Abstract
Rheumatoid arthritis (RA) is characterized by hyperplastic pannus formation mediated by activated synovial fibroblasts that cause joint destruction. We have shown earlier that RA synovial fibroblasts (RASFs) exhibit resistance to apoptosis, primarily as a result of enhanced expression of myeloid cell leukemia-1 (Mcl-1). In this study, we discovered that ursolic acid (UA), a plant-derived pentacyclic triterpenoid, selectively induces B-cell lymphoma 2 homology 3-only protein Noxa in human RASFs. We observed that UA-induced Noxa expression was followed by a consequent decrease in Mcl-1 expression in a dose-dependent manner. Subsequent evaluation of the signaling pathways showed that UA-induced Noxa is primarily mediated by the JNK pathway in human RASFs. Chromatin immunoprecipitation (IP) studies into the promoter region of Noxa indicated the role of transcription factor specificity protein 1 in JNK-mediated Noxa expression. Furthermore, the results from IP studies and proximity ligation assays indicated that UA-induced Noxa colocalizes and associates with Mcl-1 to prime it for proteasomal degradation through K48-linked ubiquitination by the selective recruitment of Mcl-1 ubiquitin ligase E3, a homologous to E6-associated protein C terminus domain-containing E3 ubiquitin ligase. These findings unveil a novel mechanism of inducing apoptosis in RASFs and a potential adjunct therapeutic strategy of regulating synovial hyperplasia in RA.—Kim, E. Y., Sudini, K., Singh, A. K., Haque, M., Leaman, D., Khuder, S., Ahmed, S. Ursolic acid facilitates apoptosis in rheumatoid arthritis synovial fibroblasts by inducing SP1-mediated Noxa expression and proteasomal degradation of Mcl-1.
Keywords: ursolic acid, synovial hyperplasia, BH3-only protein, ubiquitination
Pathogenesis of rheumatoid arthritis (RA) is characterized by persistent, chronic inflammation of the synovial tissue, leading to bone damage in the symmetrical joints (1). One of the hallmarks of RA, aside from leukocyte infiltration, is the pannus formation in which the synovial hyperplasia invades into the adjacent articular cartilage and bone, causing joint destruction. A recent understanding of the molecular mechanism that governs tumor-like expansion of synovial tissue suggests that RA synovial fibroblasts (RASFs) express abnormally high levels of myeloid cell leukemia-1 (Mcl-1) and thus, are resistant to extrinsic activation of death receptors TNF-related apoptosis-inducing ligand (TRAIL)- and Fas-induced apoptosis (2, 3).
Mcl-1 belongs to the anti-apoptotic B-cell lymphoma (Bcl)-2 protein subfamily that includes Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and Bfl-1/A1 and plays a pivotal role in the regulation of both intrinsic and extrinsic cell death signals (4, 5). Mcl-1 critically blunts apoptotic signals of sensitivity and resistance to apoptosis (6). The cells expressing high levels of Mcl-1 are resistant to a newly developed Bcl-2/Bcl-xL/Bcl-w inhibitor, and knockdown of Mcl-1 dramatically reduced resistance in these cells to undergo apoptosis (7). Mcl-1 had been implicated in Bcl-2 homologous antagonist/killer (Bak) regulation, a proapoptotic member of the Bcl-2 family, by its formation of complexes with Bak and its degradation early (8). Intriguingly, the Bcl-2 homology 3 (BH3)-only subfamily of Bcl-2 proteins exhibits proapoptotic functions, and 1 of its members, Noxa, selectively binds to Mcl-1, to initiate its proteasome-dependent degradation (9). However, investigation into the role of Noxa in regulating Mcl-1 expression has been limited to cancer cells and is yet to be tested in other cell types.
With the consideration of the importance of synovial hyperplasia in the pathogenesis of RA, several experimental approaches for RA therapy to regulate activated synovium to decelerate joint destruction have been successful, but this success has not yet been transformed to human clinical application (10–13). This is significantly a result of the lack of identified molecular targets to suppress the uncontrolled growth of RASFs (14, 15). Thus, selective targeting of RASF Mcl-1, either by modulation of the signaling pathways or upregulation of the BH3-only family proteins, may serve as a potential therapeutic strategy to reduce tissue destruction in RA (2, 4, 6, 16).
Ursolic acid (UA) is a pentacyclic triterpenoid of plant origin that is known for its hepatoprotective and anti-inflammatory effects (17). Furthermore, UA has been shown to inhibit cell growth and induce apoptosis in cancer cells through a variety of mechanisms (18). Whether UA can modulate the resistance in the primary human cells or cell lines by stabilizing proapoptotic proteins and its possible mechanism of action is yet to be tested. As RASFs overexpress Mcl-1 and resist TRAIL-induced apoptosis and as the suppression of Mcl-1 sensitizes these cells to apoptosis (19–21), the present study was carried out to evaluate the ability of UA, alone or in addition to TRAIL, to promote cell death in human RASFs.
MATERIALS AND METHODS
Antibodies and reagents
Recombinant human TRAIL was purchased from R&D Systems (Minneapolis, MN, USA). Rabbit polyclonal antibodies against poly-ADP-ribose polymerase (PARP), Bcl-2, phospho-p38 (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-c-Jun (Ser73), phospho-PKB (Akt; Ser473), phospho-p70S6K (Thr389), phospho- activating transcription factor (ATF)2 (Thr69/71), K48-ubiquitin, K63-ubiquitin, and anti-rabbit/mouse horseradish peroxide-linked secondary antibodies were procured from Cell Signaling Technology (Danvers, MA, USA). Rabbit mAb against Noxa and Dynabeads were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Rabbit pAb and mAb against Mcl-1, mouse specificity protein 1 (SP1) mAb, rabbit hypoxia-inducible factor 1α (HIF-1α) pAb, and HIF-1α inhibitor were acquired from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-ubiquitin antibody was obtained from Enzo Life Sciences (Farmingdale, NY, USA). UA; rabbit anti-β-actin pAb; colorimetric caspase-3 activity assay kits; rabbit polyclonal acetyl histone H4 antibody; and the signaling inhibitors of p38 (SB203580), JNK (SP600125), Akt (LY294002), and proteasomal degradation (MG-132) were purchased fromMilliporeSigma (Burlington, MA, USA). A reversible and highly selective inhibitor of JNK (JNK-IN-8; Cat. 1410880-22-6) was purchased from Cayman Chemical (Ann Arbor, MI, USA).
Isolation and culture of human RASFs
RASFs were isolated from synovium obtained from patients with RA, according to the Washington State University and University of Toledo Institutional Review Board-approved protocol in compliance with the Helsinki Declaration, that had undergone total joint replacement surgery or synovectomy and fulfilled the criteria set by the American College of Rheumatology. Fresh synovial tissues were minced and digested in a solution of dispase, collagenase, and DNase (22). RASFs were grown in Roswell Park Memorial Institute (RPMI) medium containing 2 mM l-glutamine with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5% CO2. Cells were used between passages 5 and 9.
Preparation of UA solution
A stock solution of 25 mM UA was prepared in sterile DMSO and stored at −20°C in aliquots. Fresh UA solution was used in each experiment and added directly to the culture medium. DMSO at the comparative volume was used as control.
Cell viability assays
To study the effect of UA on cell viability, RASFs (2 × 104/well) were plated in 96-well, flat-bottomed tissue-culture plates (Corning, Corning, NY, USA) and cultured in RPMI 1640 plus 10% FBS for 6 h. This was then replaced with fresh medium and culture continued for 24 h. UA (0.625–20 μM) was added to RASFs in serum-free medium, and the culture was incubated at 37°C for another 24–72 h. Two hours before termination of each time point, 20 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye (5 mg/ml in sterile PBS; Thermo Fisher Scientific) was added to each well and further incubated at 37°C. At the end of incubation, cells were washed twice with PBS, solubilized in 100 μl DMSO at 37°C for 5 min, and read at an optical density of 570 nm. For studying the synergistic effect of UA with TRAIL on the cell viability, confluent RASFs in 96-well plates were cultured in RPMI–1% FBS, containing DMSO or 2.5–10 μM UA and/or TRAIL (100 ng/ml) for up to 24 h and analyzed by the colorimetric method described above using MTT dye. For morphologic studies, RASFs were fixed in 1% glutaraldehyde in RPMI after the similar treatment, as described above for 24 h, and evaluated microscopically to study morphologic changes in the presence of UA, TRAIL, or UA plus TRAIL.
Western immunoblotting
Cells were lysed in lysis buffer (100 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM NaP2O4, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 1 mM PMSF) and protease inhibitors (Roche Diagnostics, IN, USA; 1 tablet/10 ml), and protein was measured using bicinchoninic acid protein assay kits (Thermo Fisher Scientific). Equal amounts of protein (15–25 μg) were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Blots were probed with antibodies for the specific protein of interest, followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Immunoreactive protein bands were visualized by ECL and were densitometrically analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Blots were stripped and reprobed for β-actin to ensure equal protein loading. Data were statistically analyzed using Prism software (GraphPad Software, La Jolla, CA, USA).
Transfection studies
Wild-type (WT) immortalized baby mouse kidney (BMK) cells, Noxa−/− cells, Noxa−/− cells transfected with empty vector, and Noxa−/− cells stably expressing WT Noxa (23) were left untreated or treated with UA at different concentrations (10, 20, and 40 μM) for 24 or 48 h. To quantify cellular viability, cells were washed with PBS, fixed with 10% trichloroacetic acid, and then stained with 0.4% sulforhodamine B (SRB). After that time, wells were washed with 1% acetic acid and air dried and dye eluted in 10 mM unbuffered Tris. Absorbance was read at 490 nm, and the absorbance data were used to calculate relative cell growth, as described in Leaman et al. (24).
Small interfering RNA studies
To study the effect of Noxa knockdown on the ability of UA to reduce cell viability, RASFs were transfected with 120 pM scrambled control small interfering RNA (siRNA) (SIC001; MilliporeSigma) and Noxa siRNA (sc-37305; Santa Cruz Biotechnology) using Lipofectamine 2000 (Thermo Fisher Scientific) for 48 h, followed by UA (10 µM) stimulation for 48 h in serum-free medium. Two hours before termination, MTT dye (5 mg/ml) was added to each well. At the time of termination, the conditioned medium were aspirated, and cells were washed with ice-cold PBS and permeabilized in DMSO. The absorbance of color developed with a solubilized MTT dye was read at 570 nm.
Chromatin immunoprecipitation assay
ChIP was performed by crosslinking with formaldehyde to a final concentration of 0.75% at room temperature for 10 min and reaction stopped by the addition of glycine (125 mM final concentration) for 5 min. Cells were washed with ice-cold PBS 2 times, scraped with 500 μl chromatin immunoprecipitation assay (ChIP) lysis buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM EDTA, 0.5% SDS, 1% Triton X-100, complete Mini, and PhosSop protease inhibitors), and placed on ice for 30 min. DNA was sheared by sonication, and the cell debris was pelleted by centrifugation at 8000 g for 10 min. Thirty liters of clear lysates was taken out to check quality controls of sonication, as well as input control. Sonication quality check was performed on 1.5% agarose gel with ethidium bromide. Immunoprecipitation (IP) was accomplished with diluted sample supernatants (5-fold) that were precleared with 30 µl protein G Dynabeads (Thermo Fisher Scientific), blocked with 2% bovine serum albumin and salmon sperm DNA (Thermo Fisher Scientific) for 60 min at 4°C. The precleared samples were incubated with preblocked and bound acetyl-histone H4 or SP1 antibodies (1 μg/sample), conjugated to protein G Dynabeads (35 μl/ sample) at 4°C overnight. The samples were washed once with low-salt wash buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, and 0.1% SDS), high-salt wash buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM EDTA, 1% Triton X-100, and 0.1% SDS), LiCl wash buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 0.25 M LiCl, and 1% Triton X-100), and finally, with Tris–EDTA buffer, pH 8. The immune complex was eluted with 120 μl elution buffer (1% SDS and 0.1 M NaHCO3). The reverse crosslinking was performed by adding 2 μl RNAse A (10 mg/ml) and 4.8 μl 5 M NaCl and incubating at 65°C overnight, followed by 2 h of 2 μl proteinase K (20 mg/ml) treatment at 65°C. After digestion, phenol/chloroform extraction, ethanol precipitation, and pellet resuspension were completed. Quantitative PCR was performed using iTaq Universal SYBR Green Mix (Bio-Rad), and specific primer sequences for human Noxa promoter regions were derived from a previousl study (25). All samples were run in duplicates and analyzed using ABI software (Thermo Fisher Scientific), provided with the instrument. For quantification, the relative abundance of each gene was normalized to input control and determined by the ΔΔCt method.
IP assays
Serum-starved RASFs were treated with UA (10 μM) for 6, 12, and 24 h and lysed by sonication in IP buffer. The lysates were precleared with control beads, and IP was performed overnight at 4°C with anti-Mcl-1 antibody-bound protein G beads or control beads. After washes, the beads were boiled with 2 times Laemmli buffer, and Western blot was performed as above.
Immunofluorescence microscopy
RASFs were seeded onto poly-l-lysine-coated sterile coverslips and allowed to grow before culturing overnight in serum-free medium and then treated with UA (10 μM) for 24 h. The cells were washed with cold PBS, fixed with 3% paraformaldehyde, quenched with 30 mM glycine/PBS, blocked with 2% horse serum/bovine serum albumin/PBS, permeabilized with 0.1% Triton X-100, incubated with anti-Mcl-1 and anti-Noxa antibodies overnight at 4°C in humidity chamber, and then incubated with secondary antibodies labeled with Alexa Fluor 488 or 594 for 1 h at room temperature after washing. After mounting the coverslip onto a slide in mounting media with DAPI, z-stack images of 0.25 μm thickness were taken under a Zeiss AxioImager M2 epifluorescence microscope with a 100 times oil objective. The images were deconvoluted using AutoQuant ×3 software using the adaptive, theoretical point spread function method, and max-projected images were shown.
Proximity ligation assay
RASFs were grown under the experimental conditions, as described for immunofluorescence (IF) with the following changes using the Duolink In Situ Fluorescence Kit (MilliporeSigma). The cells were blocked with commercially provided blocking buffer, antibodies were diluted in antibody diluent, and coverslips were washed with commercially provided wash buffers. After 4°C overnight incubation with primary antibodies, proximity ligation assay (PLA) plus and minus stock (i.e., secondary antibodies) was incubated in a humidity chamber at 37°C for 1 h, and then ligation and polymerase amplification steps were performed before mounting the coverslips onto a slide. The images were taken using the same microscope and software but with a ×40 objective.
Statistical analysis
Statistical analyses of quantitative results were performed using an unpaired 2-tailed Student’s t test for comparison of 2 groups. A value of P < 0.05 and below was determined as significant.
RESULTS
UA induces apoptosis in RASFs by PARP cleavage
To evaluated the efficacy of UA in inducing cytotoxicity in RASFs in vitro, cells were treated with UA (0.625–20 μM) for 24, 48, and 72 h in RPMI-1% FBS and evaluated for the cell viability using an MTT-based cell viability assay (19, 22). DMSO (2 μl) was used as a vehicle control. The result of this experiment showed that UA, up to 5 μM concentration, had no cytotoxic effect on RASFs (Fig. 1A). Interestingly, 10 and 20 μM concentrations of UA at 24 h treatment caused a dose-dependent decrease in viability of RASFs by ∼22 and ∼61%, respectively (Fig. 1A; P < 0.05). However, there was a marked, time-dependent increase in the efficacy of UA 10 μM to enhance cytotoxicity, as observed over time.
Figure 1.
UA induces apoptosis in RASFs by PARP cleavage. A) RASFs were treated with different concentrations of UA (0.625–20 μM) for 24, 48, and 72 h, and MTT assay was performed to determine cell viability. B) RASFs were treated with UA (2.5, 5, or 10 μM), alone or with TRAIL (100 ng/ml), for 24 h, and then MTT assay was performed. C) RASFs were treated in a similar protocol as B, with higher concentrations of UA (10 or 20 μM), and then immunoblotting was performed for PARP cleavage. Experiments were repeated using RASFs from at least 3 to 4 different patients. *P < 0.05.
To examine whether TRAIL by itself induces apoptosis and if UA presence further enhances the sensitivity of RASFs for TRAIL-induced cytotoxicity, cells were exposed to UA (5–10 μM) and/or TRAIL (100 ng/ml) for 24 h, and the viability was evaluated. The result obtained from the study using RASFs from 4 different donors showed that UA 10 μM was able to induce ∼35% loss of viability in RASFs (Fig. 1B; P < 0.05, n = 4). Importantly, instead of inducing the loss of cell viability, TRAIL maintained the survival of these cells when compared with the DMSO controls (Fig. 1B). Interestingly, when TRAIL was incubated with UA 5 μM for 24 h, there was a significant ∼21% further decrease in the viability of RASFs compared with UA 5 μM-alone treatment (Fig. 1B; P < 0.05, n = 4). This observation prompted us to evaluate the molecular mechanism of RASF sensitization for TRAIL-induced apoptosis by UA.
Studies have shown UA as a potent inducer of apoptosis in cancer cells (18). We evaluated the effect of UA, alone or with TRAIL, on the activation and cleavage of PARP, which is a marker for DNA fragmentation and apoptosis (19, 26). When RASFs were exposed to UA (10 and 20 μM), we observed a marked, dose-dependent cleavage of PARP protein, resulting in the expression of a 85 kDa fragment (Fig. 1C; P < 0.05, n = 3), suggesting that UA induces DNA fragmentation and apoptosis in RASFs in vitro. Treatment of RASFs with TRAIL (100 ng/ml) was not able to induce any cleavage in PARP, implicating that these diseased cells are resistant to TRAIL-induced apoptosis. Importantly, when TRAIL was incubated in the presence of UA, a significantly high degree of PARP cleavage was observed with the increasing concentration of UA compared with the TRAIL- or UA-alone treatments (Fig. 1C; P < 0.05 for UA 20 μM). Hence, not only can UA alone induce apoptosis in RASFs, but also, it enhances TRAIL-induced apoptosis by PARP cleavage.
Noxa is induced by UA and is essential for UA-mediated cell death
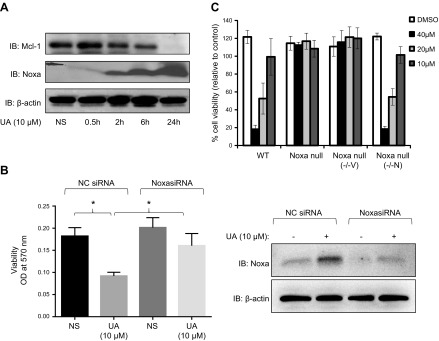
Multiple mechanisms of the down-regulation of anti-apoptotic proteins, especially the Bcl-2 family, have been proposed in earlier studies to curb down the uncontrolled proliferation rate of cancer cells and RASFs (4, 9, 16, 27). To determine whether UA treatment modulated Mcl-1 and/or Noxa levels, we treated RASFs with UA (5 and 10 μM) for 24 h and detected both Mcl-1 and Noxa, an endogenous, Mcl-1 regulatory, BH3-only protein. Western blot analysis showed that RASFs have no or very weak Noxa expression at the constitutive levels (Fig. 2A). Interestingly, the presence of UA induced Noxa in a dose-dependent fashion, whereas simultaneously decreasing the levels of Mcl-1. Together, these data suggests that UA modulates certain signaling pathways that result in enhanced Noxa mRNA and protein levels and degradation of Mcl-1.
Figure 2.
Noxa is induced by UA and is necessary for UA-mediated cell death. A) RASFs were treated with UA (10 μM) and harvested at different time points (0.5, 2, 6, and 24 h). Immunoblotting (IB) was performed with antibodies for Noxa, Mcl-1, and β-actin. B) RASFs were transfected with scrambled (NC) or Noxa siRNA (120 pM), followed by UA (10 μM) for 48 h. Cell viability of RASFs were determined using the MTT dye-based assay. Cell lysates from similarly treated RASFs were used to determine Noxa silencing using siRNA (right). OD, optical density; NS, non-stimulated. C) WT BMK, Noxa−/−, Noxa−/− transfected with empty vector (−/−V), and Noxa−/− cells stably expressing WT Noxa (−/−N) were treated with UA at different concentrations (10, 20, and 40 μM) for 24 or 48 h. Cellular viability was quantified by staining with sulforhodamine B. *P < 0.05.
To examine if UA-induced Noxa is critical for its ability to induce cell death, RASFs were transfected with scramble [negative control (NC)] or Noxa siRNA before exposure to UA for 48 h. As expected, UA treatment under NC siRNA induced cell death in RASFs by >50% (Fig. 2B; P < 0.05, n = 4). Interestingly, UA-induced cell death was significantly inhibited by the knockdown of Noxa in RASFs compared with the viability of the NC siRNA group (Fig. 2B; P < 0.05, n = 4), suggesting that UA heavily relies on Noxa to mediate cell death in RASFs.
Forcefully inducing Noxa overexpression in cancer cells has been a successful experimental approach in breaking down the resistance of tumor cells to undergo apoptosis (28, 29). The use of immortalized BMK cell lines that are WT, Noxa−/−, or stably transfected with Noxa plasmid included treatment with varying concentrations of UA. Without Noxa, UA was clearly unable to promote cell death, whereas only in the presence of Noxa was UA able to induce apoptosis (Fig. 2C). Overall, these results suggest that in multiple cell types, UA-mediated apoptosis depends on Noxa.
UA-induced Noxa upregulation is mediated through JNK pathway in RASFs
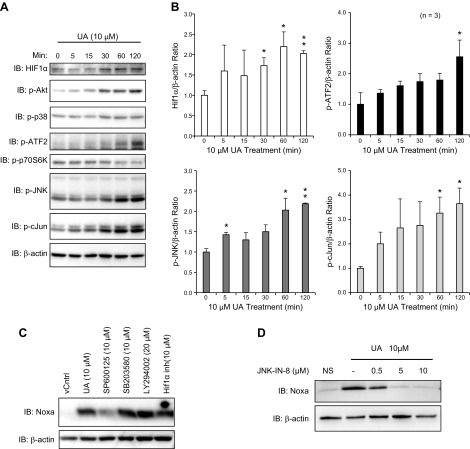
Noxa has been shown to be upregulated in cancer cells by p53 and also by HIF-1α under hypoxic conditions (27, 30). Recent studies have also suggested ATF2, ATF3, and ATF4 as potential transcription factors of Noxa expression (31, 32). To understand how Noxa is upregulated after UA treatment, we tested a variety of signaling pathways that may induce Noxa in RASFs, including hypoxia and JNK/stress-activated protein kinase pathways, as well as pathways that regulate survival, such as Akt and activation of c-Jun. Within 30–120 min after 10 μM UA treatment, UA upregulated total protein levels of HIF-1α by ∼2-fold and also induced phosphorylation of p38 and especially the p46 isoform of JNK1 (Fig. 3A, B). Downstream transcription factors, ATF2 and c-Jun, were also activated by phosphorylation at Thr69/71 and Ser73 by 2.5- and 3.5-fold, respectively. For the Akt/mammalian target of rapamycin pathway, whereas the activation loop of Ser473 of Akt was phosphorylated within 30 min and increased to 120 min after UA treatment, p70S6K was not activated at all by UA. As many pathways were activated by UA, we used chemical inhibitors, including SP600125 (JNK), SB203580 (p38), LY294002 (Akt), or the HIF-1α inhibitor, to block various pathways and examined upregulation of Noxa by UA through immunoblotting (Fig. 3C). Surprisingly, only SP600125 prevented Noxa induction by UA, indicating that Noxa is transcriptionally activated through the JNK pathway. To confirm this finding for the role of JNK in UA-induced Noxa expression, we pretreated RASFs with different concentrations (0.5–10 µM) of another highly selective chemical inhibitor of JNK (JNK-IN-8), followed by UA (10 µM) for 24 h. Western blot analysis of the cell lysates further confirmed that the inhibition of JNK completely abrogates UA-induced Noxa expression (Fig. 3D), suggesting its important role in UA-induced apoptosis.
Figure 3.
UA-mediated Noxa upregulation requires JNK pathway. A) RASFs were treated with UA (10 μM) for times indicated (5–120 min), and immunoblotting was performed with indicated antibodies. Three separate patient cells were used. p, phosphorylated. B) Densitometric analysis from experiment A was done to measure statistical differences to the control. C) RASFs were pretreated with signaling pathway inhibitors for 2 h before being treated with UA (10 μM) for 24 h and then harvested for immunoblotting. inh, inhibitor; vCntrl, vehicle control. D) RASFs were pretreated with a novel JNK inhibitor (JNK-IN-8) at different concentrations for 2 h before being treated with UA (10 μM) for 24 h and then harvested for Western blotting. Experiments were repeated using RASFs from at least 3 different patients. *P < 0.05, **P < 0.01.
Noxa expression by UA is mediated by the SP1 transcription factor
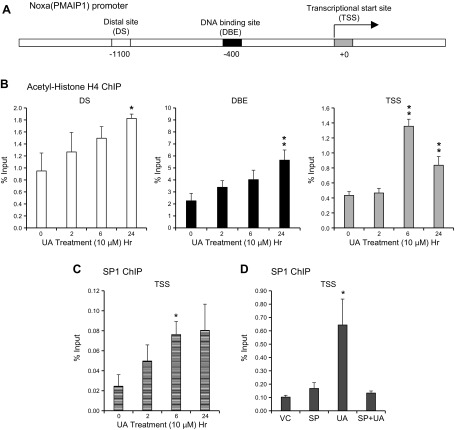
To examine further UA-mediated transcriptional activation of Noxa, we performed ChIP assays and amplified human Noxa promoter regions at the distal site (DS) and recently discovered the forkhead O1 DNA-binding element (DBE) (25), as well as the transcriptional start site (TSS), which includes c-MYC, adjacent to E2F1-binding sites (30, 33) (Fig. 4A). Interestingly, all of these sites include or are adjacent to putative SP1-binding sites. When acetylated histone H4 (K5, -8, -12, and -16) was immunoprecipitated, 2- to 3-fold enrichment was observed in all 3 sites of the Noxa promoter within 24 h of UA treatment (Fig. 4B). These results confirmed that UA treatment of RASFs induces polyacetylated chromatin signatures at the Noxa promoter region that serves as binding sites for bromo-domain-containing proteins, including chromatin remodelers, thereby opening up the chromatin for transcriptional activation of the Noxa promoter (34).
Figure 4.
Noxa expression by UA is mediated by the SP1 transcription factor. A) Graphic representation of the human Noxa promoter region. PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1. B, C) RASFs were treated with UA (10 μM) at different time points (2, 6, and 24 h), and ChIP was performed with anti-acetyl H4 antibody or anti-SP1 antibody. The results are presented as percent input, and the experiment was repeated thrice. D) RASFs were pretreated with SP600125 (SP; 10 μM) and then treated with UA (10 μM) for 24 h, and ChIP was performed with anti-SP1 antibody. VC, vehicle. The results are presented as percent input, and the experiment was repeated twice. *P < 0.05, **P < 0.01.
As the JNK pathway can activate the SP1 transcription factor (35, 36), and there are putative SP1-binding sites at DS, DBE, and TSS of the Noxa promoter, we tested whether SP1 can bind to these potential sites after UA treatment. Indeed, SP1 does bind to the Noxa promoter in RASFs upon UA treatment but only in the TSS (Fig. 4C). To ensure that SP1 binding is JNK dependent, we treated RASFs with SP600125, followed by UA stimulation. As expected, we observed a 2-fold increase in SP1 binding to TSS of the Noxa promoter, which was downregulated by SP600125 (Fig. 4D). These data confirmed that SP1 recruitment to the Noxa promoter is dependent on JNK activation.
UA promotes interaction between Mcl-1 and Noxa and induces K48-linked ubiquitination of Mcl-1
Noxa has the potential to interact selectively with Mcl-1 and facilitate its proteasomal degradation (5). However, the post-translational mechanism of this interaction is not yet known in human RASFs. First, we immunoprecipitated Mcl-1 after treatment with UA at different time points and analyzed for Noxa expression (Fig. 5A, B). As early as 6 h and most prominently, by 24 h, Noxa was pulled down with Mcl-1. The IF examination at the constitutive levels showed the presence of Mcl-1 in the cytosolic region, whereas Noxa was located mostly within the nucleus of RASFs (Fig. 5C). After UA treatment, Mcl-1 and Noxa interaction was observed primarily outside of the nucleus, as identified in large prominent dots. Finally, we also confirmed, through the PLA, that Mcl-1 and Noxa increased in interaction by ∼3-fold after UA treatment (Fig. 5D).
Figure 5.
UA promotes interaction between Noxa and Mcl-1. RASFs were treated with UA (10 μM) for different time points (6, 12, and 24 h), and IP with anti-Mcl-1 antibody was performed. A, B) Immunoblotting of input (A) and IP (B) samples is shown. The experiment was repeated thrice. RASFs that were grown on glass coverslips were treated with UA (10 μM) for 24 h and then fixed. C, D) Either IF (C) or PLA (D) was performed with anti-Mcl-1 antibody and anti-Noxa antibody. These experiments were done with cells from 3 different patients. HC, heavy chain; mIgG, ; vC, vehicle control. **P < 0.01.
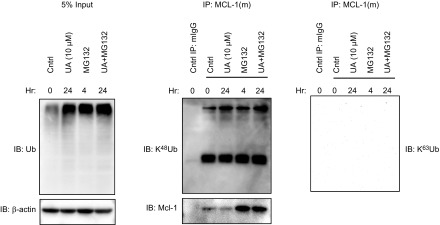
After Noxa binds to Mcl-1, E3 ligases are recruited to the complex to promote proteasomal degradation of Mcl-1. It has been shown that either Mcl-1 ubiquitin ligase E3 (Mule) or Skp, Cullin, F-box-containing complex β-transducin repeats-containing protein (β-TrCP) is recruited to ubiquitinate Mcl-1 in different cell lines (37, 38). Interestingly, in RASFs, UA treatment increased the expression of Mule (Fig. 5A) and its association with Mcl-1, as early as at 6 h of UA treatment (Fig. 5B), without altering Skp, Cullin, F-box-containing complexβ-TrCP expression. Furthermore, an IP evaluation of Mcl-1 revealed that UA enhances ubiquitination of Mcl-1, especially when under additional treatment with the proteasomal inhibitor MG-132 (Fig. 6), and that it is K48-linked ubiquitination. Taken together with data that UA treatment reduced the total protein levels of Mcl-1 in a time-dependent manner (Fig. 2B), the results indicated that UA induces proteasomal degradation of Mcl-1 by promoting Noxa expression and enhancing Noxa/Mcl-1/Mule complex formation to stimulate K48-linked ubiquitination of Mcl-1.
Figure 6.
UA induces K48-linked ubiquitination (Ub) of Mcl-1. RASFs were pretreated with MG-132 (10 μM) for 4 h, followed by UA (10 μM) treatment for 24 h. The cells were lysed in denatured conditions (1% SDS and boiling) and then diluted before IP with anti-Mcl-1 antibody. Immunoblotting was performed with anti-Ub, K48 Ub, K63 Ub, or Mcl-1 antibodies. The experiment was performed twice.
DISCUSSION
The pannus formation, by hyperproliferating RASFs in the arthritic joints, is a hallmark of RA and thus, the targeting of these cells for programmed cell death may be a potential therapeutic approach. The findings from the present study identify a novel mechanism of inducing apoptosis in RASFs to limit their invasiveness by enhancing BH3-only Noxa expression. Furthermore, we provide the molecular mechanism of Noxa induction by UA that facilitated Mcl-1 degradation to sensitize RASFs to TRAIL-induced apoptosis. These findings provide a platform to test and develop further molecules, such as UA, as an adjunct treatment option that could potentially reduce synovial hyperplasia associated with RA in an effort to minimize bone and cartilage damage.
UA is a compound that is present in wide variety of medicinal herbs and exhibits anti-inflammatory, anti-oxidative, anti-histamine, hepatoprotective, and anti-tumor effects (17). Recent studies suggest that UA uptake by Caco-2 cells is primarily through passive diffusion and was not saturated across the concentrations up to 20 µM (39). In addition, multiple intracellular, as well as extracellular, targets for UA have been identified that play role in apoptosis, angiogenesis, and inflammatory processes (40). Some of these proteins, including TRAIL, TGF-β, and PI3K/Akt, have been identified to play role in the pharmacological effects of UA (40, 41). Important relevance to RA is the fact that inhibition of these pathways has been shown to reduce inflammation and tissue remodeling in RA (1, 19, 42). Administration of UA has been shown to reduce significantly the symptoms of arthritis in preclinical models (43, 44). However, the underlying molecular mechanism of its efficacy was not clearly understood, and only immune cells were examined. In our study, we found that UA induced apoptosis in RASFs and further sensitized these cells to TRAIL-mediated apoptosis. Furthermore, even though RASFs did have a high ratio of Mcl-1:Noxa protein levels, thus making them more resistant to apoptosis, UA treatment was able to reverse this trend to induce apoptosis in RASFs. Several previous studies have identified downregulation of anti-apoptotic proteins, including Mcl-1, Bcl-xL, and Bcl-2, as a primary mechanism of its biologic activity (45–49). With the consideration that Mcl-1 prevents apoptosis by directly binding Bak or sequestering Bim, which can activate Bak to form pores for mitochondrial outer-membrane permeabilization and thus, releasing cytochrome c and caspase activation (5), downregulation of Mcl-1 is critical for cell death, especially in cells with high levels of endogenous Mcl-1, such as RASFs (19). However, these studies primarily examined transcriptional regulation of Mcl-1 by inhibition of signal transducer and activator of transcription 3 activation (45). A recent study showed that the synthetic analogs of UA can upregulate Noxa protein levels (50), but the association between Noxa upregulation and Mcl-1 downregulation remains elusive. Hence, this is the first study showing that UA-induced apoptosis in human RASFs is caused by Noxa-mediated Mcl-1 degradation.
Noxa has initially been observed to be induced by DNA damage from γ-irradiation in a p53-dependent manner, and the human Noxa promoter was discovered to contain a p53 response element (51). Subsequent studies also revealed that the Noxa promoter contained hypoxia-response element (52), c-Myc (53), E2F1 (54), forkhead box O1 (25), and Sal-like protein 2-binding sites (55). In this study, we showed that UA-mediated induction of Noxa is dependent on the JNK pathway by using the JNK-specific inhibitor, SP600125, and that JNK-mediated activation of the SP1 transcription factor binds to TSS of the Noxa promoter. Whereas our initial investigation into the Noxa promoter in RASFs focused on SP1, there are likely other transcription factors that form complexes that mediate transcriptional activation of Noxa. For example, Sal-like protein 2 and SP1 share similar DBEs and properties and may cooperatively bind together (56). Furthermore, apoptosis mediated by BNC105, a microtubule-disrupting compound, also led to JNK activation that promoted phosphorylation of ATF2 and induction of ATF3 and Noxa (31). Another study using proteasome inhibitor MG-132 for the induction of apoptosis in MEFs also discovered that JNK1 is needed to trigger cell death and required the presence of c-Myc, but the knockdown did not affect the expression of Noxa (32). However, c-Myc, along with ERα, has been shown to bind to the Noxa promoter when breast cancer has been treated with E2 (33), suggesting that c-Myc can potentially regulate Noxa expression after UA treatment in RASFs. In addition, the JNK pathway, which was triggered after apoptotic stimuli in HeLa and NIH3T3 cells, has been shown to phosphorylate c-Myc at Ser62/71 residues directly (57). Further detailed studies are required to determine whether UA-mediated Noxa expression in RASFs is also mediated by c-Myc and/or ATF2/3 in conjunction with SP1, as the Noxa promoter has a confirmed c-Myc-binding site and a potential cAMP-response element.
Mcl-1 is critical for survival of cells, which has been shown in multiple lineages and also in embryonic development (5). Human RASFs have been shown also to express high levels of Mcl-1 (20, 21), and we showed that UA treatment can ubiquitinate Mcl-1 via interaction with Noxa. As this is the first study examining the therapeutic potential of Noxa in RASF apoptosis and its interaction with Mcl-1, our efforts, using different experimental approaches, validated that UA treatment of RASFs promotes apoptosis in these cells by enhancing Noxa and Mcl-1 interaction and providing opportunity for E3 ubiquitin ligases to bind to Mcl-1 and trigger its proteasomal degradation. Among the known Mcl-l-binding E3 ligases, we found that in human RASFs, the Noxa/Mcl-1 complex facilitated the interaction with Mule and a consequent K48-linked ubiquitination of Mcl-1 leading to its proteasomal degradation.
Apoptosis has been studied in the synovium of RA patients since 1995 (58), and the targeting of human RASFs for apoptosis as a potential therapy has been proposed as early as 1997 (16, 59). However, even though much basic science research has gone into promoting apoptosis in cancer, as well as other invasive diseases, only 1 has been translated into clinics: venetoclax, a BH3 mimetic that inhibits the function of Bcl-2 for the treatment of chronic lymphocytic leukemia associated with 17-p deletion (60). In this regard, UA, as a pentacyclic triterpenoid, provides a platform for further testing as apoptosis-inducing, natural compounds for reducing synovial hyperplasia as an adjunct treatment strategy for RA.
ACKNOWLEDGMENTS
The authors thank the Cooperative Human Tissue Network (CHTN) and National Disease Research Interchange (NDRI) for providing RA synovial tissues. The authors thank Carolyn Zielinski and Maria Beamer (University of Toledo) for their technical help. This study was funded by the U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-068517 (to S.A.) and Start-Up Funds from Washington State University (to S.A.). The authors declare no conflicts of interest.
Glossary
- Akt
PKB
- ATF
activating transcription factor
- Bcl
B-cell lymphoma
- BH3
Bcl 2 homology 3
- BMK
baby mouse kidney
- ChIP
chromatin immunoprecipitation
- DBE
DNA-binding element
- DS
distal site
- FBS
fetal bovine serum
- HIF-1α
hypoxia-inducible factor 1α
- IF
immunofluorescence
- IP
immunoprecipitation
- Mcl-1
myeloid cell leukemia-1
- Mule
Mcl-1 ubiquitin ligase E3
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NC
negative control
- PARP
poly-ADP-ribose polymerase
- PLA
proximity ligation assay
- RA
rheumatoid arthritis
- RASF
RA synovial fibroblast
- RPMI
Rosewell Park Memorial Institute
- β-TrCP
β-transducin repeats-containing protein
- siRNA
small interfering RNA
- Sp1
specificity protein 1
- TRAIL
TNF-related apoptosis-inducing ligand
- TSS
transcriptional start site
- UA
ursolic acid
- WT
wild type
AUTHOR CONTRIBUTIONS
E. Y. Kim and S. Ahmed designed research and wrote the paper; E. Y. Kim performed the majority of the experiments and analyzed the data; A. K. Singh and M. Haque performed the experiments and analyzed the data; K. Sudini and D. Leaman conducted and analyzed Noxa studies in BMK cells and participated in drafting the manuscript; and S. Khuder performed statistical designing and analysis of the experimental findings.
REFERENCES
- 1.Bartok B., Firestein G. S. (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H., Pope R. M. (2004) Apoptosis in rheumatoid arthritis: friend or foe. Rheum. Dis. Clin. North Am. 30, 603–625, x [DOI] [PubMed] [Google Scholar]
- 3.Meinecke I., Rutkauskaite E., Gay S., Pap T. (2005) The role of synovial fibroblasts in mediating joint destruction in rheumatoid arthritis. Curr. Pharm. Des. 11, 563–568 [DOI] [PubMed] [Google Scholar]
- 4.Shamas-Din A., Brahmbhatt H., Leber B., Andrews D. W. (2011) BH3-only proteins: orchestrators of apoptosis. Biochim. Biophys. Acta 1813, 508–520 [DOI] [PubMed] [Google Scholar]
- 5.Mojsa B., Lassot I., Desagher S. (2014) Mcl-1 ubiquitination: unique regulation of an essential survival protein. Cells 3, 418–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelin A. M., II, Pope R. M. (2007) Myeloid cell leukemia-1 as a therapeutic target. Expert Opin. Ther. Targets 11, 363–373 [DOI] [PubMed] [Google Scholar]
- 7.Dai Y., Grant S. (2007) Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 67, 2908–2911 [DOI] [PubMed] [Google Scholar]
- 8.Cuconati A., Mukherjee C., Perez D., White E. (2003) DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17, 2922–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis S. N., Adams J. M. (2005) Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis L. S. (2003) A question of transformation: the synovial fibroblast in rheumatoid arthritis. Am. J. Pathol. 162, 1399–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grodzicky T., Elkon K. B. (2000) Apoptosis in rheumatic diseases. Am. J. Med. 108, 73–82 [DOI] [PubMed] [Google Scholar]
- 12.Huber L. C., Distler O., Tarner I., Gay R. E., Gay S., Pap T. (2006) Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 45, 669–675 [DOI] [PubMed] [Google Scholar]
- 13.Zhang H. G., Wang Y., Xie J. F., Liang X., Liu D., Yang P., Hsu H. C., Ray R. B., Mountz J. D. (2001) Regulation of tumor necrosis factor alpha-mediated apoptosis of rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis Rheum. 44, 1555–1567 [DOI] [PubMed] [Google Scholar]
- 14.Lipsky P. E. (2007) Why does rheumatoid arthritis involve the joints? N. Engl. J. Med. 356, 2419–2420 [DOI] [PubMed] [Google Scholar]
- 15.Perlman H., Pope R. M. (2010) The synovial lining micromass system: toward rheumatoid arthritis in a dish? Arthritis Rheum. 62, 643–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope R. M. (2002) Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2, 527–535 [DOI] [PubMed] [Google Scholar]
- 17.Liu J. (1995) Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 49, 57–68 [DOI] [PubMed] [Google Scholar]
- 18.Bishayee A., Ahmed S., Brankov N., Perloff M. (2011) Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 16, 980–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S., Silverman M. D., Marotte H., Kwan K., Matuszczak N., Koch A. E. (2009) Down-regulation of myeloid cell leukemia 1 by epigallocatechin-3-gallate sensitizes rheumatoid arthritis synovial fibroblasts to tumor necrosis factor alpha-induced apoptosis. Arthritis Rheum. 60, 1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Eksarko P., Temkin V., Haines G. K., III, Perlman H., Koch A. E., Thimmapaya B., Pope R. M. (2005) Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J. Immunol. 175, 8337–8345 [DOI] [PubMed] [Google Scholar]
- 21.Liu H., Huang Q., Shi B., Eksarko P., Temkin V., Pope R. M. (2006) Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 54, 3174–3181 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed S., Pakozdi A., Koch A. E. (2006) Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 54, 2393–2401 [DOI] [PubMed] [Google Scholar]
- 23.Rosebeck S., Sudini K., Chen T., Leaman D. W. (2011) Involvement of Noxa in mediating cellular ER stress responses to lytic virus infection. Virology 417, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaman D. W., Chawla-Sarkar M., Vyas K., Reheman M., Tamai K., Toji S., Borden E. C. (2002) Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J. Biol. Chem. 277, 28504–28511 [DOI] [PubMed] [Google Scholar]
- 25.Valis K., Prochazka L., Boura E., Chladova J., Obsil T., Rohlena J., Truksa J., Dong L. F., Ralph S. J., Neuzil J. (2011) Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 71, 946–954 [DOI] [PubMed] [Google Scholar]
- 26.Hafeez B. B., Ahmed S., Wang N., Gupta S., Zhang A., Haqqi T. M. (2006) Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol. Appl. Pharmacol. 216, 11–19 [DOI] [PubMed] [Google Scholar]
- 27.Cottier K. E., Fogle E. M., Fox D. A., Ahmed S. (2014) Noxa in rheumatic diseases: present understanding and future impact. Rheumatology (Oxford) 53, 1539–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas K. M., Mohana-Kumaran N., Lau D., Zhang X. D., Hersey P., Huang D. C., Weninger W., Haass N. K., Allen J. D. (2012) Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin. Cancer Res. 18, 783–795 [DOI] [PubMed] [Google Scholar]
- 29.Jeong J. H., Kim K., Lim D., Jeong K., Hong Y., Nguyen V. H., Kim T. H., Ryu S., Lim J. A., Kim J. I., Kim G. J., Kim S. C., Min J. J., Choy H. E. (2014) Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS One 9, e80050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploner C., Kofler R., Villunger A. (2008) Noxa: at the tip of the balance between life and death. Oncogene 27(Suppl 1), S84–S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates D., Feris E. J., Danilov A. V., Eastman A. (2016) Rapid induction of apoptosis in chronic lymphocytic leukemia cells by the microtubule disrupting agent BNC105. Cancer Biol. Ther. 17, 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietkiewicz S., Sohn D., Piekorz R. P., Grether-Beck S., Budach W., Sabapathy K., Jänicke R. U. (2013) Oppositional regulation of Noxa by JNK1 and JNK2 during apoptosis induced by proteasomal inhibitors. PLoS One 8, e61438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W., Swetzig W. M., Medisetty R., Das G. M. (2011) Estrogen-mediated upregulation of Noxa is associated with cell cycle progression in estrogen receptor-positive breast cancer cells. PLoS One 6, e29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filippakopoulos P., Picaud S., Mangos M., Keates T., Lambert J. P., Barsyte-Lovejoy D., Felletar I., Volkmer R., Müller S., Pawson T., Gingras A. C., Arrowsmith C. H., Knapp S. (2012) Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benasciutti E., Pagès G., Kenzior O., Folk W., Blasi F., Crippa M. P. (2004) MAPK and JNK transduction pathways can phosphorylate Sp1 to activate the uPA minimal promoter element and endogenous gene transcription. Blood 104, 256–262 [DOI] [PubMed] [Google Scholar]
- 36.Chuang J. Y., Wang Y. T., Yeh S. H., Liu Y. W., Chang W. C., Hung J. J. (2008) Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Mol. Biol. Cell 19, 1139–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Q., Gao W., Du F., Wang X. (2005) Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 38.Ding Q., He X., Hsu J. M., Xia W., Chen C. T., Li L. Y., Lee D. F., Liu J. C., Zhong Q., Wang X., Hung M. C. (2007) Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 27, 4006–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiang Z., Ye Z., Hauck C., Murphy P. A., McCoy J. A., Widrlechner M. P., Reddy M. B., Hendrich S. (2011) Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 137, 1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashyap D., Tuli H. S., Sharma A. K. (2016) Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 146, 201–213 [DOI] [PubMed] [Google Scholar]
- 41.Murakami S., Takashima H., Sato-Watanabe M., Chonan S., Yamamoto K., Saitoh M., Saito S., Yoshimura H., Sugawara K., Yang J., Gao N., Zhang X. (2004) Ursolic acid, an antagonist for transforming growth factor (TGF)-beta1. FEBS Lett. 566, 55–59 [DOI] [PubMed] [Google Scholar]
- 42.Singh A. K., Umar S., Riegsecker S., Chourasia M., Ahmed S. (2016) Regulation of transforming growth factor β-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: suppression of K(63)-linked autoubiquitination of tumor necrosis factor receptor-associated factor 6. Arthritis Rheumatol. 68, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S. Y., Yoon S. Y., Roh D. H., Jeon M. J., Seo H. S., Uh D. K., Kwon Y. B., Kim H. W., Han H. J., Lee H. J., Lee J. H. (2008) The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J. Pharm. Pharmacol. 60, 1347–1354 [DOI] [PubMed] [Google Scholar]
- 44.Baek S. Y., Lee J., Lee D. G., Park M. K., Lee J., Kwok S. K., Cho M. L., Park S. H. (2014) Ursolic acid ameliorates autoimmune arthritis via suppression of Th17 and B cell differentiation. Acta Pharmacol. Sin. 35, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak A. K., Bhutani M., Nair A. S., Ahn K. S., Chakraborty A., Kadara H., Guha S., Sethi G., Aggarwal B. B. (2007) Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 5, 943–955 [DOI] [PubMed] [Google Scholar]
- 46.Gao N., Cheng S., Budhraja A., Gao Z., Chen J., Liu E. H., Huang C., Chen D., Yang Z., Liu Q., Li P., Shi X., Zhang Z. (2012) Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 165, 1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J. H., Kwon H. Y., Sohn E. J., Kim K. A., Kim B., Jeong S. J., Song J. H., Koo J. S., Kim S. H. (2013) Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol. Rep. 65, 1366–1374 [DOI] [PubMed] [Google Scholar]
- 48.Chuang W. L., Lin P. Y., Lin H. C., Chen Y. L. (2016) The apoptotic effect of ursolic acid on SK-Hep-1 cells is regulated by the PI3K/Akt, p38 and JNK MAPK signaling pathways. Molecules 21, 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z., Jiang J., Liu X. S. (2016) Ursolic acid-mediated apoptosis of K562 cells involves Stat5/Akt pathway inhibition through the induction of Gfi-1. Sci. Rep. 6, 33358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leal A. S., Wang R., Salvador J. A., Jing Y. (2012) Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg. Med. Chem. 20, 5774–5786 [DOI] [PubMed] [Google Scholar]
- 51.Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 [DOI] [PubMed] [Google Scholar]
- 52.Kim J. Y., Ahn H. J., Ryu J. H., Suk K., Park J. H. (2004) BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J. Exp. Med. 199, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikiforov M. A., Riblett M., Tang W. H., Gratchouck V., Zhuang D., Fernandez Y., Verhaegen M., Varambally S., Chinnaiyan A. M., Jakubowiak A. J., Soengas M. S. (2007) Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc. Natl. Acad. Sci. USA 104, 19488–19493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hershko T., Ginsberg D. (2004) Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J. Biol. Chem. 279, 8627–8634 [DOI] [PubMed] [Google Scholar]
- 55.Escobar D., Hepp M. I., Farkas C., Campos T., Sodir N. M., Morales M., Álvarez C. I., Swigart L., Evan G. I., Gutiérrez J. L., Nishinakamura R., Castro A. F., Pincheira R. (2015) Sall2 is required for proapoptotic Noxa expression and genotoxic stress-induced apoptosis by doxorubicin. Cell Death Dis. 6, e1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu H., Li D., Sung C. K., Yim H., Troke P., Benjamin T. (2011) DNA-binding and regulatory properties of the transcription factor and putative tumor suppressor p150(Sal2). Biochim. Biophys. Acta 1809, 276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noguchi K., Kitanaka C., Yamana H., Kokubu A., Mochizuki T., Kuchino Y. (1999) Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase. J. Biol. Chem. 274, 32580–32587 [DOI] [PubMed] [Google Scholar]
- 58.Firestein G. S., Yeo M., Zvaifler N. J. (1995) Apoptosis in rheumatoid arthritis synovium. J. Clin. Invest. 96, 1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migita K., Eguchi K., Ichinose Y., Kawabe Y., Tsukada T., Aoyagi T., Nagataki S. (1997) Effects of rapamycin on apoptosis of rheumatoid synovial cells. Clin. Exp. Immunol. 108, 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gentile M., Petrungaro A., Uccello G., Vigna E., Recchia A. G., Caruso N., Bossio S., De Stefano L., Palummo A., Storino F., Martino M., Morabito F. (2017) Venetoclax for the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 26, 1307–1316 [DOI] [PubMed] [Google Scholar]