Abstract
Pathogenesis of alcohol-related diseases such as alcoholic hepatitis involves gut barrier dysfunction, endotoxemia, and toxin-mediated cellular injury. Here we show that Lactobacillus plantarum not only blocks but also mitigates ethanol (EtOH)-induced gut and liver damage in mice. L. plantarum blocks EtOH-induced protein thiol oxidation, and down-regulation of antioxidant gene expression in colon L. plantarum also blocks EtOH-induced expression of TNF-α, IL-1β, IL-6, monocyte chemotactic protein 1 (MCP1), C-X-C motif chemokine ligand (CXCL)1, and CXCL2 genes in colon. Epidermal growth factor receptor (EGFR) signaling mediates the L. plantarum–mediated protection of tight junctions (TJs) and barrier function from acetaldehyde, the EtOH metabolite, in Caco-2 cell monolayers. In mice, doxycycline-mediated expression of dominant negative EGFR blocks L. plantarum–mediated prevention of EtOH-induced TJ disruption, mucosal barrier dysfunction, oxidative stress, and inflammatory response in colon. L. plantarum blocks EtOH-induced endotoxemia as well as EtOH-induced pathologic lesions, triglyceride deposition, oxidative stress, and inflammatory responses in the liver by an EGFR-dependent mechanism. L. plantarum treatment after injury accelerated recovery from EtOH-induced TJ, barrier dysfunction, oxidative stress, and inflammatory response in colon, endotoxemia, and liver damage. Results demonstrate that L. plantarum has both preventive and therapeutic values in treatment of alcohol-induced tissue injury, particularly in alcoholic hepatitis.—Shukla, P. K., Meena, A. S., Manda, B., Gomes-Solecki, M., Dietrich, P., Dragatsis, I., Rao, R. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor–dependent mechanism.
Keywords: probiotics, barrier function, colon, alcoholic hepatitis, cytokines
The mechanism involved in alcoholic tissue injury is poorly understood. A significant body of evidence indicates that alcoholic liver disease (ALD) is associated with the disruption of intestinal epithelial apical junctional complexes (AJCs), mucosal barrier dysfunction, and absorption of gut bacteria-derived LPS or endotoxins from the colonic lumen into the systemic circulation (1). AJCs determine the gut barrier function that impedes diffusion of macromolecules, including LPS, from the gut lumen into mucosal tissue and systemic circulation (2, 3). AJCs comprise multiprotein complexes, the tight junction (TJ), the adherens junction (AJ) and the perijunctional actomyosin belt, to which TJ and AJ proteins adhere. Occludin, claudins, and zonula occludens (ZO)-1, ZO-2, and ZO-3 are some of the major proteins of TJ; the interactions between these proteins and the TJ integrity are regulated by the intracellular signaling mechanism (4). Interactions between E-cadherin, α-catenin, β-catenin, and γ-catenin are involved in the assembly of AJ, the integrity of which is also regulated by signal transduction (4). Alcohol consumption is known to disrupt TJ in the human intestine, and ethanol (EtOH) feeding disassembles the TJ and AJ in mice and rats (1). Acetaldehyde (the toxic metabolite of EtOH) disrupts TJ and AJ in Caco-2 cell monolayers (the cell culture model of the intestinal epithelium) by protein phosphorylation–dependent mechanisms (1). EtOH and acetaldehyde, each at subresponsive concentrations, synergistically elevate intracellular calcium and induce mitochondrial oxidative stress leading to TJ disruption and barrier dysfunction (5). Disruption of AJCs leads to diffusion of LPS from the gut lumen into the intestinal mucosa and delivered to liver via portal veins. LPS acts as a key mediator of inflammation in the liver and forms an important trigger in the development of ALD (6). Endotoxin activates Kupffer cells by a TLR-4–dependent mechanism to release inflammatory cytokines and reactive oxygen species, which are known to play key roles in alcoholic liver damage and pathogenesis of ALD.
Factors that prevent and mitigate alcoholic tissue damage are likely to have therapeutic benefit in the treatment of alcohol-related diseases. Lactobacilli, the lactic acid–producing probiotics, are promising therapeutic agents in the treatment of many gastrointestinal disorders (7). These probiotics exhibit a variety of biologic actions, including boosting mucosal immune system, balancing the gut microbiota, and diminishing oxidative stress. Evidence indicates that alcohol consumption causes dysbiosis with quantitative and qualitative alterations in gut microbiota (8). Reduction of gram-negative bacteria in rat gut by treatment with Lactobacillus rhamnosus ameliorates alcoholic liver damage (9). Whereas the mucosal protective effect of L. rhamnosus has been characterized, little is known about the potential role of L. plantarum in prevention or mitigation of alcoholic gut and liver injury. A previous pilot study in ALD patients suggested that probiotics may have beneficial effects in management of ALD (10), although an optimized formulation and treatment strategy may be required for more promising results. It is therefore crucial to understand the molecular mechanisms involved in the beneficial effect of probiotics in ALD to define the best treatment strategy. Whereas L. casei and L. rhamnosus are present in dairy products, L. plantarum is present in plant-based fermented food products. Therefore, dietary choices may determine which species of Lactobacillus is helpful to alcoholics. Additionally, L. plantarum has been shown to be a better species for vaccination with tetanus toxin fragment C than L. casei or L. lactis (11). This species has been used to develop an effective oral vaccine for Lyme disease (12) and an oral treatment that prevents severe kidney damage in mice infected with Leptospira interrogans (13).
In the present study, we evaluated the effects of L. plantarum in prevention and mitigation of EtOH-induced AJC disruption, gut permeability, endotoxemia, and liver injury in mice in vivo and Caco-2 cell monolayers in vitro. We found that L. plantarum blocks and mitigates EtOH-induced gut and liver injury by epidermal growth factor receptor (EGFR)-mediated suppression of oxidative stress and inflammatory responses.
MATERIALS AND METHODS
Chemicals
Maltose dextrin was purchased from Bioserv (Flemington, NJ, USA). Regular Lieber-DeCarli EtOH diet was purchased from Dyets (710260; Bethlehem, PA, USA). Hoechst 33342 dye and Bodipy FL-N-(2-aminoethyl)Waltham, MA, USA). N-ethylmaleimide and Tris(2-carboxyethyl)phosphine were from MilliporeSigma (Burlington, MA, USA). Alexa Fluor 488–conjugated phalloidin and all other chemicals were purchased from either MilliporeSigma or Thermo Fisher Scientific.
Antibodies
Anti–ZO-1, anti-occludin, anti–claudin-2, and anti–claudin-3 antibodies were purchased from Invitrogen (Thermo Fisher Scientific). Anti–E-cadherin and anti–β-catenin antibodies were purchased from BD Biosciences (San Jose, CA, USA). Horseradish peroxidase–conjugated anti-mouse IgG and anti-rabbit IgG and anti-actin antibodies were obtained from MilliporeSigma. Alexa Fluor 488–conjugated phalloidin, Alexa Fluor 488–conjugated anti-mouse IgG, and Cy3-conjugated anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR, USA). AG1478, SB203580 and NSC23766 were purchased from EMD Biosciences (San Diego, CA, USA).
Animals and diets
Female C57BL/6 (8–10 wk old; Harlan Laboratories, Houston, TX, USA) were used in the first part of these studies. B6;SJL-Tg(tetO-Egfr*)2-9Jek/J (EGFR*Tg) and B6.Cg-Gt(ROSA)26Sortm1(rtTA*M2)Jae/J (rtTA) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and crossbred to generate rtTA-B6;SJL-Tg(tetO-Egfr*)2-9Jek/J (rtTA-EGFR*Tg). These mice were fed doxycycline in a liquid diet to induce the EGFR* gene, which was confirmed by PCR. The rtTA-EGFR*Tg and corresponding rtTA mice were used for the second set of experiments. The rtTA-EGFR*Tg mice express a dominant negative, cytoplasmic tyrosine kinase domain–deleted mutant form of epidermal growth factor receptor (EGFR-tr) regulated by the tetracycline operator [tetO; also called tetracycline-responsive element (TRE, TetRE) or tet-operator] and cytomegalovirus minimal promoter. The expression is global but affects only those cells that express EGFR, an epithelial receptor. EGFR-tr expression disrupts subsequent activation of signal transduction pathways that are involved in regulating cell proliferation, differentiation, and survival. These tetO-EGFR-tr mice were bred to generate bitransgenic mutant mice with conditional (inducible/reversible) expression of a dominant negative epidermal growth factor (EGF) receptor, and used in studying alcohol-mediated receptor tyrosine kinase function of the EGF receptor.
All animal experiments were performed according to the protocols approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee. Animals were housed in an institutional animal care facility with 12:12 h light–dark cycles. All mice had free access to regular laboratory chow and water until the start of experiments. During the experiments, they were fed a liquid diet.
EtOH feeding
Two types of EtOH feeding models were used, chronic EtOH feeding (CEF) and chronic + binge EtOH feeding. For CEF, adult female (8–10 wk old) mice, wild type (WT), rtTA, and rtTA-EGFR*Tg, were fed Lieber-DeCarli liquid diet that contained alcohol with or without L. plantarum [strain 256, 106 colony-forming units (CFU)/ml] or isocaloric maltodextrin for 4 wk. Animals were gradually adapted to an EtOH-containing diet (0% for 2 d, 1% for 2 d, and 2% EtOH for 2 d followed by 4% EtOH for 1 wk, 5% for 1 wk, and 6% for 1 wk). Control animals were pair fed with diets with isocalorically substituted maltodextrin for EtOH. For chronic + binge EtOH feeding, WT adult female mice were fed a Lieber-DeCarli liquid diet as above but with 5% EtOH for 4 wk. During the last 3 d, animals received additional EtOH gavage (5 g/kg body weight/d). After EtOH feeding, animals were divided into 3 groups. One group was humanely killed immediately, while the other 2 groups were switched to EtOH-free diet with or without L. plantarum (106 CFU/ml) for 24 h before they were humanely killed. The pair-fed control group was included in this model.
In all experiments, animals were maintained in pairs to facilitate body temperature maintenance. At the end of the experiment, gut permeability was measured. Blood samples were collected by cardiac puncture (into heparinized tubes) and centrifuged at 3000 g for 10 min at 4°C to prepare plasma samples. Pieces (∼0.5 cm long) of distal colon were fixed in buffered formalin or cryofixed in Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA) and the remainder of the tissues was stored in RNALater for RNA isolation.
Gut permeability in vivo
At the end of each set of experiments, mice were intravenously injected with FITC-inulin (100 mg/ml solution; 2 μl/g body weight) via tail vein using a restrainer. One hour after injection, blood samples were collected by cardiac puncture under isoflurane anesthesia to prepare plasma. Mice were humanely killed by cervical dislocation under isoflurane anesthesia. Luminal contents of intestinal segments were flushed with 0.9% saline. Fluorescence in plasma and luminal flushing samples was measured using a fluorescence plate reader. Fluorescence values in the luminal flushing were normalized to fluorescence values in corresponding plasma samples and calculated as the percentage of inulin load. This in vivo method to assess intestinal mucosal permeability measures serosal-to-lumen flux of inulin, which involves no physical manipulation of the intestinal segment.
Immunofluorescence microscopy
Cryosections (10 μm thick) were fixed in acetone:methanol (1:1) at −20°C for 2 min and rehydrated in PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 10 mM disodium hydrogen phosphate, and 1.8 mM potassium dihydrogen phosphate). Sections were permeabilized with 0.2% Triton X-100 in PBS for 10 min and blocked in 4% nonfat milk in Triton-Tris buffer (150 mM sodium chloride containing 10% Tween 20 and 20 mM Tris, pH 7.4). It was then incubated for 1 h with the primary antibodies (mouse monoclonal anti-occludin and rabbit polyclonal anti–ZO-1 antibodies or mouse monoclonal E-cadherin and rabbit polyclonal anti–β-catenin antibodies), followed by incubation for 1 h with secondary antibodies (Alexa Fluor 488–conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies) and 10 min incubation with Hoechst 33342. Actin cytoskeleton was stained with Alexa Fluor 488–conjugated phalloidin. The fluorescence was examined using a Zeiss 710 confocal microscope (Carl Zeiss GmbH, Jena, Germany), and images from x–y sections (1 μm) were collected by LSM 5 Pascal software (Carl Zeiss Microscopy). Images were stacked by ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/) and processed by Adobe Photoshop (Adobe Systems, San Jose, CA, USA). All images for tissue samples from different group were collected and processed under identical conditions.
PCR
Total RNA (1.5 μg) was used for generation of cDNAs using the ThermoScript RT-PCR System for first-strand synthesis (Thermo Fisher Scientific). Quantitative PCR (qPCR) reactions were performed using cDNA mix (cDNA corresponding to 35 ng RNA) with 300 nM of primers in a final volume of 25 μl of 2 times concentrated RT2 Real-Time SYBR Green/Rox Master Mix (Qiagen, Germantown, MD, USA) in an Applied Biosystems QuatStudio 6-Flex Real-Time PCR instrument (Thermo Fisher Scientific). The cycle parameters were as follows: 50°C for 2 min, 1 denaturation step at 95°C for 10 min and 40 cycles of denaturation at 95°C for 10 s followed by annealing and elongation at 60°C. Relative gene expression of each transcript was normalized to GAPDH using the ΔΔCt method. Sequences of primers used for qPCR are provided in Table 1.
TABLE 1.
Primers used for RT-qPCR
| Primer, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| CXCL9/MIG | GGAGTTCGAGGAACCCTAGTG | GGGATTTGTAGTGGATCGTG |
| CXCL10/IP-10 | CCAAGTGCTGCCGTCATTTTC | GGCTCGCAGGGATGATTTCAA |
| CCL5/RANTES | GCTGCTTTGCCTACCTCTCC | TCGAGTGACAAACACGACTGC |
| TLR4 | ATGGCATGGCTTACACCACC | GAGGCCAATTTTGTCTCCACA |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| MCP-1/CCL2 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| CXCL1 | CTGGGATTCACCTCAAGAACATC | CAGGGTCAAGGCAAGCCTC |
| CXCL2 | CCAACCACCAGGCTACAGG | GCGTCACACTCAAGCTCTG |
| TXN1 | CATGCCGACCTTCCAGTTTTA | TTTCCTTGTTAGCACCGGAGA |
| SOD1 | AACCAGTTGTGTTGTCAGGAC | CCACCATGTTTCTTAGAGTGAGG |
| SOD2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| CAT | AGCGACCAGATGAAGCAGTG | TCCGCTCTCTGTCAAAGTGTG |
| GPX1 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| GPX3 | CCTTTTAAGCAGTATGCAGGCA | CAAGCCAAATGGCCCAAGTT |
| NRF2 | CTCGCTGGAAAAAGAAGTG | CCGTCCAGGAGTTCAGAGG |
| GAPDH | CTGCACCACCAACTGCTTAG | GGGCCATCCACAGTCTTCT |
Protein thiol assay
Protein thiols in intestinal sections were assessed as previously described (14). Reduced protein thiols were evaluated by staining cryosections of colon and ileum with Bodipy FL-N-(2-aminoethyl)maleimide and confocal microscopy at excitation and emission wavelengths of 490 and 534 nm, respectively. For oxidized protein thiols, the reduced protein thiol was first alkylated with N-ethylmaleimide, followed by reduction of oxidized protein thiols with Tris(2-carboxyethyl)phosphine before staining with FL-N-(2-aminoethyl)maleimide. Control staining is done after N-ethylmaleimide treatment. Fluorescence images were collected, and fluorescence was quantitated by ImageJ software.
Plasma endotoxin assay
Plasma endotoxin concentrations were measured using the Pierce LAL Chromogenic Endotoxin Quantitation Kit (88282; Thermo Fisher Scientific) according to the manufacturer’s instructions.
Liver histopathology
Liver and colon tissues was fixed in 10% buffered formalin, and 8 µm thick paraffin-embedded sections were stained with hematoxylin and eosin (H&E). Stained sections were imaged by a Nikon 80Ti Microscope (Nikon, Tokyo, Japan) using a ×40 objective lens and a color camera.
Plasma transaminase assay
Plasma alanine transaminase (ALT) activities were measured by colorimetric assay using ALT (700260; Cayman Chemicals, Ann Arbor, MI, USA) assay kits according to the manufacturer’s instructions.
Oil Red O staining
To detect fat deposition in the liver, frozen liver sections from both control and EtOH-treated groups were fixed for 10 min in 4% paraformaldehyde, stained with Oil Red O (MilliporeSigma), and rinsed with 60% isopropanol. Nuclei were lightly stained with hematoxylin. Images were taken by a Nikon 80Ti Microscope using a ×40 objective lens and a color camera.
Triglyceride assay
Quantitation of liver triglycerides was determined by using the glycerol phosphate oxidase method using an assay kit from Beckman Coulter (Brea, CA, USA). Liver lipids were extracted by digesting the tissue with 3 M potassium hydroxide (in 65% EtOH) for 1 h at 70°C at room temperature for 24 h. Hepatic triglycerides were measured by enzymatic hydrolysis of triglycerides to glycerol and free fatty acids followed by colorimetric measurement (at 540 nm wavelength) of glycerol. Values for hepatic triglycerides were expressed as mg triglyceride/g liver tissue.
Cell culture
Caco-2bbe2 cells (American Type Culture Collection, Manassas, VA, USA, Manassas, VA, USA) were grown under standard cell culture conditions as previously described (5). Experiments were conducted using cells grown in Transwell inserts of varying diameters (6.5 or 12 mm; Corning, Corning, NY, USA) for 10 to 12 d.
Cell treatments
Cell monolayers in Transwell inserts were incubated with acetaldehyde (400 μM) with or without live or dead L. plantarum (103–105 CFU/ml). L. plantarum was killed by heating at 100°C for 10 min and confirmed by culture of the treated samples. In some experiments, cell monolayers were treated with AG1478, SB203580 (0–0.1 μM), and NSC23766 (0–100 μM).
Epithelial barrier function
Transepithelial electrical resistance (TER) was measured using a Millicell-ERS Electrical Resistance System (MilliporeSigma), and macromolecular permeability was evaluated by measuring unidirectional flux of FITC-inulin as previously described (5). The basal TER values for Caco-2 cell monolayers in these experiments were 400 to 500 Ω/cm2.
Statistical analyses
All data are expressed as means ± sem. The differences among multiple groups were first analyzed by ANOVA by GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA). When a statistical significance was detected, Tukey’s t test was used to determine the statistical significance between multiple testing groups and the corresponding control. Statistical significance was established at 95%.
RESULTS
L. plantarum attenuates alcohol-induced disruption of mouse colonic epithelial AJCs, mucosal barrier dysfunction, and endotoxemia
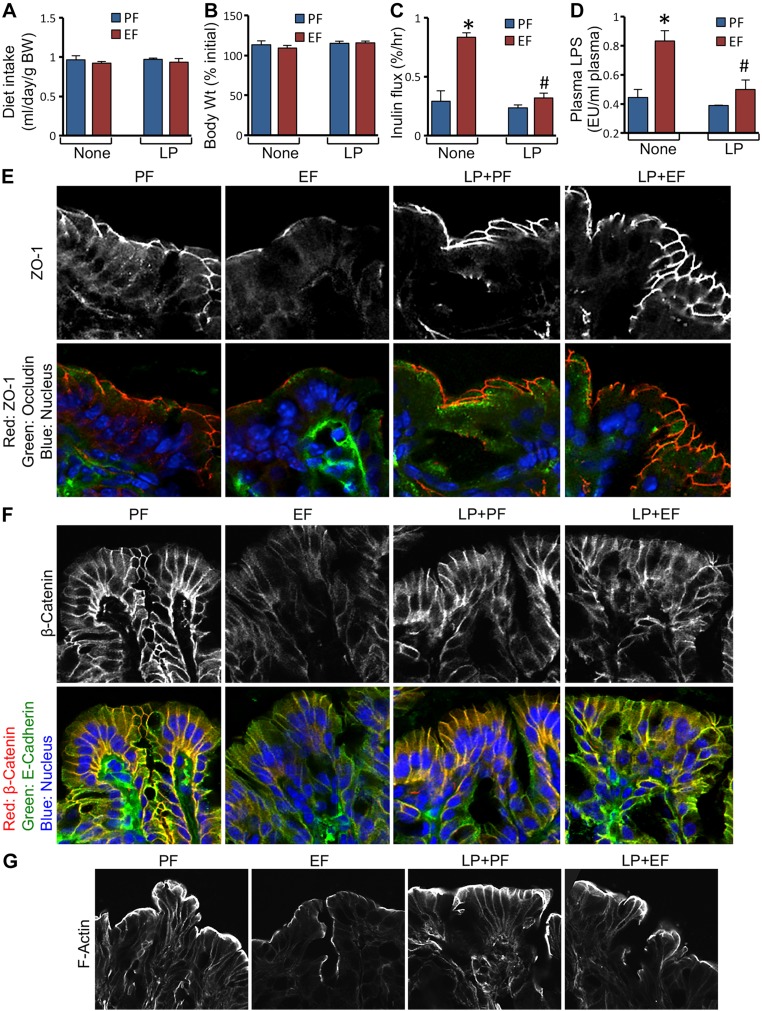
We used a mouse model of CEF to determine the effect of dietary supplementation of L. plantarum on EtOH-induced mucosal barrier dysfunction and disruption of epithelial junctions. Similar diet intake was maintained by pair feeding of mice in different groups (Fig. 1A). There were no significant differences between the groups in their body weight changes (Fig. 1B). Mucosal permeability to FITC-inulin in vivo was nearly 3-fold higher in EtOH fed mice compared to pair-fed control mice (Fig. 1C). EtOH feeding, however, failed to increase inulin permeability when diet was supplemented with L. plantarum. EtOH feeding significantly elevated plasma LPS levels, which was attenuated by L. plantarum in the diet (Fig. 1D). EtOH caused redistribution of TJ proteins (ZO-1 and occludin) from the colonic epithelial junctions in the absence of L. plantarum, but not in the presence of this probiotic (Fig. 1E). Similarly, EtOH triggered redistribution of AJ proteins (E-cadherin and β-catenin) (Fig. 1F) and disruption of actin cytoskeleton (Fig. 1G) in the colonic epithelium in the absence of L. plantarum, but not in the presence of it.
Figure 1.
L. plantarum prevents CEF-induced barrier dysfunction and disruption of AJCs in mouse colonic epithelium. Adult female mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF) and with or without L. plantarum (106 CFU/ml) (LP). Diet intake (A) and body weight (B) were recorded. Colonic mucosal permeability in vivo (C) and plasma LPS levels (D) were measured. Cryosections of distal colon were stained for TJ proteins (E), AJ proteins (F), and actin cytoskeleton (G). Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
Alcohol induces oxidative stress and down-regulates antioxidant gene expression, and L. plantarum blocks these alcohol effects
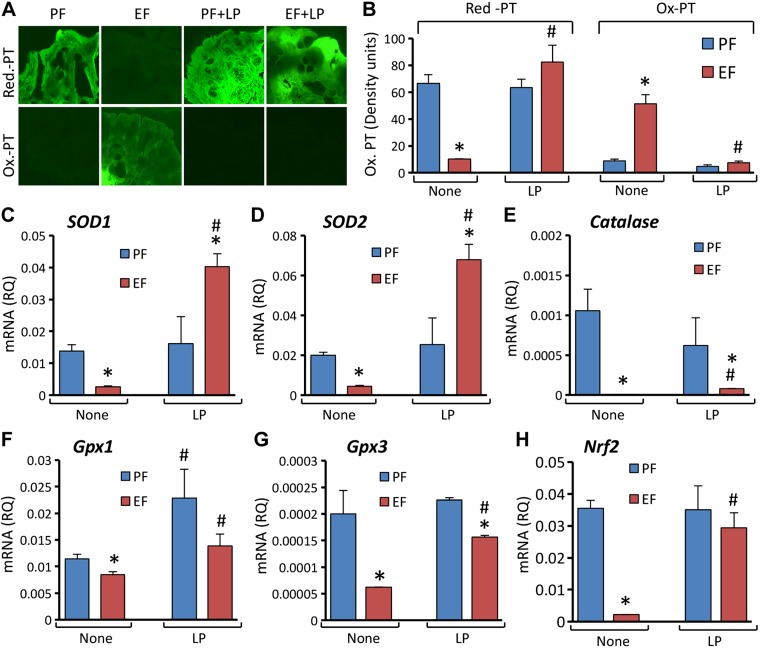
Our recent study showed that EtOH and acetaldehyde induces a synergistic production of reactive oxygen species in Caco-2 cell monolayers (5). Chronic alcohol consumption is known to induce oxidative stress in multiple organs in vivo (15, 16). We evaluated the effect of L. plantarum on EtOH-induced oxidative stress and antioxidant gene expression in mouse colon. EtOH feeding caused a dramatic depletion of reduced-protein thiols in mouse colon with a concomitant increase in oxidized-protein thiols (Fig. 2A). However, EtOH had no effect on the levels of reduced or oxidized-protein thiols in mice fed an L. plantarum–supplemented diet. Quantitative analyses confirmed that EtOH-induced protein thiol oxidation is absent in L. plantarum–fed mice (Fig. 2B). The effect of EtOH on antioxidant gene expression in mouse colon is poorly understood, and we therefore evaluated the effect of EtOH on antioxidant gene expression with or without L. plantarum supplementation. Our data showed that EtOH feeding significantly reduced the levels of mRNA for superoxide dismutase 1 (SOD1; Fig. 2C), SOD2 (Fig. 2D), Catalase (Fig. 2E), and glutathione peroxidase 1 (Gpx1; Fig. 2F) and Gpx3 (Fig. 2G) genes in mouse colon. L. plantarum feeding significantly blocked these effects of EtOH on antioxidant gene expression. Interestingly, SOD1 and SOD2 mRNA levels in the colon of L. plantarum–supplemented EtOH-fed mice were several fold greater than those in pair-fed mice without L. plantarum supplementation. Nuclear factor–like 2 (Nrf2) is an important transcription factor that plays a role in regulation of antioxidant gene expression. EtOH feeding caused a dramatic reduction in mRNA for the Nrf2 gene in colonic mucosa (Fig. 2H), and L. plantarum effectively blocked this effect of EtOH.
Figure 2.
L. plantarum attenuates CEF-induced protein thiol oxidation and suppression of antioxidant gene expression in mouse colon. Adult female mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF) and with or without L. plantarum (106 CFU/ml) (LP). Cryosections of distal colon were stained for reduced and oxidized protein thiols (A) and fluorescence densities quantitated (B). RNA extracted from colonic mucosa was subjected to qPCR for SOD1 (C), SOD2 (D), Catalase (E), Gpx1 (F), Gpx3 (G), and Nrf2 (H) genes. Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
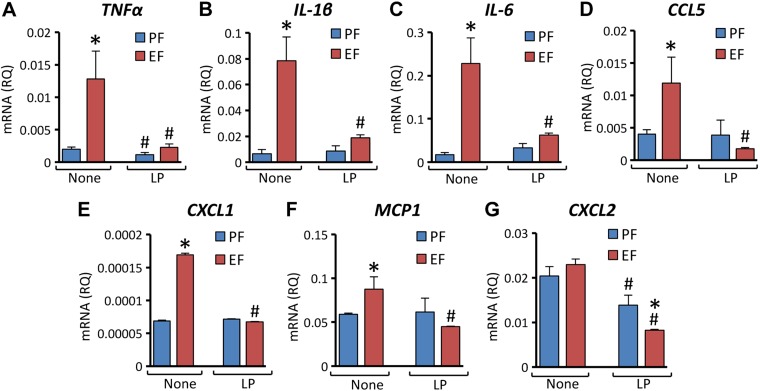
L. plantarum blocks alcohol-induced expression of inflammatory cytokines and chemokines in mouse colon
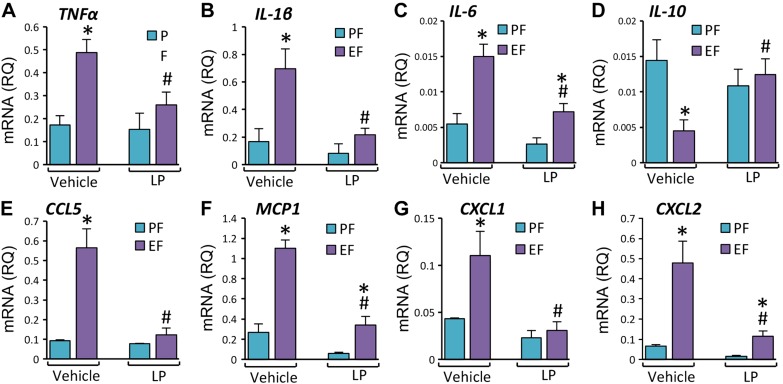
Chronic alcohol consumption is known to induce inflammation in multiple organs (14, 17, 18). However, the effect of EtOH feeding on expression of proinflammatory cytokines in the colon are poorly understood. We evaluated the effects of EtOH and L. plantarum on mRNA for several cytokines in mouse colon. EtOH feeding elevated TNF-α (Fig. 3A), IL-1β (Fig. 3B), and IL-6 (Fig. 3C) mRNA by 7- to 15-folds, and dietary supplementation of L. plantarum completely blocked these effects of EtOH. Because there is no information available regarding the effect of EtOH or L. plantarum on chemokine gene expression in colon, we evaluated the levels of mRNA for a few proinflammatory chemokines. EtOH feeding elevated chemokine ligand 5 (CCL5; Fig. 3D), C-X-C motif chemokine ligand 1 (CXCL1; Fig. 3E), and monocyte chemotactic protein 1 (MCP1; Fig. 3F) mRNA, which was completely blocked by L. plantarum in the diet. CXCL2 mRNA was unaffected by EtOH, but L. plantarum significantly reduced CXCL2 mRNA in the presence or absence of EtOH in the diet (Fig. 3G).
Figure 3.
L. plantarum attenuates CEF-induced expression of proinflammatory cytokines and chemokines in mouse colon. Adult female mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF) and with or without L. plantarum (106 CFU/ml) (LP). RNA extracted from colonic mucosa was subjected to qPCR for TNF-α (A), IL-1β (B), IL-6 (C), Rantes/CCL5 (D), CXCL1 (E), MCP1 (F) and CXCL2 (G) genes. Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
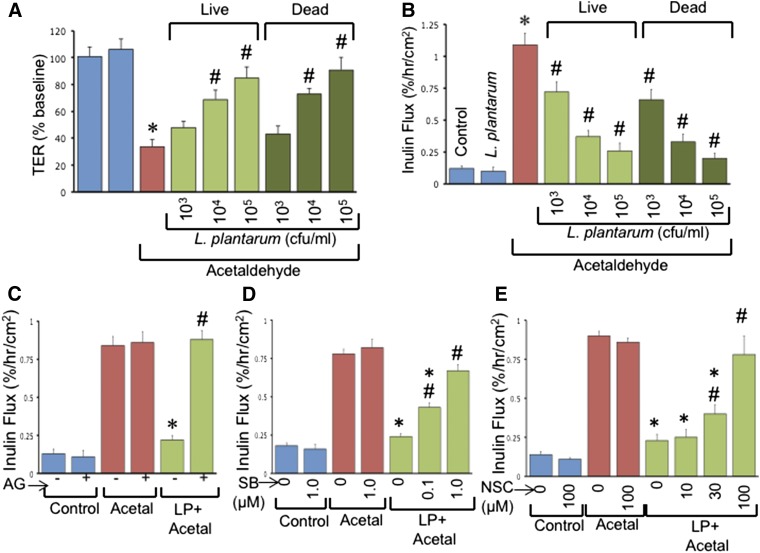
L. plantarum blocks acetaldehyde-induced barrier dysfunction in Caco-2 cell monolayers by EGFR-dependent mechanism
To determine the direct interaction of L. plantarum with the intestinal epithelium, we evaluated the effect of L. plantarum on acetaldehyde-induced barrier dysfunction in Caco-2 cell monolayers grown on Transwell inserts. Our previous studies indicated that acetaldehyde but not EtOH disrupts barrier function in Caco-2 cell monolayers because these cells do not express alcohol dehydrogenase. Administration of live or UV-treated L. plantarum 30 min before administration of acetaldehyde dose-dependently blocked acetaldehyde-induced decrease in TER (Fig. 4A) and increased inulin permeability (Fig. 4B). Pretreatment with AG1478 (an EGFR tyrosine kinase inhibitor) did not affect inulin flux in control or acetaldehyde-treated cell monolayers, but it effectively blocked L. plantarum–mediated prevention of acetaldehyde-induced inulin permeability (Fig. 4C). Rac1 and p38-MAP kinase are some of the important signaling elements downstream of EGFR activation. Treatment of cell monolayers with SB203580 (p38-MAP kinase inhibitor) (Fig. 4D) or NSC23766 (Rac1 inhibitor) (Fig. 4E) also dose-dependently attenuated L. plantarum effect on acetaldehyde-induced inulin permeability.
Figure 4.
L. plantarum prevents acetaldehyde-induced barrier dysfunction in Caco-2 cell monolayers by EGFR-dependent mechanism. A, B) Caco-2 cell monolayers were pretreated with or without live or UV-treated L. plantarum (LP) 30 min before incubation with acetaldehyde. After 4 h, TER (A) and inulin permeability (B) were measured. Values are means ± sem (n = 4). *P < 0.05, significantly different from corresponding control values; #value different from corresponding acetaldehyde values. C–E) Caco-2 cell monolayers were pretreated with or without AG1478 (C), SB203580 (D), or NSC23766 (E) 30 min before L. plantarum (106 CFU/ml) treatment. Acetaldehyde (Acetal) was administered 30 min after LP and inulin permeability measured after 4 h. Values are means ± sem (n = 4). *P < 0.05, significantly different from corresponding acetaldehyde values; #LP + acetal + inhibitor values different from corresponding LP + acetal values.
EGFR plays a role in L. plantarum–mediated prevention of alcohol-induced barrier dysfunction and AJC disruption in mouse colonic epithelium
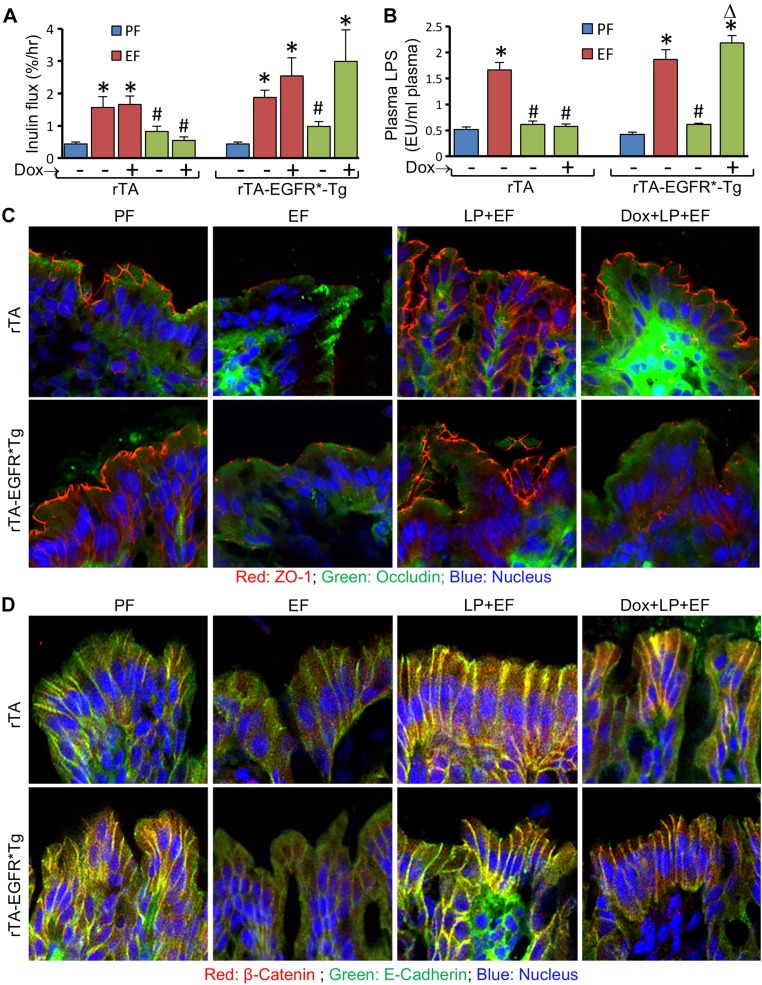
The results of studies in Caco-2 cell monolayers suggest that EGFR tyrosine kinase is involved in L. plantarum–mediated prevention of acetaldehyde-induced barrier dysfunction. To determine the role of EGFR in L. plantarum effect in vivo, we performed a CEF experiment in rtTA-EGFR*Tg mice where expression of dominant negative EGFR is induced by doxycycline administration. Similar to its effects in WT mice, L. plantarum supplementation blocked EtOH-induced inulin permeability in the absence or presence of doxycycline in rtTA mice (Fig. 5A). However, in rtTA-EGFR*Tg mice, L. plantarum blocked EtOH-induced colonic mucosal permeability to inulin in the absence, but not in the presence, of doxycycline. We measured plasma LPS levels to evaluate alcoholic endotoxemia and its prevention by L. plantarum. L. plantarum blocked EtOH-induced elevation of plasma LPS in rtTA mice in the presence or absence of doxycycline (Fig. 5B). However, in rtTA-EGFR*Tg mice, L. plantarum was effective in reducing plasma LPS only in the absence of doxycycline. EtOH-induced redistribution of TJ (Fig. 5C) and AJ (Fig. 5D) proteins from the colonic epithelial junctions was blocked by L. plantarum in rtTA mice in the presence or absence of doxycycline. However, in rtTA-EGFR*Tg mice, L. plantarum blocked EtOH-induced redistribution of TJ and AJ proteins in the absence but not in the presence of doxycycline.
Figure 5.
Expression of dominant negative EGFR attenuates L. plantarum–mediated prevention of CEF-induced barrier dysfunction and disruption of AJCs in mouse colonic epithelium. Adult rtTA and rtTA-EGFR*-Tg mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF), with or without L. plantarum (106 CFU/ml) (LP) and with or without doxycycline (Dox). Colonic mucosal permeability to inulin in vivo (A) and plasma LPS levels (B) were measured as described in Materials and Methods. Cryosections of distal colon were stained for TJ (C) and AJ (D) proteins. Values are means ± sem (n = 4 for PF and EF and 6 for LP + EF and Dox + LP + EF groups). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
EGFR is required for L. plantarum–mediated prevention of alcohol-induced oxidative stress and inflammatory responses in mouse colonic epithelium
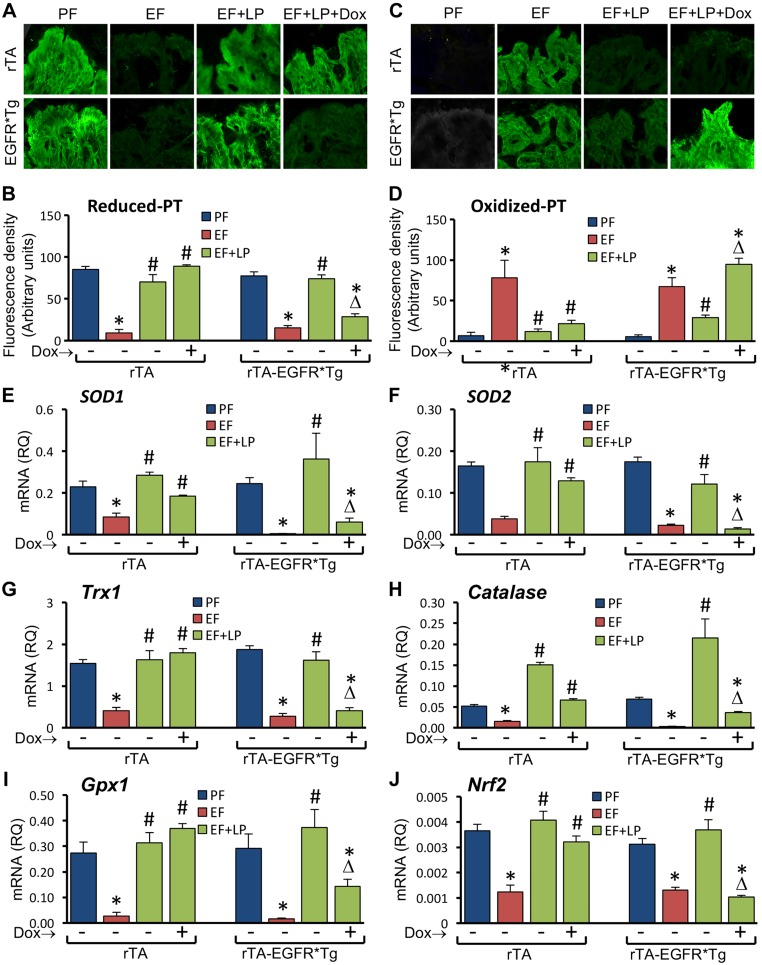
L. plantarum supplementation blocked EtOH-induced depletion of reduced-protein thiols (Fig. 6A, B) and elevation of oxidized-protein thiols (Fig. 6C, D) in colonic mucosa in the presence or absence of doxycycline in rtTA mice. In rtTA-EGFR*Tg mice, L. plantarum blocked EtOH-induced protein thiol oxidation in the absence but not in the presence of doxycycline. Similar to its effects in WT mice, L. plantarum attenuated EtOH-induced depletion of mRNA for SOD1 (Fig. 6E), SOD2 (Fig. 6F), Trx1 (Fig. 6G), Catalase (Fig. 6H), Gpx1 (Fig. 6I), and Nrf2 (Fig. 6J) in rtTA mice in the presence or absence of doxycycline. However, in rtTA-EGFR*Tg mice, L. plantarum blocked EtOH-induced reduction of mRNA for antioxidant genes in the absence but not in the presence of doxycycline.
Figure 6.
Expression of dominant negative EGFR attenuates L. plantarum–mediated prevention of CEF-induced protein thiol oxidation and suppression of antioxidant gene expression in mouse colon. Adult rtTA and rtTA-EGFR*-Tg mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF), with or without L. plantarum (106 CFU/ml) (LP) and with or without doxycycline (Dox). Cryosections of distal colon were stained for reduced and oxidized protein thiols (A, C) and fluorescence densities quantitated (B, D). RNA extracted from colonic mucosa was subjected to qPCR for SOD1 (E), SOD2 (F), Trx1 (G), Catalase (H), Gpx1 (I), and Nrf2 (J) genes. Values are means ± sem (n = 4 for PF and EF and 6 for LP + EF and Dox + LP + EF groups). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values; Δvalue different from corresponding values for EF + LP without Dox.
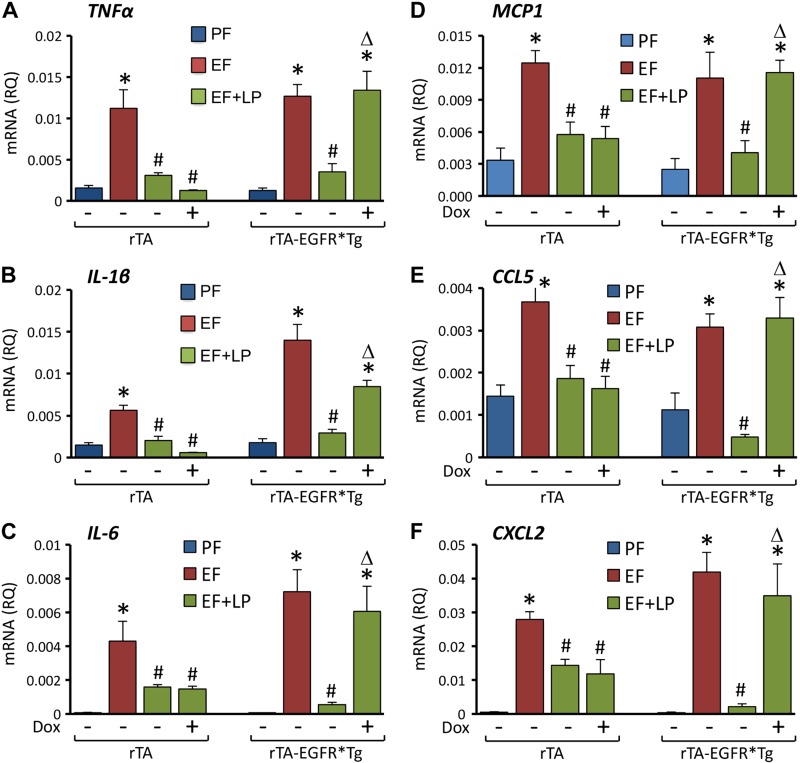
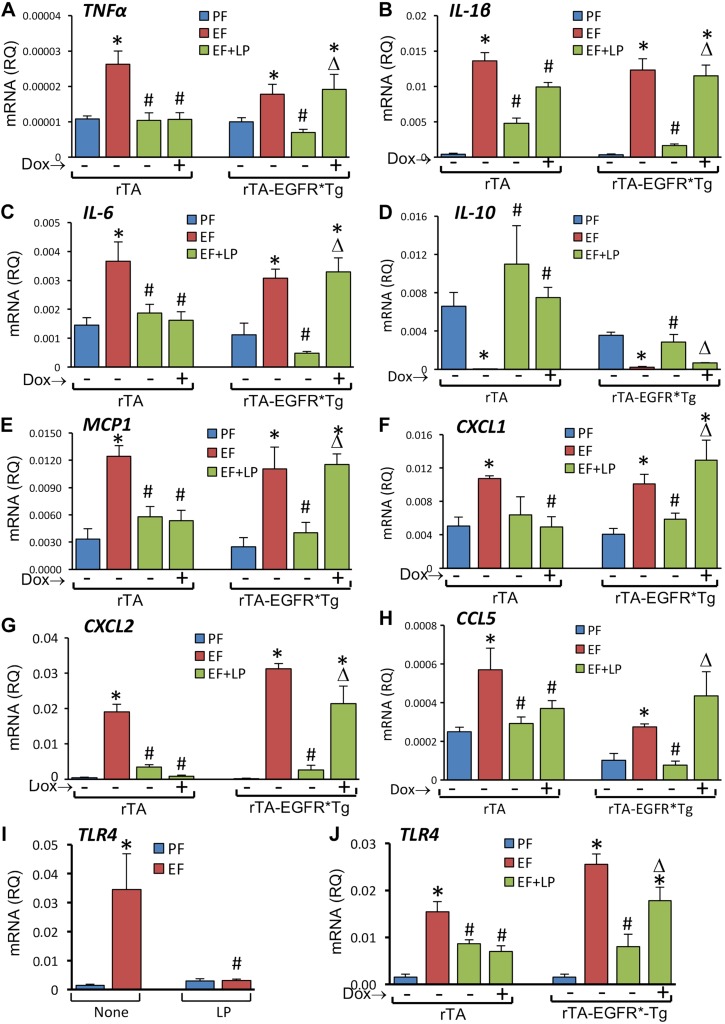
L. plantarum supplementation blocked EtOH-induced elevation of mRNA for TNF-α (Fig. 7A), IL-1β (Fig. 7B), IL-6 (Fig. 7C), MCP1 (Fig. 7D), CCL5 (Fig. 7E), and CXCL2 (Fig. 7F) genes in the colon of rtTA mice in the presence or absence of doxycycline. In rtTA-EGFR*Tg mice, L. plantarum blocked EtOH-induced cytokine and chemokine mRNA elevation in the absence but not in the presence of doxycycline.
Figure 7.
Expression of dominant negative EGFR attenuates L. plantarum–mediated prevention of CEF-induced expression of inflammatory cytokine and chemokine genes in mouse colon. Adult rtTA and rtTA-EGFR*-Tg mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF), with or without L. plantarum (106 CFU/ml) (LP) and with or without doxycycline (Dox). RNA extracted from colonic mucosa was subjected to qPCR for TNF-α (A), IL-1β (B), IL-6 (C), MCP1 (D), CCL5 (E), and CXCL2 (F) genes. Values are means ± sem (n = 4 for PF and EF and 6 for LP + EF and Dox + LP + EF groups). *P < 0.05, significatnly different from corresponding PF values; #value different from corresponding EF values; Δvalue different from corresponding values for EF + LP without Dox.
L. plantarum attenuates alcohol-induced liver damage by EGFR-dependent mechanism
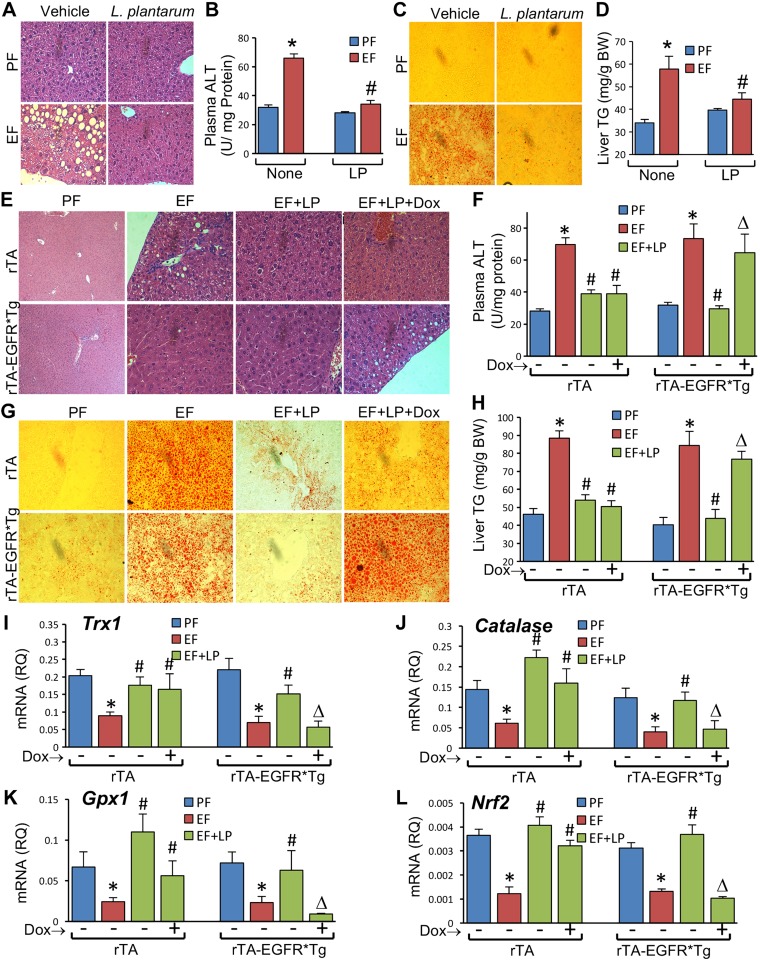
Chronic alcohol consumption is known to affect the gut–liver axis. Alcohol-induced gut permeability plays an important role in alcoholic liver damage. We examined the effect of L. plantarum on EtOH-induced liver damage and the potential role of EGFR in this effect of L. plantarum. Histopathologic examination revealed a chicken-wire pattern in the liver of EtOH-fed mice, similar to the symptoms of steatohepatitis. Liver injury was not present in mice fed an L. plantarum–supplemented diet (Fig. 8A). EtOH-induced liver injury was associated with a significant elevation of plasma ALT activity, which was completely blocked by L. plantarum supplementation (Fig. 8B). Staining liver sections with Oil Red O indicated that EtOH feeding caused deposition of lipid droplets in liver, which was attenuated by L. plantarum supplementation (Fig. 8C). L. plantarum–mediated prevention of EtOH-induced fat deposition in the liver was confirmed by measuring liver triglyceride levels (Fig. 8D). Similar to its effects in WT mice, L. plantarum attenuated EtOH-induced liver injury in rtTA mice in the presence or absence of doxycycline (Fig. 8E). However, in rtTA-EGFR*Tg mice, L. plantarum attenuated EtOH-induced liver histopathology in the absence but not in the presence of doxycycline. This was supported by similar effects of L. plantarum on plasma ALT activity in rtTA and rtTA-EGFR*Tg mice (Fig. 8F). Both fat deposition (Fig. 8G) and elevation of liver triglyceride content (Fig. 8H) caused by EtOH feeding was attenuated by L. plantarum in rtTA mice with or without doxycycline, but L. plantarum was effective only in the absence of doxycycline in rtTA-EGFR*Tg mice.
Figure 8.
L. plantarum attenuates CEF-induced liver damage by EGFR-dependent mechanism. A–D) Adult female mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF) and with or without L. plantarum (106 CFU/ml) (LP). Paraffin-embedded sections of formalin-fixed liver were stained with H&E (A). Plasma was assayed for ALT activity (B). Cryosections of liver were stained with Oil Red O (C) and liver extracts assayed for triglyceride (TG) (D). Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values. E–L) Adult rtTA and rtTA-EGFR*-Tg mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF), with or without L. plantarum (106 CFU/ml) (LP) and with or without doxycycline (Dox). Paraffin-embedded sections of formalin-fixed liver were stained with H&E (E). Plasma was assayed for ALT activity (F). Cryosections of liver were stained with Oil Red O (G) and liver extracts assayed for triglyceride (TG) (H). RNA extracted from liver was subjected to qPCR for Trx1 (I), Catalase (J), Gpx1 (K), and Nrf2 (L) genes. Values are means ± sem (n = 4 for PF and EF and 6 for LP + EF and Dox + LP + EF groups). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values; Δvalue different from corresponding values for EF + LP without Dox.
Data indicate that EtOH feeding significantly reduced the mRNA for Catalase (Fig. 8J), Gpx1 (Fig. 8K), Trx1 (Fig. 8I), and Nrf2 (Fig. 8L) genes in the liver. Dietary supplementation of L. plantarum blocked these effects of EtOH. L. plantarum attenuated EtOH-induced depletion of Trx1 (Fig. 8I), Catalase (Fig. 8J), Gpx1 (Fig. 8K), and Nrf2 (Fig. 8L) mRNA in the liver of rtTA mice in the absence or presence of doxycycline. In rtTA-EGFR*Tg mice, however, L. plantarum failed to block EtOH-induced depletion of mRNA for antioxidant genes in the presence of doxycycline.
EGFR is required for L. plantarum–mediated attenuation of alcohol-induced expression of cytokine and chemokine genes in liver
EtOH feeding increased the levels of mRNA for TNF-α (Fig. 9A), IL-1β (Fig. 9B), IL-6 (Fig. 9C), CCL5 (Fig. 9E), MCP1 (Fig. 9F), CXCL1 (Fig. 9G), and CXCL2 (Fig. 9H) genes in the liver, which was blocked by L. plantarum in the diet. The level of IL-10 mRNA, however, was reduced by EtOH (Fig. 9D), and this effect of EtOH was blocked by L. plantarum. L. plantarum attenuated EtOH-induced elevation of TNF-α (Fig. 10A), IL-1β (Fig. 10B), IL-6 (Fig. 10C), IL-10 (Fig. 10D), MCP1 (Fig. 10E), CXCL1 (Fig. 10F), CXCL2 (Fig. 10G), and CCL5 (Fig. 10H) mRNA in rtTA mouse liver in the absence or presence of doxycycline. In rtTA-EGFR*Tg mice, however, L. plantarum failed to block EtOH-induced elevation of mRNA for these cytokine and chemokine genes in the presence of doxycycline.
Figure 9.
L. plantarum attenuates CEF-induced cytokine and chemokine gene expression in liver. Adult female mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF) and with or without L. plantarum (106 CFU/ml) (LP). RNA extracted from liver was subjected to qPCR for TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), CCL5 (E), MCP1 (F), CXCL1 (G), and CXCL2 (H) genes. Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
Figure 10.
L. plantarum attenuates CEF-induced cytokine, chemokine, and TLR-4 gene expression in liver by EGFR-dependent mechanism. A–H) Adult rtTA and rtTA-EGFR*-Tg mice were fed liquid diet with EtOH (EF) or isocaloric maltodextrin (PF), with or without L. plantarum (106 CFU/ml) (LP) and with or without doxycycline (Dox). RNA extracted from liver was subjected to qPCR for TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), MCP1 (E), CXCL1 (F), CXCL2 (G), and CCL5 (H) genes. I, J) TLR4 mRNA (M) was evaluated in liver of WT mice (I) or rtTA mice with or without EGFR* expression (J). Values are means ± sem (n = 4 for PF and EF and 6 for LP + EF and Dox + LP + EF groups). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values; Δvalue different from corresponding values for EF + LP without Dox.
Endotoxemia plays a crucial role in initiating alcoholic damage in the liver and other organs (1). Intestinal microflora is the main source of plasma endotoxins in alcoholics. Our data revealed that EtOH feeding elevates plasma LPS and that L. plantarum prevents this effect of EtOH by an EGFR-dependent mechanism. LPS exposure has been shown to increase the expression of its receptor, TLR4, in different tissues, including the liver (19, 20). Therefore, we evaluated TLR4 mRNA levels in liver. Data indicated that EtOH feeding causes a marked increase in TLR4 mRNA in the liver, which was completely attenuated by L. plantarum in the diet (Fig. 10I). L. plantarum significantly reduced EtOH-induced elevation of TLR4 mRNA in the liver of rtTA mice, and doxycycline had no influence on this effect of L. plantarum (Fig. 10J). In rtTA-EGFR*Tg mice, however, L. plantarum failed to block EtOH-induced elevation of liver TLR4 mRNA in the presence of doxycycline.
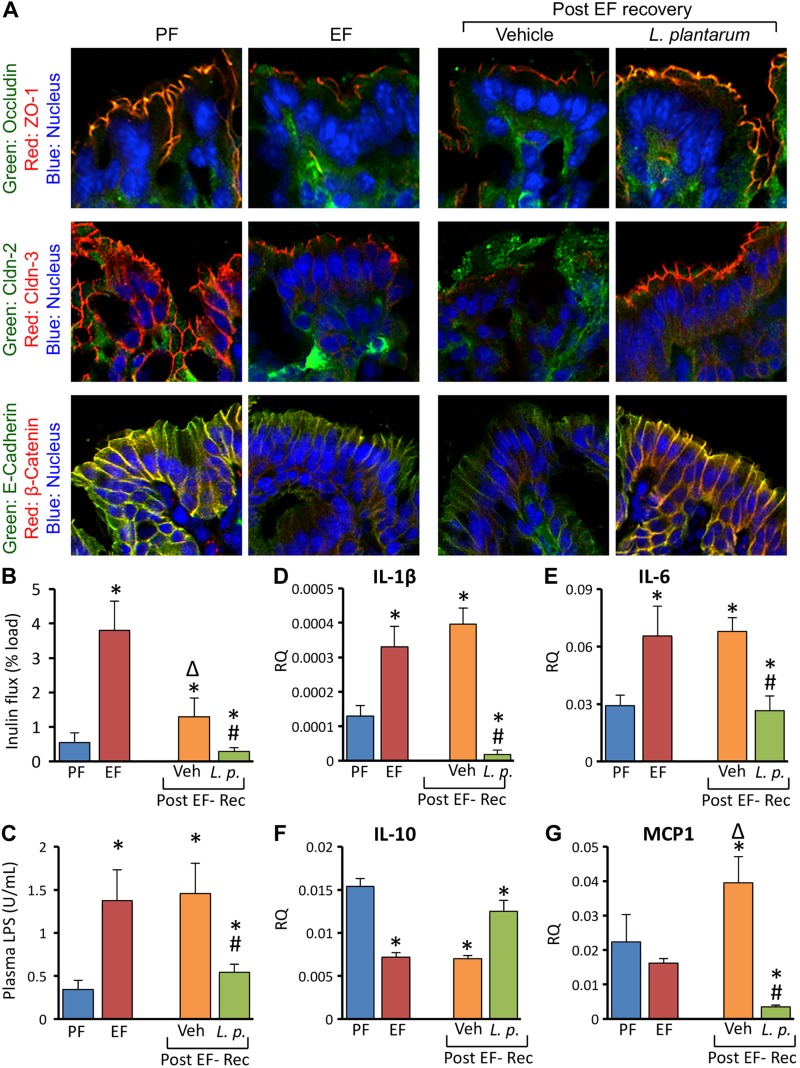
L. plantarum accelerates restitution of alcohol-induced mucosal damage in mouse colon
In order to determine the effect of L. plantarum on recovery from EtOH-induced colonic mucosal damage, we applied a chronic + binge mouse model of EtOH feeding. Mice were fed a 5% EtOH diet for 4 wk with an additional daily gavage of EtOH (5 g/kg body weight) provided during the last 3 d. After EtOH feeding, mice were fed EtOH-free diet with or without L. plantarum for 24 h. Redistribution of TJ and AJ proteins from the junctions continued to be present even after 24 h after receiving an EtOH-free diet in the absence of L. plantarum (Fig. 11A). However, in mice fed an L. plantarum–supplemented diet, junctional organization of TJ and AJ proteins was completely restored in 24 h. EtOH-induced colonic mucosal permeability was partially reduced by the EtOH-free diet in the absence of L. plantarum, but the permeability was lower than that in pair-fed control mice when EtOH-free diet was supplemented with L. plantarum (Fig. 11B). Plasma LPS level was not reduced by EtOH-free diet in the absence of L. plantarum, but the EtOH-free diet in the presence of L. plantarum significantly reduced plasma LPS level (Fig. 11C). EtOH-induced elevation of IL-1β (Fig. 11D) and IL-6 (Fig. 11E) and reduction of IL-10 (Fig. 11F) mRNA in colonic mucosa was unaffected by feeding animals an EtOH-free diet without L. plantarum. On the other hand, L. plantarum supplementation of the diet completely restored the normal levels of these cytokines in 24 h. MCP1 mRNA in colonic mucosa was not elevated during EtOH-free diet feeding, but it was significantly reduced to lower than basal levels by L. plantarum supplementation (Fig. 11G).
Figure 11.
L. plantarum accelerates restitution of CEF-induced mucosal damage in mouse colon. Adult female mice were fed liquid diet with 5% EtOH (EF) or isocaloric maltodextrin (PF) for 4 wk, and EF mice received additional gavage of EtOH (g/body weight) once daily during last 3 d. EF mice were then fed EtOH-free diet with (L.p.) or without (Veh) L. plantarum (106 CFU/ml) for 24 h for recovery from EtOH damage (Post EF-Rec). Cryosections of distal colon were stained for TJ and AJ proteins (A). Colonic mucosal permeability in vivo (B) and plasma LPS levels (C) were measured. RNA extracted from liver was subjected to qPCR for IL-1β (D), IL-6 (E), IL-10 (F), and MCP1 (G) genes. Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values; Δvalue significantly different from corresponding EF value.
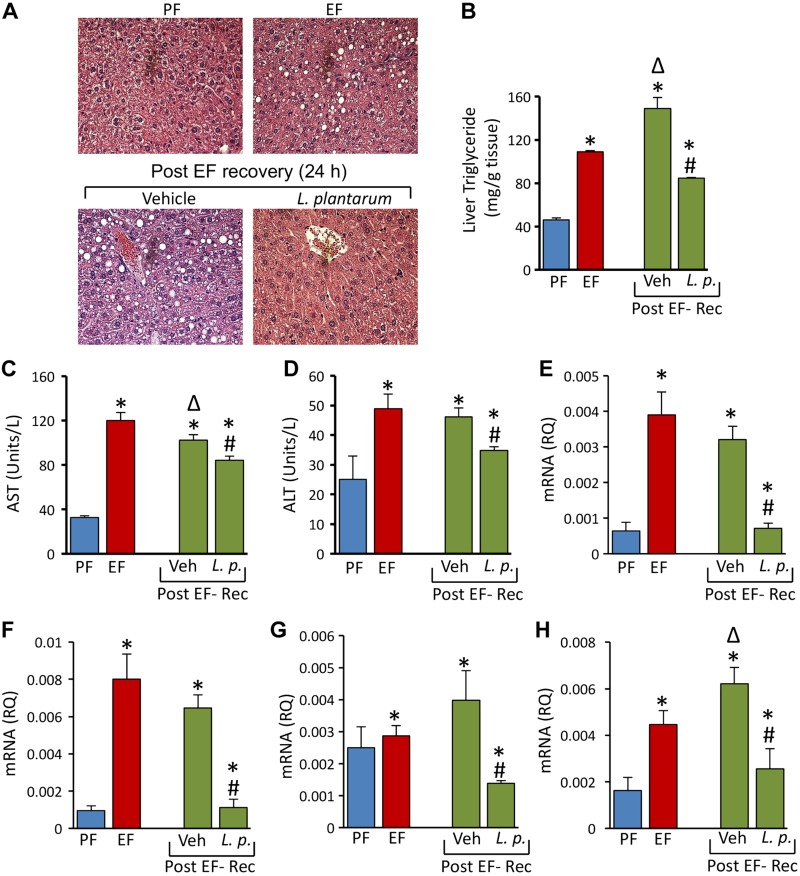
L. plantarum accelerates healing of alcohol-induced liver damage
Histopathologic evaluation of liver showed no recovery from liver damage by an EtOH-free diet in the absence of L. plantarum (Fig. 12A). In fact, the liver triglyceride content was significantly elevated during the 24 h EtOH-free diet (Fig. 12B). EtOH-induced elevation of plasma AST (Fig. 12C) and ALT (Fig. 12D) was also unaffected by an EtOH-free diet in the absence of L. plantarum. However, L. plantarum supplementation of an EtOH-free diet almost completely attenuated EtOH-induced lesions in the liver and partially but reduced liver triglyceride and plasma AST and ALT activities. An EtOH-free diet in the absence of L. plantarum did not affect EtOH-induced elevation of mRNA for IL-1β (Fig. 12E) and IL-6 (Fig. 12F) in the liver. However, L. plantarum in the diet reduced IL-1β and IL-6 mRNA to normal levels. The levels of MCP1 mRNA in liver was unaffected by EtOH feeding or by 1 d recovery from EtOH (Fig. 12G). L. plantarum, however, reduced MCP1 mRNA to significantly lower than the normal levels. TLR4 mRNA level after 1 d of an EtOH-free diet was significantly higher than before an EtOH-free diet, but L. plantarum supplementation of an EtOH-free diet significantly reduced TLR4 mRNA (Fig. 12H).
Figure 12.
L. plantarum accelerates healing of CEF-induced liver damage in mice in vivo. A–D) Adult female mice were fed liquid diet with 5% EtOH (EF) or isocaloric maltodextrin (PF) for 4 wk and EF mice received additional gavage of EtOH (g/BW) once daily during last 3 d. EF mice were then fed EtOH free diet with (L.p.) or without (Veh) L. plantarum (106 CFU/ml) for 24 h for recovery from EtOH damage (Post EF-Rec). A) Paraffin sections of formalin-fixed liver were stained with H&E. B–D) Liver extracts were assayed for triglyceride (TG) levels (B) and plasma was assayed for AST (C) and ALT (D) activities. Values are means ± sem (n = 4). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding Veh values; Δvalue significantly different from corresponding EF value. E–H) RNA extracted from liver was subjected to qPCR for IL-1β (E), IL-6 (F), MCP1 (G), and TLR4 (H) genes. Values are means ± sem (n = 6). *P < 0.05, significantly different from corresponding PF values; #value different from corresponding EF values.
DISCUSSION
Identifying the agents that prevent and mitigate alcohol-induced tissue injury and understanding the mechanisms involved in their effects are essential for the development of novel therapeutics for prevention and treatment of alcohol-related diseases such as ALD. The present study demonstrated that dietary supplementation with the probiotic L. plantarum effectively attenuated and mitigated EtOH-induced gut barrier dysfunction, endotoxemia, and liver damage by a mechanism that involves EGFR-dependent prevention of oxidative stress and inflammatory responses.
CEF in the present study resulted in disruption of TJ, AJ, and the actin cytoskeleton in mouse colonic epithelium, which has been associated with a significant increase in colonic mucosal permeability to inulin (21). Furthermore, our data show that supplementation of diet with L. plantarum almost completely blocks EtOH-induced colonic mucosal barrier dysfunction and AJC disruption. Previous studies have shown the role of small intestinal permeability in alcoholic endotoxemia and liver damage (22). However, in our model, we consistently found that the alcoholic intestinal permeability was confined to colon. We speculate that the vivarium-related variability in the basal microbiome status may have contributed to this discrepancy. To investigate the mechanisms involved in the EtOH-induced tissue damage and its prevention by L. plantarum, we evaluated the effects of EtOH and L. plantarum on oxidative stress in colon. Oxidative stress is known to disrupt the intestinal epithelial AJCs and cause barrier dysfunction (4). The dramatic depletion of reduced-protein thiols and the elevation of oxidized-protein thiols in the colon of EtOH fed mice indicated that EtOH-induced oxidative stress in colon involves glutathione oxidation. Oxidized glutathione is known to induce thiol oxidation in many cellular proteins (23). Dietary supplementation of L. plantarum completely attenuated EtOH-induced protein thiol oxidation. Thiol oxidation of signaling molecules such as protein tyrosine phosphatase 1B may increase tyrosine phosphorylation of AJC proteins, leading to disruption of TJ and AJ (23). The mechanism of EtOH-induced oxidative stress may involve influence on generation of reactive oxygen species such as hydrogen peroxide as well as depletion of antioxidants. Results of our present study show that EtOH down-regulates the expression of antioxidant genes such as SOD1, SOD2, Catalase, Gpx1, and Gpx3. L. plantarum blocks the effect of EtOH on antioxidant gene expression. L. plantarum elevated SOD1 and SOD2 mRNA several fold greater than that in control mice, but its effect on Catalase expression was weak. The transcription factors ARE and Nrf2 are known to control the antioxidant gene expression in many tissues. A dramatic suppression of Nrf2 mRNA by EtOH and its complete prevention by L. plantarum suggest that down-regulation of Nrf2 may play an important role in EtOH-induced oxidative stress and AJC disruption in colon, and that L. plantarum–mediated blockage of Nrf2 down-regulation may prevent EtOH-induced oxidative stress and AJC disruption.
Previous studies indicated that acute or chronic EtOH administration in mice (24) results in elevated mRNA for cytokines such as TNF-α, IL-1β, and IL-6 in the small intestine, but the effect of EtOH on cytokine expression in colon is unknown. Furthermore, no information is available on the effects of EtOH on expression of chemokines in the small intestine or colon. Our study found that CEF induces a dramatic elevation of mRNA for cytokines (TNF-α, IL-1β, and IL-6) and chemokines (CCL5, MCP1/CCL2, CXCL1, and CXCL2) in colonic mucosa, and that L. plantarum feeding blocks these effects of EtOH. These data indicate that L. plantarum prevents EtOH-induced inflammatory responses in the colon. The mechanism of EtOH-induced inflammatory responses is unclear. It is likely that EtOH-induced oxidative stress disrupts epithelial TJ and barrier function, which in turn results in leakage of luminal LPS into the colonic mucosa. LPS in mucosa may stimulate the hematopoietic and epithelial cells to secrete cytokines and chemokines. IL-6 (25, 26), IL-1β (27), and TNF-α (28) have been previously shown to disrupt intestinal epithelial TJ. Therefore, the LPS-mediated release of cytokines may further damage the epithelial and endothelial junctions, widen the paracellular space, and enhance LPS flux into the mucosa and portal circulation. The effects of chemokines on epithelial or endothelial TJ and barrier function are unknown. Hence, the sources and targets of chemokines released by EtOH remain to be clarified. Data in this study demonstrate that L. plantarum blocks EtOH-induced expression of cytokines and chemokines in mouse colon. The effects of L. plantarum on colonic cytokines and chemokines may be indirect as a result of its effects on EtOH-induced oxidative stress and epithelial barrier dysfunction. However, it is also possible that L. plantarum directly targets the expression of cytokine and chemokine genes by releasing soluble factors into the medium that may penetrate the intestinal epithelium.
Data from the above in vivo study raised the question whether L. plantarum directly affects the intestinal epithelium to preserve AJC integrity and barrier function from EtOH-induced damage. Acetaldehyde is the most toxic metabolite of EtOH. The highest concentration of acetaldehyde was recorded in the colon as a result of EtOH metabolism by intestinal microflora, which express high levels of alcohol dehydrogenase (29) but very low levels of aldehyde dehydrogenase (30). Our previous studies demonstrated that acetaldehyde disrupts TJ and AJ in Caco-2 cell monolayers (1). In the present study, the prevention of acetaldehyde-induced barrier dysfunction by L. plantarum in Caco-2 cell monolayers in vitro indicates that L. plantarum may directly interact with the intestinal epithelium to prevent EtOH-induced gut barrier dysfunction in colon. Protective activity of UV-treated dead L. plantarum indicates that certain protective factors produced by these bacteria are responsible for this beneficial effect. The inhibition of L. plantarum effect on acetaldehyde-induced barrier dysfunction by AG1478 indicated that the protective activity of L. plantarum involves EGFR tyrosine kinase activity. Our previous studies showed that EGFR activation by EGF protects epithelial junctions from acetaldehyde in Caco-2 cell monolayers (31) and human colonic mucosa (32). The present data also show that the activities of EGFR downstream signaling molecules such as p38-MAPK and Rac1 are involved in the mechanism of L. plantarum–mediated prevention of acetaldehyde-induced barrier dysfunction in Caco-2 cell monolayers.
To determine the role of EGFR in L. plantarum effect on EtOH-induced gut permeability in vitro, we used rtTA-EGFR*Tg mice, in which a dominant negative EGFR is expressed by doxycycline-inducible mechanism; corresponding rtTA mice were used as control groups for comparison. The effects of EtOH and L. plantarum in rtTA mice in the absence or presence of doxycycline were similar to those in WT mice, but doxycycline blocked the effect of L. plantarum in rtTA-EGFR*Tg mice. Although doxycycline had no influence on EtOH effects, it effectively blocked L. plantarum–mediated prevention of EtOH-induced barrier dysfunction, disruption of AJCs, protein thiol oxidation, down-regulation of antioxidant gene expression, and up-regulation of cytokine and chemokine gene expression in colon of rtTA-EGFR*Tg mice. These data confirm the role of EGFR in the mechanism of L. plantarum in vivo.
A significant body of evidence indicates that gut barrier dysfunction and resulting endotoxemia play crucial role in the pathogenesis of ALD and likely in alcoholic injury in other organ systems. Our data show that L. plantarum in the diet attenuates EtOH-induced liver damage, as indicated by the prevention of hepatic lesions and elevation of plasma ALT activity. L. plantarum also blocks EtOH-induced fat deposition in the liver. It is likely that this effect of L. plantarum on liver is indirect, mediated by the prevention of gut permeability and endotoxemia. However, a direct effect of L. plantarum on the liver cannot be ruled out at this time. Although L. plantarum is confined to the intestinal lumen, it is possible that the protective factors released from the bacteria may be delivered to the liver via portal circulation. L. plantarum attenuates EtOH-induced down-regulation of antioxidant gene expression and up-regulation of inflammatory cytokine and chemokine gene expression in the liver. These data collectively demonstrate that dietary supplementation of L. plantarum prevents EtOH-induced liver damage. The results from rtTA-EGFR*-Tg mice further demonstrate that L. plantarum prevents alcoholic liver damage by an EGFR-dependent mechanism.
Although the prevention of liver damage by L. plantarum in the gut lumen provides strong support for the role of gut permeability and endotoxin flux in alcoholic liver damage, a direct effect of EtOH on liver cannot be ruled out. To gather further support on LPS-mediated liver damage, we evaluated the effect of EtOH and L. plantarum on the levels of plasma LPS and liver mRNA for TLR4 and MYD88, both known to be up-regulated by LPS exposure (19, 20). L. plantarum in the diet attenuated EtOH-induced elevation of plasma LPS in WT and rtTA mice, which was blocked by doxycycline-mediated expression of dominant negative EGFR in rtTA-EGFR*-Tg mice. Therefore, the effects of EtOH, L. plantarum and EGFR* expression on colonic mucosal permeability and liver damage are parallel to each other. EtOH elevated the expression of TLR4 and MYD88 genes, which was attenuated by L. plantarum. L. plantarum effects on TLR4 and MYD88 were blocked by doxycycline-mediated induction of EGFR* expression in rtTA-EGFR*-Tg mice. These data further support the role of colon-derived LPS in alcoholic liver damage.
The blocking effect of L. plantarum on EtOH-induced gut and liver damage is important for the prevention of alcoholic organ damage. However, the healing effect of L. plantarum during postalcoholic tissue injury would be of tremendous therapeutic value in treatment of alcohol-related diseases. Therefore, we used a chronic + binge model of EtOH feeding in mice and evaluated the effect of L. plantarum fed after the EtOH injury. Data showed that switching to an EtOH-free diet for 24 h did not restore the integrity of TJ or AJ, as evidenced by continued disruption of junctional organization of occludin, ZO-1, claudin-3, E-cadherin, and β-catenin in the colonic epithelium. However, supplementation of EtOH-free diet with L. plantarum completely restored the junctional organization of TJ and AJ proteins, indicating that the EtOH-induced disruption of TJ and AJ can be restored in 24 h by feeding L. plantarum to the animals. This was confirmed by the complete restoration of colonic mucosal barrier function by L. plantarum. The L. plantarum–supplemented diet, but not the diet without L. plantarum, also reversed EtOH-induced endotoxemia, up-regulation of proinflammatory cytokines, and down-regulation of anti-inflammatory cytokine in the colonic mucosa.
Feeding animals an EtOH-free diet for 24 h did not correct EtOH-induced histopathologic lesions in the liver. In fact, there was a further increase in liver damage, which was supported by significant increase in the triglyceride content of the liver and TLR4 expression during the EtOH-free diet. The EtOH-free diet did not reduce plasma AST/ALT levels or mRNA for proinflammatory cytokines in the liver. Supplementation of diet with L. plantarum almost completely reversed histopathologic lesions and mRNA for proinflammatory cytokines, and partially reduced plasma AST/ALT, liver triglyceride, and mRNA for TLR4. These observations demonstrate that L. plantarum supplementation during post-EtOH injury alleviates liver injury by 24 h and suggest that this probiotic has potential therapeutic value.
In summary, this study provides evidence for the potential roles of oxidative stress and inflammatory responses in EtOH-induced disruption of colonic AJCs, gut permeability, and endotoxemia, and that dietary supplementation with L. plantarum prevents alcoholic gut permeability, endotoxemia, and liver damage by EGFR-mediated suppression of EtOH-induced down-regulation of antioxidant gene expression and up-regulation of inflammatory cytokine and chemokine gene expression. Interestingly, our data also demonstrate that L. plantarum effectively mitigates alcoholic tissue injury in colon and liver, thus demonstrating its potential therapeutic value in treatment of alcoholic tissue injury.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health Grants AA12307 (National Institute on Alcohol Abuse and Alcoholism) and DK55532 (National Institute of Diabetes and Digestive and Kidney Diseases) to R.R. The authors declare no conflicts of interest.
Glossary
- AJ
adherens junction
- AJC
apical junctional complex
- ALD
alcoholic liver disease
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- CCL5
chemokine ligand 5
- CEF
chronic ethanol feeding
- CFU
colony-forming unit
- CXCL1/2
C-X-C motif chemokine ligand 1/2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EGFR-tr
tyrosine kinase domain–deleted mutant form of epidermal growth factor receptor
- EtOH
ethanol
- Gpx
glutathione peroxidase
- H&E
hematoxylin and eosin
- MCP1
monocyte chemotactic protein 1
- Nrf2
nuclear factor–like 2
- qPCR
quantitative PCR
- rtTA
Gt(ROSA)26Sortm1(rtTA*M2)Jae/J
- SOD
superoxide dismutase
- TER
transepithelial electrical resistance
- tetO
tetracycline operator
- TJ
tight junction
- WT
wild type
- ZO
zonula occludens
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. K. Shukla and A. S. Meena performed research, analyzed data, and wrote the report; B. Manda assisted P. K. Shukla and A. S. Meena in performing some of the experiments; M. Gomes-Solecki contributed by providing L. plantarum strain 256 and guidance in growth of probiotics; P. Dietrich and I. Dragatsis were involved in producing EGFR*-Tg mice; and R. Rao contributed by designing research and writing the report.
REFERENCES
- 1.Rao R. (2009) Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50, 638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balda M. S., Matter K. (2016) Tight junctions as regulators of tissue remodelling. Curr. Opin. Cell Biol. 42, 94–101 [DOI] [PubMed] [Google Scholar]
- 3.Quiros M., Nusrat A. (2014) RhoGTPases, actomyosin signaling and regulation of the epithelial apical junctional complex. Semin. Cell Dev. Biol. 36, 194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao R. (2008) Oxidative stress–induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 13, 7210–7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samak G., Gangwar R., Meena A. S., Rao R. G., Shukla P. K., Manda B., Narayanan D., Jaggar J. H., Rao R. (2016) Calcium channels and oxidative stress mediate a synergistic disruption of tight junctions by ethanol and acetaldehyde in Caco-2 cell monolayers. Sci. Rep. 6, 38899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magdaleno F., Blajszczak C. C., Nieto N. (2017) Key events participating in the pathogenesis of alcoholic liver disease. Biomolecules 7, 1–14 [Google Scholar]
- 7.Wilkins T., Sequoia J. (2017) Probiotics for gastrointestinal conditions: a summary of the evidence. Am. Fam. Physician 96, 170–178 [PubMed] [Google Scholar]
- 8.Bull-Otterson L., Feng W., Kirpich I., Wang Y., Qin X., Liu Y., Gobejishvili L., Joshi-Barve S., Ayvaz T., Petrosino J., Kong M., Barker D., McClain C., Barve S. (2013) Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 8, e53028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanji A. A., Khettry U., Sadrzadeh S. M. (1994) Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc. Soc. Exp. Biol. Med. 205, 243–247 [DOI] [PubMed] [Google Scholar]
- 10.Kirpich I. A., Solovieva N. V., Leikhter S. N., Shidakova N. A., Lebedeva O. V., Sidorov P. I., Bazhukova T. A., Soloviev A. G., Barve S. S., McClain C. J., Cave M. (2008) Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw D. M., Gaerthé B., Leer R. J., Van Der Stap J. G., Smittenaar C., Heijne Den Bak-Glashouwer M., Thole J. E., Tielen F. J., Pouwels P. H., Havenith C. E. (2000) Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 100, 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Rio B., Dattwyler R. J., Aroso M., Neves V., Meirelles L., Seegers J. F., Gomes-Solecki M. (2008) Oral immunization with recombinant Lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin. Vaccine Immunol. 15, 1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potula H. H., Richer L., Werts C., Gomes-Solecki M. (2017) Pre-treatment with Lactobacillus plantarum prevents severe pathogenesis in mice infected with Leptospira interrogans and may be associated with recruitment of myeloid cells. PLoS Negl. Trop. Dis. 11, e0005870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilg H., Moschen A. R., Szabo G. (2016) Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64, 955–965 [DOI] [PubMed] [Google Scholar]
- 15.Hernández J. A., López-Sánchez R. C., Rendón-Ramírez A. (2016) Lipids and oxidative stress associated with ethanol-induced neurological damage. Oxid. Med. Cell. Longev. 2016, 1543809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louvet A., Mathurin P. (2015) Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 12, 231–242 [DOI] [PubMed] [Google Scholar]
- 17.De Timary P., Stärkel P., Delzenne N. M., Leclercq S. (2017) A role for the peripheral immune system in the development of alcohol use disorders? Neuropharmacology 122, 148–160 [DOI] [PubMed] [Google Scholar]
- 18.Kane C. J., Drew P. D. (2016) Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J. Leukoc. Biol. 100, 951–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y., Liu B., Feng X., Liu Z., Liang D., Li F., Li D., Cao Y., Feng S., Zhang X., Zhang N., Yang Z. (2013) Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol. 151, 20–27 [DOI] [PubMed] [Google Scholar]
- 20.Huang X. Y., Ansari A. R., Huang H. B., Zhao X., Li N. Y., Sun Z. J., Peng K. M., Zhong J., Liu H. Z. (2017) Lipopolysaccharide mediates immuno-pathological alterations in young chicken liver through TLR4 signaling. BMC Immunol. 18, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhry K. K., Shukla P. K., Mir H., Manda B., Gangwar R., Yadav N., McMullen M., Nagy L. E., Rao R. (2016) Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J. Nutr. Biochem. 27, 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirpich I. A., Feng W., Wang Y., Liu Y., Barker D. F., Barve S. S., McClain C. J. (2012) The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 36, 835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R. K., Li L., Baker R. D., Baker S. S., Gupta A. (2000) Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G332–G340 [DOI] [PubMed] [Google Scholar]
- 24.Fleming S., Toratani S., Shea-Donohue T., Kashiwabara Y., Vogel S. N., Metcalf E. S. (2001) Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol. Clin. Exp. Res. 25, 579–589 [PubMed] [Google Scholar]
- 25.Al-Sadi R., Ye D., Boivin M., Guo S., Hashimi M., Ereifej L., Ma T. Y. (2014) Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One 9, e85345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki T., Yoshinaga N., Tanabe S. (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 286, 31263–31271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Sadi R., Guo S., Dokladny K., Smith M. A., Ye D., Kaza A., Watterson D. M., Ma T. Y. (2012) Mechanism of interleukin-1β–induced increase in mouse intestinal permeability in vivo. J. Interferon Cytokine Res. 32, 474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchiando A. M., Shen L., Graham W. V., Weber C. R., Schwarz B. T., Austin J. R., II, Raleigh D. R., Guan Y., Watson A. J., Montrose M. H., Turner J. R. (2010) Caveolin-1–dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 189, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salaspuro M. (1997) Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addict. Biol. 2, 35–46 [DOI] [PubMed] [Google Scholar]
- 30.Visapää J. P., Tillonen J., Salaspuro M. (2002) Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 37, 322–326 [DOI] [PubMed] [Google Scholar]
- 31.Sheth P., Seth A., Thangavel M., Basuroy S., Rao R. K. (2004) Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 28, 797–804 [DOI] [PubMed] [Google Scholar]
- 32.Basuroy S., Sheth P., Mansbach C. M., Rao R. K. (2005) Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and l-glutamine. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G367–G375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.