DOI] [PubMed] [Google Scholar]
- [21].Wang TB, Deng MH, Qiu WS, et al. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 2007;13:1794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baek SK, Jung KY, Lee SH, et al. Prognostic significance of vascular endothelial growth factor-C expression and lymphatic vessel density in supraglottic squamous cell carcinoma. Laryngoscope 2009;119:1325–30. [DOI] [PubMed] [Google Scholar]


