Abstract
Proper arteriovenous morphogenesis is crucial for maintaining normal tissue perfusion. However, our understanding of how arterial morphogenesis is regulated in the CNS is incomplete. In this study, we asked whether vascular basement membrane (BM) laminins, specifically the γ3-containing isoforms, regulate retinal arterial morphogenesis. We provide evidence that laminin-γ3 is deposited at both arterial and venous BMs during arteriogenesis. Vascular BM laminin-γ3 bound dystroglycan (DG), a laminin receptor preferentially expressed by arterial endothelial cells (ECs) during arteriogenesis. Blockade of laminin–DG binding in vitro led to decreased Delta-like ligand (DLL)-4 expression in ECs. Moreover, genetic deletion of the laminin-γ3- and EC-specific deletion of DG led to similar defects in retinal arteriogenesis, including reduced Dll4 expression, hyperbranching and reduced smooth muscle coverage. These results implicate a newly identified laminin-γ3–DG signaling cascade that regulates arterial Dll4/Notch signaling to specify and stabilize retinal arteries.—Biswas, S., Watters, J., Bachay, G., Varshney, S., Hunter, D. D., Hu, H., Brunken, W. J. Laminin–dystroglycan signaling regulates retinal arteriogenesis.
Keywords: extracellular matrix, basement membrane, CNS angiogenesis, vascular remodeling
Although several studies focused on the developmental mechanisms of arteriogenesis in the trunk, a detailed understanding of how arteriogenesis is regulated in the CNS is incomplete. The retina is an ideal model to address this question as the planar patterning of retinal vessels can be easily visualized both in vivo and ex vivo. In the murine retina, the superficial vasculature forms over the retinal surface between postnatal day 1 (P1) and P8. Eventually, 2 other plexuses form in the deeper layers: at the outer plexiform layer and then at the inner plexiform layer, completing retinal vascular development by P21 (1).
Our prior works have shown that astrocytes and microglia regulate retinal vascular expansion and branching in response to the extracellular cue (2, 3). One unresolved question is how retinal arteriovenous morphogenesis is regulated. During the initial phase of retinal angiogenesis, all endothelial cells (ECs) exhibit venous signatures. Distinct molecular signatures for arterial ECs are not apparent until P3–4 (4, 5). At this time, arteries arise from immediately behind the nascent plexus toward the optic nerve head, maturing in a spatiotemporal gradient. The mechanisms underlying this shift from venous to arterial signature is poorly understood.
Arteriogenesis involves a series of developmental steps including adoption of arterial identity by ECs, mural cell recruitment, arterial elongation, and establishment of an arterial branching pattern (6). One study, outside of the CNS, suggested that Delta-like ligand (Dll)-4/Notch signaling regulates arterial identity (7). Arterial ECs express Dll4, which activates the Notch pathway. Downstream of Dll4/Notch signaling, arterial ECs express Ephrin-B2 (8). Dll4/Notch signaling is also involved in establishing the arterial branching pattern (9). However, how Dll4 expression is regulated in retinal arteries is not understood completely. According to the conventional model, VEGF signals through VEGF receptor (VEGFR)-2 to induce Dll4 expression in arterial ECs (10). However, 2 studies reported that retinal arteries form despite pharmacological or genetic disruption of VEGF/VEGFR2 pathway (11, 12). These observations suggest that VEGF-mediated signaling is not the sole regulator of arterial Dll4 expression in the retina.
Laminin, a basement membrane (BM) component, has been implicated in several key processes during vascular development. Laminins are heterotrimeric glycoproteins made of α-, β-, and γ-chains. Five α-, 3 β- and 3 γ-chains have been identified, combining into 16 different isoforms (13, 14). Laminin binding and signaling are mediated by various transmembrane receptors, including integrins and dystroglycan (DG). CNS ECs express several integrins, as well as DG (15). A recent report demonstrated that laminin–integrin signaling could directly induce Dll4 expression in endothelial tip cells (16). However, whether laminin-mediated signaling has any regulatory role in Dll4/Notch signaling–mediated arteriogenesis in the retina or in any other tissue is unknown. Moreover, it is unknown whether signaling though other laminin receptors, such as DG, can induce endothelial Dll4 expression.
We have reported that γ3-laminins are expressed in the retinal vascular BM (2, 17). Laminin-γ3–null retinae exhibit a hyperbranched and hyperproliferative superficial vasculature (2, 3) which phenocopies Dll4+/− vasculature (18, 19). Based on these observations, we asked whether the loss of laminin-γ3 chain affects arterial Dll4/Notch signaling and arteriogenesis in the retina.
In this study, we demonstrate that γ3-laminins are deposited in both arterial and venous BMs during retinal vascular development and that they bind DG. Arterial ECs heavily express DG during retinal arteriogenesis, with little expression in venous ECs. Genetic deletion of either the laminin-γ3 chain (Lamc3−/−) or EC-specific deletion of the DG gene (Dag1ΔEC) leads to similar retinal arterial dysgenesis, including hyperbranching and reduced smooth muscle coverage. Arterial Dll4 expression is down-regulated in Lamc3−/− and Dag1ΔEC retinae, suggesting a regulatory role of laminin γ3–DG signaling in arterial Dll4 expression. Finally, in vitro blockade of laminin–DG binding also leads to reduced DLL4 expression in aortic ECs. These results provide the first description of a novel pathway where γ3-laminins signal through DG to regulate retinal arteriogenesis by inducing Dll4/Notch signaling in arterial ECs.
MATERIALS AND METHODS
Animals
All procedures involving mice were performed in accordance with the Animal Care and Use Committee of State University of New York (SUNY), Upstate Medical University. Targeted deletion of the laminin γ3-gene (Lamc3) has been previously described (17, 20). The laminin-γ3–null mouse line was backcrossed over 9 generations to C57bl/6J. To generate EC-specific DG (Dag1)–knockout mice, Tie2-Cre mice [B6.Cg-Tg(Tek-cre)1Ywa/J (21)] were crossed with Dag1-floxed mice [129-Dag1tm2Kcam/J (22)]. Both strains were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Tie2-Cre.Dag1fl/+ breeders gave birth to pups in the Mendelian ratio. Littermate WT (Tie2-Cre.Dag1+/+) and EC-specific Dag1 knockout (Tie2-Cre.Dag1fl/fl or Dag1ΔEC) mice were used for the experiments. Generation of protein O-mannose β-1,2-N-acetylglucosaminyltransferase (POMGnT1)–deficient mice was described by Liu et al. (23). Littermate WT, POMGnT1+/−, and POMGnT1−/− mice were used for experiments.
Immunohistochemistry
To prepare retinal flat mounts, eyes were fixed in paraformaldehyde (4% PFA in 1× PBS) for 10 min, unless otherwise specified. Retinae were then dissected, flat mounted, and treated with absolute methanol at −20°C for 10 min. For laminin-γ3, Ephrin-B2, and α-DG immunostaining, eyes were fixed in 2% PFA for 5 min. To label laminin in vitro, cells were fixed in absolute methanol at −20°C for 10 min. After 1× PBS washes, samples were incubated overnight at 4°C in blocking buffer (5% goat or donkey serum, 0.3% Triton X-100 in 1× PBS). Samples were incubated with primary antibodies in antibody-diluting solution (5% goat or donkey serum; 0.01% Triton X-100 in 1× PBS) for 48 h at 4°C, washed, and incubated with secondary antibodies for 24 h. For radial section staining for VEGF, fresh-frozen eyes in optimal cutting temperature compound were sectioned at a thickness of 16 μm. For radial section staining for pVEGFR2, eyes were fixed in 4% PFA for 10 min at 4°C, washed in 1× PBS, cryoprotected in 20% sucrose for 1 h, embedded in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Torrance, CA, USA), and sectioned at a thickness of 16 μm. After absolute methanol fixation for 10 min at −20°C, sections were blocked for 2 h at room temperature followed by primary antibody incubation overnight at 4°C. Following 1× PBS washes, sections were incubated with secondary antibodies for 4 h at room temperature. Samples were imaged with an Orca-R2 camera (Hamamatsu Ltd., Hamamatsu City, Japan) mounted on an Eclipse Ni-U microscope (Nikon, Tokyo, Japan). The Volocity software package (v.6.3; Quorum, Puslinch, ON, Canada) was used to acquire, process, and analyze images.
Antibodies
Primary antibodies used were rat anti-PDGF receptor (PDGFR)-α (1:500) and rat anti-CD31 (1:250) BD Biosciences, San Jose, CA, USA); rabbit anti-γ3-laminin (R96) [specificity reported by Li et al. (17); 1:10000]; mouse anti-γ-tubulin (1:10,000), rabbit anti-collagen IV (1:200), FITC-conjugated mouse anti-α-smooth muscle actin (1: 500) (α-SMA), mouse anti-α-DG (VIA4-1, 1:100), goat anti-Ephrin-B2 (1:100), rabbit anti-VEGFA (1:1000), rabbit anti-pan laminin (1: 500), and FITC-conjugated isolectin B4 (IB4, 1:250) (MilliporeSigma, Burlington, MA, USA); goat anti-Dll4 (1:200) and goat anti-EphB4 (1:100) (R&D Systems, Minneapolis, MN, USA); rabbit anti-phospho-VEGFR2Tyr1175 (1:1000, for immunoblot analysis; Cell Signaling Technology, Danvers, MA, USA); rabbit anti-phospho-VEGFR2Tyr1175 (1:100, for immunohistochemistry; Abcam, Cambridge, MA, USA); mouse anti-α-DG (IIH6; 1:1000. a gift from Kevin P. Campbell, University of Iowa, Iowa City, IA, USA); rabbit anti-β-DG (JAF; 1:250; a gift from Dominique Mornet, Université de Montpellier, Montpellier, France); goat anti-Brn3 (1:250; Santa Cruz Biotechnology, Dallas, TX, USA); rat anti-F4/80 (1:250; Thermo Fisher Scientific); and rabbit anti-GFAP (1:500; Chemicon, Billerica, MA, USA). Secondary antibodies used for immunohistochemistry were goat anti-rabbit 488 and 594; goat anti-rat 488 and 568; donkey anti-rabbit 488, 594, and 647; and donkey anti-rat 594 (1:250; Thermo Fisher Scientific). Secondary antibodies used for immunoblot analysis were donkey anti-mouse 800CW and donkey anti-rabbit 680RD (1:10,000; Li-Cor Biosciences, Lincoln, NE, USA).
Laminin overlay
P5 brains were dissolved in lysis buffer [150 mM NaCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris (pH 8.0)] containing EDTA-free 1× protease inhibitor cocktail (Thermo Fisher Scientific), incubated with wheat germ agglutinin (WGA) beads (A-2101-5; EY Laboratories, San Mateo, CA, USA) overnight at 4°C and centrifuged at 1000 rpm for 5 min. The beads were washed in the lysis buffer followed by elution of bound glycoproteins in the loading buffer [2× Laemmli sample buffer: 65.8 mM Tris-HCl (pH 6.8), 26.3% glycerol, 2.1% SDS, and 0.01% bromophenol blue with 5% 2-ME] at 90°C for 5 min. The samples were centrifuged and the supernatant run through 12% SDS polyacrylamide gels, and transferred to PVDF membranes. The membranes were blocked in Odyssey blocking buffer (Li-Cor Biosciences) and incubated with either wild-type (WT; contains γ3-laminin) or Lamc3−/− brain lysate overnight at 4°C. Henceforth, all washing buffers contained 1 mM CaCl2 and 1 mM MgCl2. Upon incubation overnight, membranes were washed and probed for specific proteins.
Primary EC culture
We obtained primary human aortic ECs (HAECs) from Sciencell Research Laboratories (Carlsbad, CA, USA). The cells were resuspended in EC medium (Sciencell Research Laboratories), containing 5% fetal bovine serum (FBS), 1% EC growth supplement (Sciencell Research Laboratories), and 1% penicillin/streptomycin solution (penicillin: 10,000 IU/ml; streptomycin: 10,000 μg/ml). For DG-function–blocking experiments on cell-derived matrix, HAECs were grown on glass coverslips (12 mm) for 3 d in the EC medium. Next, either only FBS or FBS with DG-function–blocking antibody (IIH6) or heat-killed IIH6 was added to the medium. HAECs were gown for 1 more day, followed by either staining or real-time quantitative PCR (qPCR).
Immunoblot analysis
For each experiment, 8 retinae per genotype were lysed together in 100 μl lysis buffer containing EDTA-free 1× protease inhibitor cocktail. Samples were centrifuged, and the supernatant was diluted in the loading buffer (1:1) and boiled for 5 min. The samples were run through 6% SDS-polyacrylamide gels, transferred to PVDF membranes, and probed for target proteins. Membranes were imaged with Odyssey CLx (Li-Cor Biosciences) and analyzed with Image Studio (v.3.1; Li-Cor Biosciences).
qPCR
Primer specificity was confirmed with the Primer-Blast tool (National Center for Biotechnology Information, Bethesda, MD, USA). Total RNA was isolated with Trizol (Thermo Fisher Scientific), according to the manufacturer’s instructions. One microgram of total RNA was converted into cDNA with the Verso cDNA Kit (Thermo Fisher Scientific). Real-time PCR was performed on an ABI 7900 system (Thermo Fisher Scientific). All data were normalized to the expression of 18S or GAPDH RNA, and the fold change was calculated with the ΔCt-method with the expression level in WT animals or heat-killed IIH6-treated controls set at 1. The following primers were used: Hes1: forward 5′-TCAACACGACACCGGACAAAC-3′ and reverse 5′-ATGCCGGGAGCTATCTTTCTT-3′; Hes5: forward 5′-AGTCCCAAGGAGAAAAACCGA-3′ and reverse 5′-GCTGTGTTTCAGGTAGCTGAC-3′; DLL4: forward 5′-TGCAACTGCCCTTATGGCTTTGTG-3′ and reverse 5′-ACAAGTTGTTCATGGCTTCCCTGC-3′; 18S rRNA: forward 5′-GTAACCCGTTGAACCCCATT-3′, and reverse 5′-CCATCCAATCGGTAGTAGCG-3′; and GAPDH: forward 5′-GGACCTGACCTGCCGTCTAGAA-3′ and reverse 5′-GGTGTCGCTGTTGAAGTCAGAG-3′.
Measurements and statistics
All measurements, unless otherwise specified, were performed with Volocity software. Data were not segregated based on sex. Each measurement was made in at least 3 quadrants of the retina and averaged. To assess arterial smooth muscle coverage, we measured lengths of arteries from the optic nerve head to the most distal point of arterial α-SMA immunoreactivity and calculated the ratio of α-SMA+arterial length to the vascular migration distance. We expressed this ratio as the percentage of arterial length covered with smooth muscle. To measure arterial expression of Dll4 and DG, individual arteries were selected from an image, and relative fluorescence intensity ratios (normalized to CD31 fluorescence intensity) were calculated with histogram analysis in ImageJ software (National Institutes of Health, Bethesda, MD, USA). To measure the relative expression of γ3-laminin, β-DG, and Dll4 between arteries and veins in WT retinae, the fluorescence intensities were calculated for each field. For β-DG and Dll4 measurements, the fluorescence intensities in veins were normalized to that of arteries within the same sample. For γ3-laminin measurements, the fluorescence intensities in arteries were normalized to that of veins within the same sample. To calculate pVEGFR2 expression in ECs, fluorescence intensities were measured in areas defined by CD31 staining in radial sections. To measure the endothelial expression level of DLL4 in vitro, we calculated relative fluorescence intensities (normalized to IB4 fluorescence intensity) from each field with ImageJ software.
Statistical analysis
Samples from at least 3 different animals (for in vivo experiments) and 3 separate experiments (for in vitro experiments) were used for statistical analysis. To test for statistical significance of any differences, a 2-tailed, unpaired Student’s t test was performed. A value of P < 0.05 was considered statistically significant.
RESULTS
Arterial Dll4/Notch signaling is affected in the Lamc3−/− retina
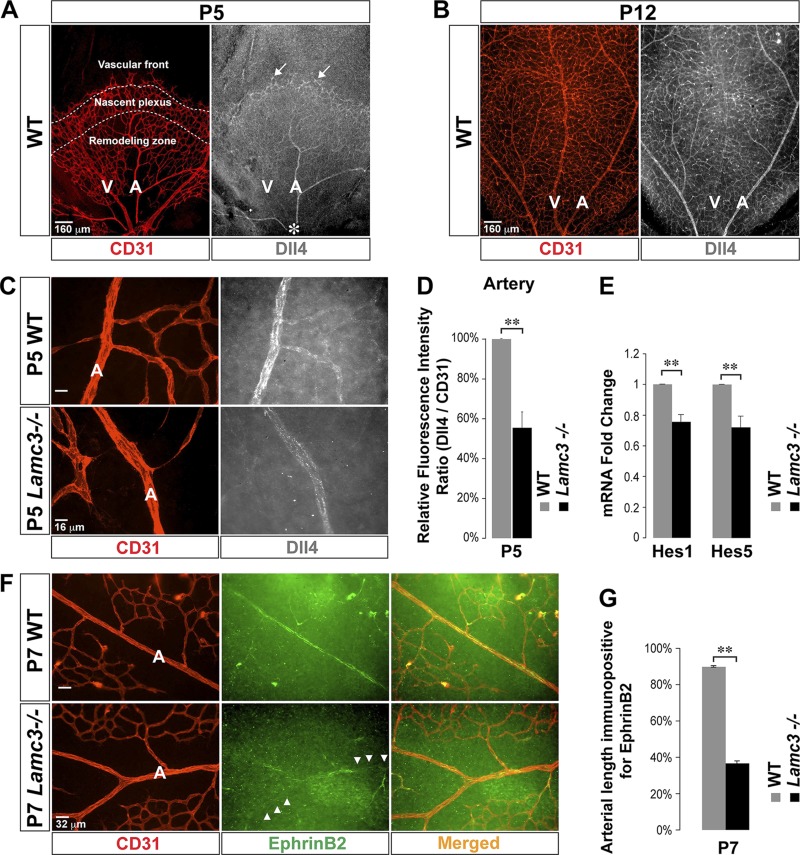
By P5, the vascular front reached more than halfway along the WT retinal surface, and all 3 distinct angiogenic zones were seen (Fig. 1A). Dll4 expression was relatively high in endothelial tip cells. Dll4 expression was relatively low in the nascent plexus. The Dll4 expression pattern became artery specific in the remodeling zone as the arteries matured from behind the nascent plexus toward the optic nerve head (Fig. 1A and Supplemental Fig. S1A). Importantly, Dll4 was not expressed in the emerging venous ECs. By P12, the vascular front reached the retinal periphery, and the nascent plexus nearly completely remodeled into adult superficial vasculature, where arteries and veins were identifiable from the optic nerve head to the retinal periphery (Fig. 1B). The artery-specific expression pattern of Dll4 was retained in this adult superficial vasculature (Fig. 1B and Supplemental Fig. S1A). The artery-specific expression of Dll4 is consistent with the critical role that the Dll4/Notch pathway plays in arteriogenesis (7, 9).
Figure 1.
Arterial Dll4/Notch signaling is disrupted in the Lamc3−/− retina. A) CD31 (EC marker: red) and Dll4 (white) labeling demonstrated high Dll4 expression in the tip cells (arrows) at the vascular front (P5). There was little Dll4 expression in the nascent plexus and the Dll4 expression pattern became artery specific in the remodeling zone as arteries mature from behind the nascent plexus toward the optic nerve head (asterisk). B) The artery-specific expression pattern of Dll4 persists in the mature superficial vasculature (P12). C) Higher power images of CD31 (red) and Dll4 (white) labeling of arteries; arterial Dll4 expression was down regulated in the Lamc3−/− retina. D) Dll4 immunofluorescence was quantified relative to CD31 fluorescence, demonstrating a significant decline in P5 Lamc3−/− arteries (n = 3). E) mRNA expression of Notch target genes; Hes1 and Hes5 mRNA levels were down-regulated in the Lamc3−/− retina (n = 3). F) CD31 (red) and Ephrin-B2 (green) labeling of arteries demonstrated that Lamc3−/− arterial regions completely lacked Ephrin-B2 expression (arrowheads). G) Quantification of arterial length immunopositive for Ephrin-B2 demonstrated significantly less extensive arterial Ephrin-B2 immunoreactivity in the Lamc3−/− retina (n = 2). A, artery; NS, not significant; V, vein. Scale bars: 160 μm (A, B), 16 μm (C), 32 μm (F). Data are means ± sem. **P < 0.02.
Next, we asked whether Dll4 expression is affected in the Lamc3−/− retinal vasculature. Dll4 immunoreactivity in tip cells was not affected (Supplemental Fig. S1B, C), but arterial Dll4 immunoreactivity was significantly reduced in the Lamc3−/− retina (Fig. 1C, D). Consistent with reduced Dll4 expression, mRNA expression of the Dll4/Notch target genes Hes1 and Hes5 (24) was significantly reduced in the Lamc3−/− retina (Fig. 1E). The expression of the arterial marker Ephrin-B2, which is downstream of Dll4/Notch signaling (8), was also disrupted in the Lamc3−/− retina, with areas of arteries devoid of Ephrin-B2 expression (Fig. 1F, G). In contrast, venous expression of EphB4 in the Lamc3−/− retina was similar to that in the WT (data not shown). These results suggest that the loss of laminin-γ3 chain specifically leads to a disruption in Dll4/Notch signaling in arterial ECs.
Arterial morphogenesis is disrupted in the Lamc3−/− retina
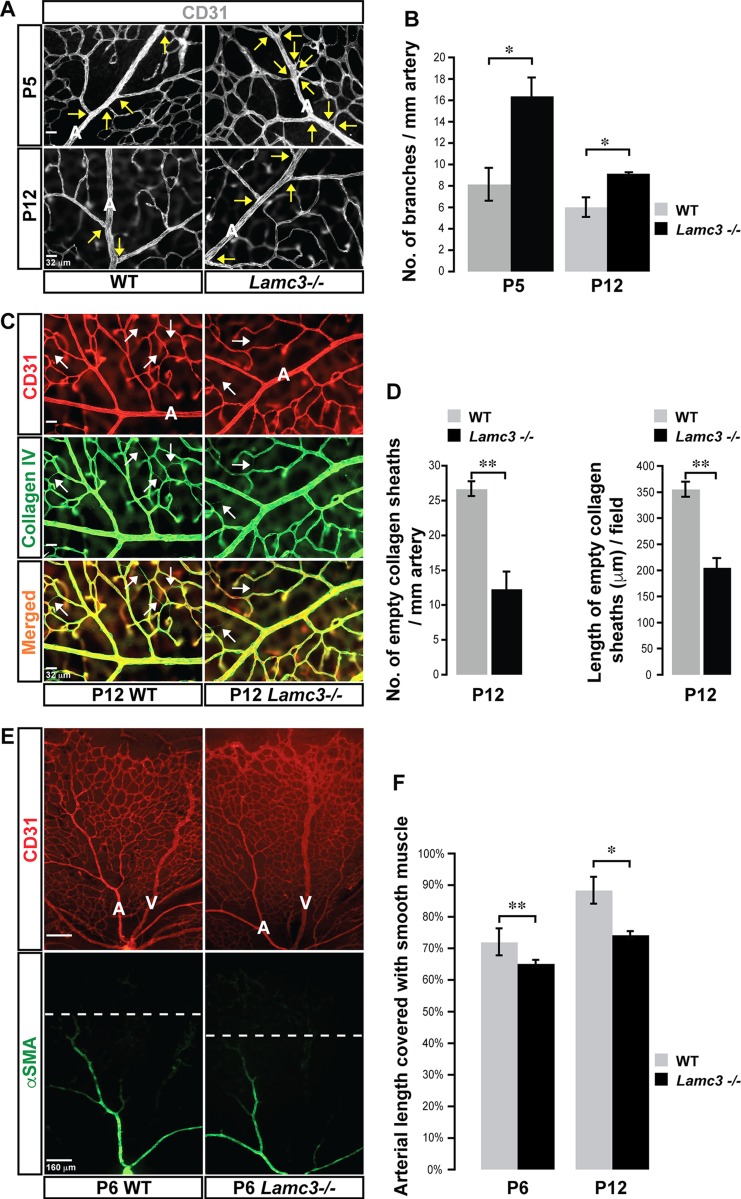
Next, we assayed several arterial morphologic parameters in the Lamc3−/− retina. Arteries and veins in both WT and Lamc3−/− retinae were distinguished morphologically, with arteries having smaller diameter and fewer branches than veins. Lamc3−/− retinal arteries were significantly hyperbranched compared to WT at P5 (Fig. 2A, B), a phenotype that persisted even after retinal arteries were fully specified and the arterial branching pattern was established (P12). Periarterial vascular pruning, visualized as empty collagen IV sheaths (25), was significantly less extensive in the Lamc3−/− retina than in the WT (Fig. 2C, D). Finally, smooth muscle coverage (i.e., the length of arterial wall with vascular smooth muscle cells) was significantly less extensive in the Lamc3−/− retina than in the WT (Fig. 2E, F). In contrast, pericyte coverage was not affected in the Lamc3−/− retina (Supplemental Fig. S2). These results suggest that artery-specific patterning (i.e., branching and mural cell coverage) is disrupted by the loss of laminin-γ3 chain, whereas generalized mural assembly (pericyte attachment) is not affected.
Figure 2.
Arterial morphogenesis is disrupted in the Lamc3−/− retina. A) CD31 (white) labeling demonstrates more primary arterial branches (arrows) in the Lamc3−/− retina. B) Arterial branching is quantified demonstrating a significant increase in the Lamc3−/− retinae (n = 3). C) CD31 (red) and collagen IV (green) labeling of arteries demonstrates less extensive vascular pruning around Lamc3−/− arteries. Arrows: empty collagen sheaths (collagen IV+/CD31−) demarking pruned vessels. D) Number and lengths of pruned vessels are quantified demonstrating a significant decrease around Lamc3−/− arteries (n = 3). E) CD31 (red) and α−SMA (green) marker labeling demonstrates less extensive vascular smooth muscle coverage of Lamc3−/− arteries. Dashed lines: extent of arterial smooth muscle coverage. F) Quantification of the extent of arterial smooth muscle coverage, relative to vascular migration distance, demonstrates significant reduction in Lamc3−/− arteries (n = 3). A, artery; V, vein. Scale bars: 32 μm (A, C), 160 μm (E). Data are means ± sem. *P < 0.05, **P < 0.02.
Next, we asked how γ3-laminins regulate arterial Dll4 expression. The conventional model postulates that VEGFA/VEGFR2 signaling induces Dll4 expression in ECs (10). Astrocytes are the main cellular source of VEGFA, which guides the retinal superficial vasculature (26). No change in VEGFA protein level, as measured by either immunoreactivity or quantitative immunoblot analysis was seen between WT and Lamc3−/− retinae (Supplemental Fig. S3A, B). Furthermore, there was no difference between WT and Lamc3−/− in the total retinal activated (phosphorylated) VEGF receptor 2 (pVEGFR2Tyr1175) levels (Supplemental Fig. S3C) or pVEGFR2Tyr1175 immunoreactivity, specifically in ECs (Supplemental Fig. S3D). These data suggest that the down-regulation of Dll4 expression in Lamc3−/− retinal arterial ECs is independent of VEGF signaling.
DG is preferentially expressed in arterial ECs during retinal arteriogenesis
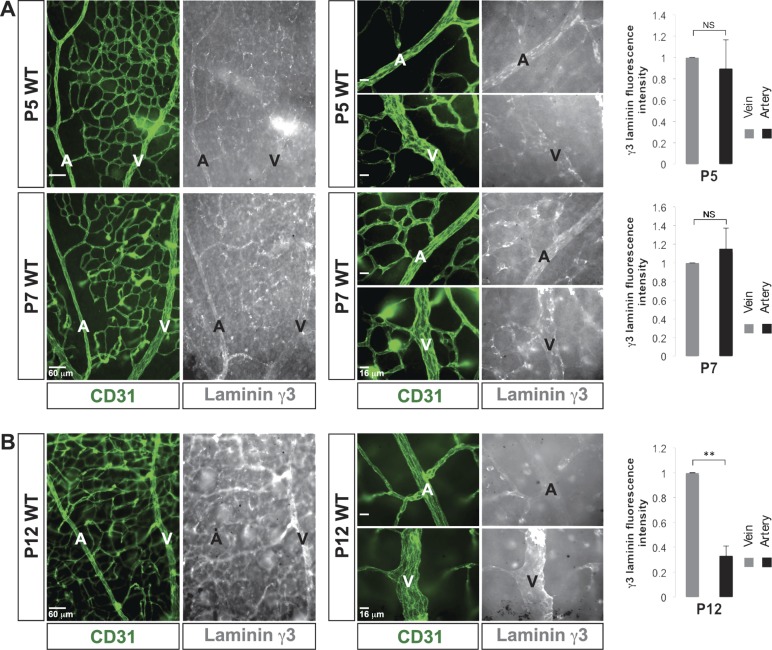
Next, we asked whether γ3-laminins could directly induce arterial Dll4 expression. At P5, γ3-laminins were absent around the endothelial tip cells, but were present in the proximal stalk cell BM (Supplemental Fig. S4A). In the nascent plexus, γ3-laminins were restricted to vascular branch points (2). In the developmentally older remodeling zone, γ3-laminins were found on microvessels, as well as in emerging arterial and venous BMs, a pattern that was retained through P7 (Fig. 3A). By P12, the nascent plexus had nearly completely remodeled into mature vasculature where retinal arteries and veins were fully specified, and their morphology was established. Laminin-γ3 immunoreactivity was drastically reduced in the arterial BM at this time, whereas it persisted in the venous and microvascular BMs (Fig. 3B).
Figure 3.
γ3-Laminin is deposited in both arterial and venous BMs during retinal arteriogenesis. A) CD31 (green) and γ3-laminin (white) labeling demonstrates that γ3-laminin was deposited in emerging arterial, venous, and microvascular BMs during retinal arteriogenesis (P5 and 7). Quantifications showed no difference in relative expression of γ3-laminin between arteries and veins at these time points (n = 3). B) In mature vessels (P12), γ3-laminins were present, mostly in venous and microvascular BMs. Quantifications showed a drastic decrease in relative expression of γ3-laminin in arteries at that time (n = 3). A, artery; NS, not significant; V, vein. Scale bars: 60 μm, 2 left columns; 16 μm, 2 right columns (A, B). Data are means ± sd. **P < 0.02.
Because γ3-laminins are present in both arterial and venous BMs during retinal arteriogenesis, we hypothesized that the γ3-laminin receptor that regulates arterial Dll4 expression would logically have exclusive or significantly higher expression in arterial ECs. To test this hypothesis, we assessed the expression of laminin receptors in the developing vasculature. Ido et al. (27) reported that γ3-laminins do not bind integrins. Thus, we examined whether they may signal via DG, another laminin receptor (23).
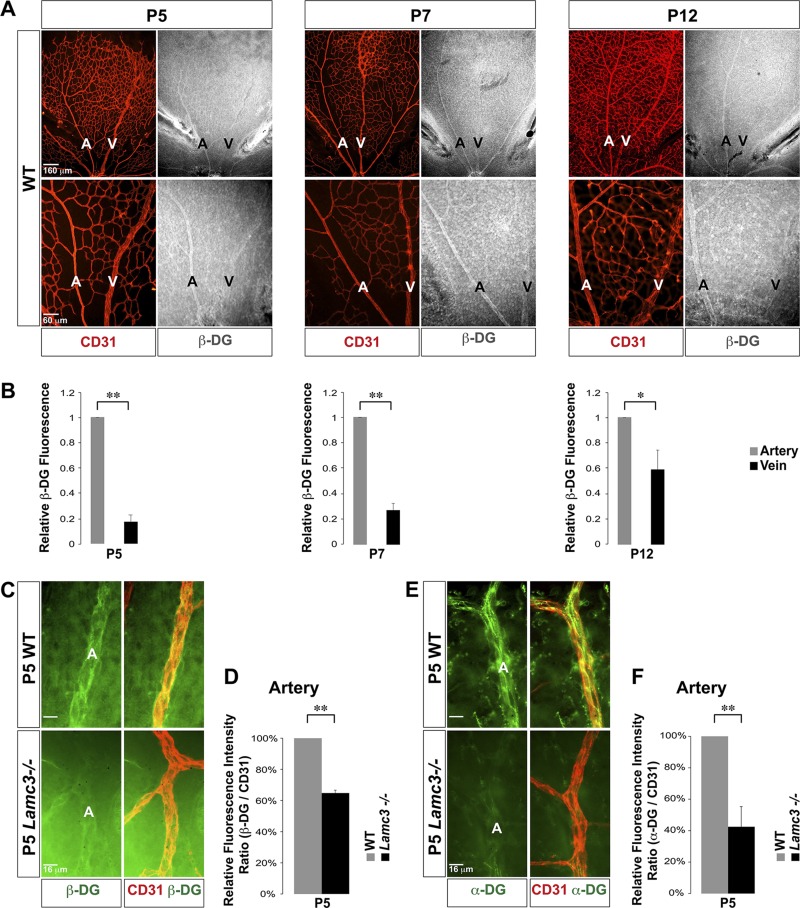
At P5, there was little DG expression at the vascular front and the nascent plexus (Supplemental Fig. S4B). In contrast, DG expression was high in the remodeling zone in the endothelium associated with the emerging arteries (Fig. 4A, B). There was little DG expression in the endothelium associated with the emerging veins, and this pattern was retained through P7. At P12, when retinal arteries and veins are fully specified and their morphology is established, DG expression remained significantly higher in arterial ECs than in venous ECs. Thus, this spatial segregation of DG expression to the arterial side of the superficial vasculature is maintained throughout the vascular maturation.
Figure 4.
Preferential DG expression in arterial ECs during arteriogenesis. A) Labeling with CD31 (red) and β-DG (white) antibodies demonstrated little to no DG expression in the tip cells and the nascent plexus (P5 and 7). The DG expression pattern became artery specific in the remodeling zone as the artery matured. The preferentially arterial expression pattern of DG persisted in the mature superficial vasculature (P12). B) Quantifications showed significantly higher expression of DG in arteries than in veins at these time points (n = 3). C) Higher power images of CD31 (red) and β-DG (green) labeling of arteries. D) Arterial β-DG immunofluorescence was quantified relative to CD31 fluorescence, demonstrating a significant decline in P5 Lamc3−/− arteries (n = 3). E) Higher power images of CD31 (red) and α-DG (green) labeling of arteries. F) Arterial α-DG immunofluorescence was quantified relative to CD31 fluorescence, demonstrating a significant decline in P5 Lamc3−/− arteries (n = 3). A, artery; V, vein. Scale bars: 160 μm, top; 60 μm, bottom (A, B); 16 μm (C, E). Data represent means ± sd (B); means ± sem (D, F). *P < 0.05, **P < 0.02.
Logically, if DG is an important receptor for laminin-γ3–mediated signaling, its expression should be altered in the in the Lamc3−/− retina, as laminins are known to induce their own receptor expression (2, 28). Expressions of both β- and α-DG were significantly down-regulated in P5 Lamc3−/− retinal arterial ECs compared to the WT (Fig. 4D–F). These results are consistent with our hypothesis that DG functions as a γ3-laminin receptor in arterial ECs.
DG-mediated signaling induces endothelial Dll4 expression
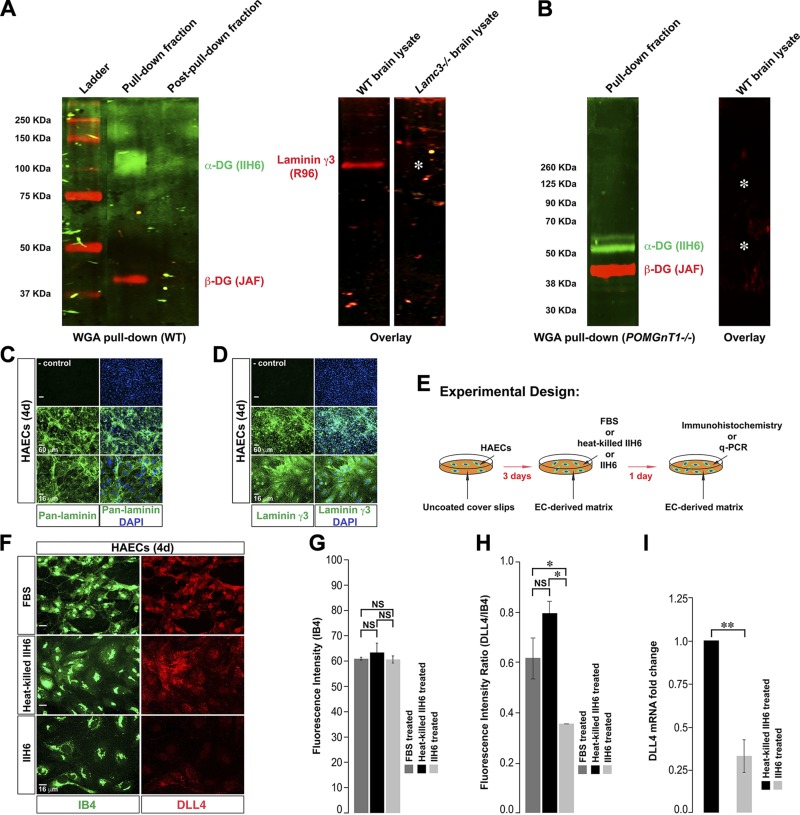
To establish whether γ3-laminins indeed bind DG, we isolated glycosylated proteins from the WT brain lysate with WGA beads. We confirmed that the WGA pulled-down fraction from the WT brain lysate contained glycosylated α- (ligand binding subunit) and β-DG (Fig. 5A). Next, we transferred the WGA pulled-down fraction from the WT brain to membranes and incubated the membranes with either WT or Lamc3−/− brain lysate and probed for laminin-γ3. Indeed, γ3-laminins from the WT brain lysate bound to the same region on the membrane as the glycosylated α-DG. As expected, the γ3 band was not detected in the membrane incubated with Lamc3−/− brain lysate. These results support our hypothesis that γ3-laminins form a complex with the glycosylated α-DG.
Figure 5.
γ3-Laminins bind DG, and blocking ligand–DG binding down-regulates endothelial Dll4 expression. A) Glycosylated proteins were pulled down with WGA beads from the WT brain. The pulled-down fraction contained α- (green) and β- (red) DG, whereas the remaining fraction after pull-down did not. The pulled-down fraction from the WT brain was transferred to PVDF membranes, followed by incubation with either WT or Lamc3−/− brain lysate. Laminin-γ3 in the WT brain lysate bound (red band) to the same position as α-DG on the membrane. The laminin-γ3 band was absent (asterisk) on the membrane incubated with Lamc3−/− brain lysate. B) The α-DG (green) band in the WGA pulled-down fraction from the POMGnT1−/− brain was at a lower molecular mass, consistent with defective α-DG glycosylation. The pulled-down fraction from the POMGnT1−/− brain was transferred to a PVDF membrane, followed by incubation with WT brain lysate. The laminin γ3-band was absent (asterisks) on the membrane incubated with WT brain lysate. C) HAECs, grown on the glass coverslip for 4 d, were stained with anti-pan-laminin (green) antibody and DAPI (nuclear marker: blue). Top: secondary antibody-only control, middle panel: low magnification and bottom panel: higher magnification. D) HAECs, grown on the glass coverslip for 4 d, were stained with anti-laminin-γ3 (green) antibody and DAPI (blue). Top: secondary antibody-only control; middle: low magnification; and bottom: higher magnification images. HAECs made a laminin-γ3–rich matrix in culture. E) The experimental design presented in (F–H). F) HAECs, grown on glass coverslips under the indicated conditions, were stained with IB4 (EC marker: green) and anti-Dll4 (red) antibody. The expression of DLL4 was reduced in HAECs upon DG blocking (IIH6 treatment). G) Quantification of IB4 fluorescence in individual HAECs grown under the indicated conditions (n = 3). IB4 fluorescence in individual endothelial cells is similar between 3 test groups. H) Quantification of DLL4 immunofluorescence relative to IB4 fluorescence demonstrates significant reduction in IIH6 treated HAECs (n = 3). I) mRNA expression of DLL4; DLL4 mRNA was down-regulated in the IIH6-treated HAECs (n = 3). NS, not significant. Scale bars: 60 μm, top 2 rows; 16 μm, bottom row (C, D); 16 μm (F). The data are means ± sem. *P < 0.05, **P < 0.02.
To further confirm whether γ3-laminins form a complex with α-DG, we performed a WGA pull-down assay from the brain lysate of POMGnT1−/− mice (Fig. 5B). These animals have disrupted α-DG glycosylation, resulting in deficient laminin binding (23). Indeed, the α-DG band migrated faster, consistent with an apparent lower molecular mass because of hypoglycosylation, in the WGA pulled-down fraction from the POMGnT1−/− brain, similar to a previous report (29). When we incubated the membrane containing the WGA pulled-down fraction from the POMGnT1−/− brain with WT brain lysate, we did not observe any γ3-laminin band (Fig. 5B). These data demonstrate that γ3-laminins do not form a complex with any proteins in the WGA pulled-down fraction from the POMGnT1−/− brain, supporting the hypothesis that DG is a potential receptor for γ3-laminins.
These results, combined with the other data reported, are consistent with the hypothesis that γ3-laminins signal via DG in arterial ECs to induce Dll4 expression. To test this hypothesis directly, we performed an in vitro assay in primary HAECs, adopting a strategy to reduce DG signaling with function-blocking antibody. Using this approach, we assayed endothelial DLL4 expression. HAECs in culture produce a laminin-rich matrix (Fig. 5C), which includes γ3-laminins (Fig. 5D). We grew HAECs on the glass coverslips for 3 d. Next, we added either only FBS or combined FBS with a DG function-blocking antibody (IIH6) or heat-killed IIH6. We allowed HAECs to grow for 1 more day and assayed DLL4 expression (Fig. 5E, F). We used isolectin (I)-B4 to mark ECs. IB4 immunofluorescence in individual ECs did not alter between the 3 test groups (Fig. 5F, G). In contrast, when we blocked laminin–DG binding (IIH6 treatment), endothelial DLL4 immunofluorescence was significantly lowered compared to both FBS and heat-killed IIH6-treated controls (Fig. 5F, H). Consistent with reduced DLL4 immunofluorescence, DLL4 mRNA expression level was significantly reduced in IIH6-treated HAECs (Fig. 5I). These results suggest that DG-mediated signaling induces Dll4 expression in ECs.
EC-specific deletion of the DG gene replicates the Lamc3−/− arterial phenotype
We next asked whether the disruption of DG-mediated signaling in vivo would have the same effect on arterial Dll4 expression. First, we examined a mouse model where DG’s ligand binding ability is disrupted because of the deletion of the POMGnT1 gene (23). Superficial vascular expansion along the retinal surface was severely inhibited in POMGnT1−/− mice, along with the persistence of hyaloid vessels (Supplemental Fig. S5). Therefore, an analysis of arterial morphology was impossible in these mutant retinae.
As an alternative, we deleted Dag1 specifically from ECs (Dag1ΔEC) by crossing Tie2-Cre mice with Dag1-floxed mice (Supplemental Fig. S6A) and assayed the consequent arterial phenotype. We confirmed genotypes by PCR (Supplemental Fig. S6B) and loss of DG expression in ECs by immunohistochemistry (Supplemental Fig. S6C). The residual DG immunoreactivity in Dag1ΔEC arteries was likely from astrocyte endfeet (Supplemental Fig. S6C), which are known to express DG (30). We also examined 3 major nonendothelial cell types that influence retinal angiogenesis: ganglion cells, microglia, and astrocytes. None of these cell types exhibited abnormal distribution in the Dag1ΔEC retina (Supplemental Fig. S6D).
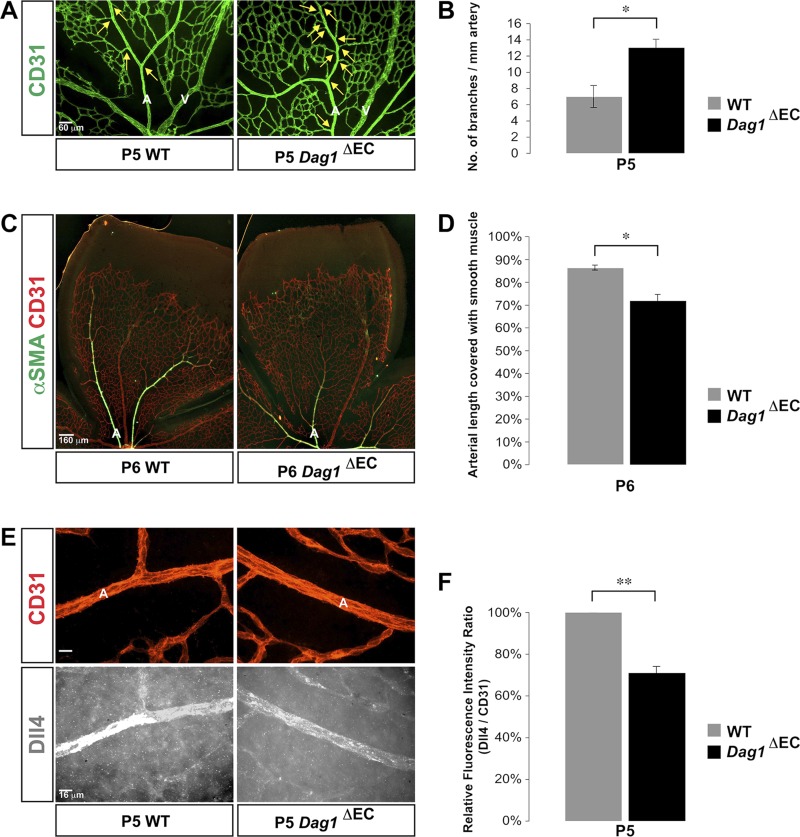
As in the Lamc3−/− retina, Dag1ΔEC retinae exhibited significantly increased arterial branching compared to the littermate controls (Fig. 6A, B). Moreover, the extent of arterial smooth muscle coverage was significantly reduced in Dag1ΔEC retinae compared to the littermate controls (Fig. 6C, D). Finally, arterial Dll4 expression was significantly down-regulated in Dag1ΔEC retinae (Fig. 6E, F), phenocopying Lamc3−/− arterial Dll4 expression. These data strongly support our hypothesis that laminin γ3–DG signaling regulates retinal arteriogenesis by inducing Dll4/Notch signaling in arterial ECs.
Figure 6.
EC-specific deletion of DG gene reproduces Lamc3−/− retinal arterial phenotype. A) CD31 (green) labeling demonstrated more arterial branches (arrows) in the Dag1ΔEC retina at P5. B) Quantification of arterial branching demonstrated a significant increase in Dag1ΔEC retinae (n = 3). C) CD31 (red) and αSMA (green) labeling demonstrated less extensive vascular smooth muscle coverage of Dag1ΔEC arteries at P6. D) Quantification of the extent of arterial smooth muscle coverage, relative to vascular migration distance, demonstrated significant reduction in Dag1ΔEC arteries compared to the littermate WT control (n = 3). E) CD31 (red) and Dll4 (white) labeling of arteries demonstrated down-regulation of arterial Dll4 expression in the Lamc3−/− retina. F) Quantification of Dll4 immunofluorescence relative to CD31 fluorescence demonstrated a significant decline in P5 Dag1ΔEC arteries (n = 3). A, artery; V, vein. Scale bars: 60 μm (A), 160 μm (C), 16 μm (E). Data are means ± sem. *P < 0.05, **P < 0.02.
DISCUSSION
VEGF/VEGFR2 and laminin γ3–DG signaling cooperate to induce arterial Dll4 expression
The CNS, including the retina, vascularizes primarily by sprouting new blood vessels (31). All ECs in retinal sprouts initially display venous character. Distinct morphologic and molecular identities of retinal arteries are not apparent until P3–4 (4, 5). The mechanisms underlying this shift from venous to arterial identity during retinal angiogenesis are still poorly understood. Moreover, these mechanisms remain active throughout life, and disruptions of these processes in pathologic conditions account for several vascular diseases in the retina that lead to abnormal arterial and venous patterning (32, 33).
The Dll4/Notch pathway has been shown to be crucial for arterial specification (7), branching (9), and vascular pruning (25). However, the molecular mechanisms that regulate arterial Dll4 expression are not completely understood. VEGF can induce arterial Dll4 expression (10). However, Pan et al. (11) reported that retinal arteries formed in mice, despite treatment with anti-VEGF or anti-Nrp1 (VEGFR2 coreceptor) antibodies. Benedito et al. (12) reported that retinal arteries still formed in mice where VEGFR2 was inducibly deleted in ECs. Even in mice that selectively expressed either VEGF isoform-188 or -120, retinal arteries formed, although their development was delayed and their number was reduced (34). These observations suggest that arterial Dll4 expression, and subsequent arteriogenesis, are regulated by yet another mechanism in concert with VEGF-mediated signaling.
BM laminin-411 has been shown to directly induce Dll4 expression in endothelial tip cells via integrin-mediated signaling (16). However, there is no evidence to date that BM laminins regulate Dll4 expression in ECs during arteriogenesis. Moreover, it is unknown whether other laminin receptors, such as DG, can induce endothelial Dll4 expression.
Our laboratory identified the laminin-γ3 chain as a component of unique laminin isoforms in the CNS, heavily deposited in the vascular BM (2, 17, 35). During retinal angiogenesis, γ3-laminins are not deposited around tip cells, but are deposited in the stalk cell BM. In the nascent plexus, γ3-laminins are restricted to the vascular branch points (2). In the remodeling zone, γ3-laminins are found on microvessels as well as emerging arterial and venous BMs. In contrast, γ3-laminins are found only in venous and microvascular BMs in adult retinal vessels (2, 17). This complex spatial and temporal regulation suggests an important functional role for γ3-laminins.
In this study, we found the first biochemical evidence that γ3-laminins bind DG. DG is not expressed at the vascular front during retinal angiogenesis. Thus, laminin-γ3–DG signaling does not take place there. Arterial ECs preferentially express high levels of DG in the remodeling zone, with little expression in the venous ECs. Thus, the arterial compartment is unique in its combined expression of both γ3-laminins and high levels of DG. Blocking DG-mediated signaling in vitro down-regulates endothelial Dll4 expression. Moreover, arterial Dll4 expression is down-regulated in both Lamc3−/− and Dag1ΔEC retinae. These results strongly suggest a regulatory role for laminin-γ3–DG signaling in arterial Dll4 expression. However, it should be noted that arterial Dll4 expression is not completely abolished in Lamc3−/− and Dag1ΔEC retinae, suggesting that other regulatory pathways (such as VEGF/VEGFR2) still induce some Dll4 expression in these ECs.
Laminin-γ3–DG signaling regulates arterial morphogenesis in the retina
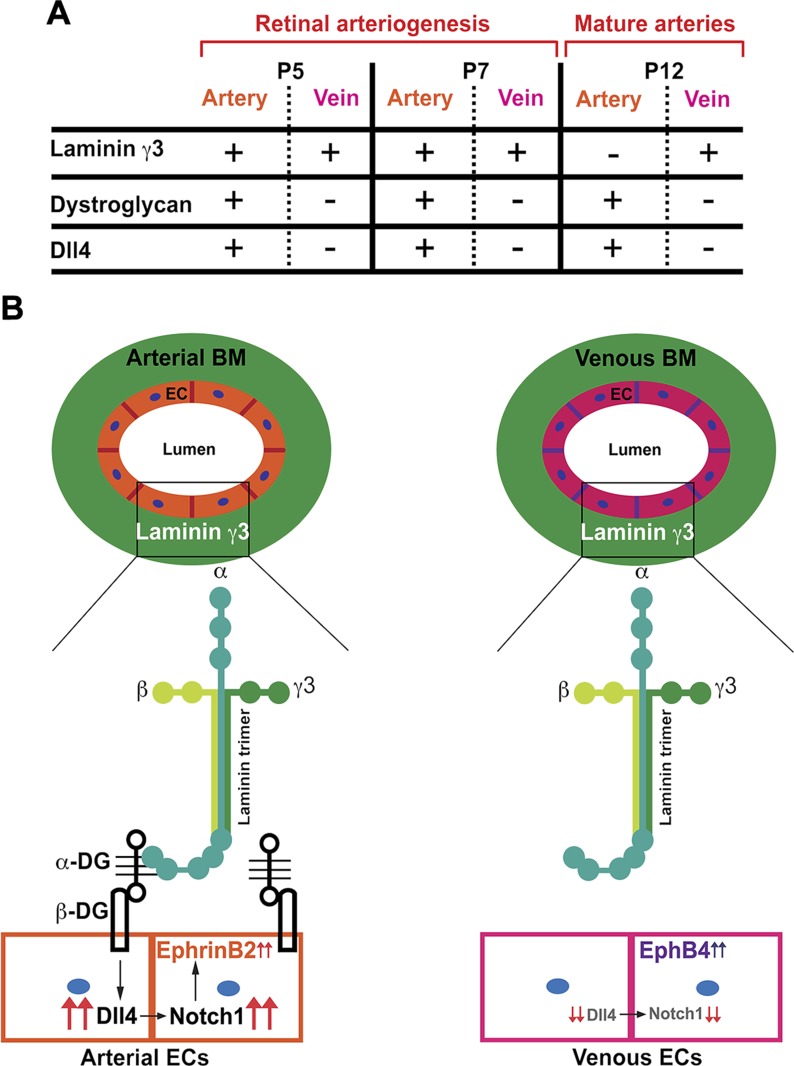
Based on our results, we postulate that γ3-laminins signal through DG to induce arterial identity in those ECs that express both DG and γ3-laminins. Laminin-γ3–DG signaling induces Dll4 expression in arterial ECs, which in turn activates Notch signaling and leads to proper arterial specification and arterial morphogenesis. In contrast, venous ECs express little DG during this period. The absence of laminin γ3–DG signaling inhibits Dll4 expression in venous ECs (Fig. 7A, B).
Figure 7.
γ3-Laminin–DG signaling regulates retinal arterial morphogenesis. A) During retinal arteriogenesis (P5 and P7), γ3-laminins are present in both arterial and venous BMs. However, only arterial ECs express DG during this time, paralleling the artery-specific expression pattern of Dll4. By P12, retinal arteries are fully specified and arterial morphology is established. Although γ3-laminin expression in the arterial BM is drastically reduced at this time, DG and Dll4 continue to be expressed preferentially by arterial ECs. B) We postulate that the γ3-laminin signal through DG to arterial ECs during arteriogenesis. This laminin–DG signaling induces Dll4 expression in arterial ECs, leading to proper arterial morphogenesis and arterial Ephrin-B2 expression. The absence of laminin–DG signaling inhibits Dll4 expression in venous endothelial cells. Consequently, venous endothelial cells express venous marker Eph-B4.
One question that remains to be elucidated is the molecular signaling downstream of laminin-γ3–DG binding. One potential pathway that can be activated by laminin-γ3–DG signaling is the extracellular signal–related kinase (ERK), also known as MAPK, which is one of the earliest arterial markers (36). β-DG acts as an adaptor for activated ERK-1, and activated ERK-1 is specifically localized at the cell membrane–matrix interface (37). Whether laminin-γ3–DG signaling induces endothelial Dll4 expression via activation of the ERK/MAPK signaling pathway remains to be tested. Another possible mechanism is that β-DG has a nuclear localization signal (38). Whether nuclear translocation of β-DG is facilitated by γ3-laminin binding and whether β-DG acts as a transcriptional regulator to induce Dll4 expression needs to be examined.
Supporting our model, Lamc3−/− and Dag1ΔEC retinae exhibit similar arterial dysgenesis, such as hyperbranching, which phenocopies Dll4+/− vasculature in the brain (9), most likely caused by reduced periarterial vascular pruning. It should be noted that vascular defects in the Lamc3−/− retina are milder than those in the Dll4+/− retina (18, 19). The milder phenotype in the Lamc3−/− relative to the Dll4+/− mouse is perhaps best explained by the spatial expression of γ3-containing laminins and DG, neither of which are present at the tip cells.
We observed a similar reduction in arterial smooth muscle coverage in Lamc3−/− and Dag1ΔEC retinae, phenocopying Eogt−/− retinae in which Dll4 binding to Notch1 receptor is disrupted (39). The arterial dysgenesis, detectable in the Lamc3−/− retina as early as P5, persisted even after retinal arteries were fully specified and arterial morphology is established (P12). However, laminin-γ3 expression drastically decreases in the arterial BM by P12. One possibility is that once the arterial phenotype is established during an early developmental window, it persists into adulthood. A previous study showed that disrupting endothelial Notch signaling after P10 does not affect the arterial phenotype in the mouse retina, which supports our interpretation (40).
In summary, our study introduces a novel laminin–DG signaling–dependent regulation of arteriogenesis in the retina, perhaps functioning in parallel to the VEGF signaling. Specifically, for the first time, we identified DG as a receptor for γ3-laminins. We propose that the spatiotemporal regulation of laminin γ3-DG signaling is important for proper arteriogenesis in the retina and perhaps throughout the CNS.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank members of the Center for Vision Research for their help and input during the study, and Dmitri Serjanov [Department of Ophthalmology, Department of Neuroscience and Physiology, and The Center for Vision Research, State University of New York (SUNY) Upstate Medical University, Syracuse, New York, USA] for reading the manuscript. This work was funded by U.S. National Institutes of Health, National Eye Institute Grant R01EY12676 (to W.J.B.), and an Unrestricted Grant to the Department of Ophthalmology (Robert Fechtner, M.D., Chair and Principal Investigator) from Research to Prevent Blindness. The authors declare no conflicts of interest.
Glossary
- α-SMA
smooth muscle actin
- BM
basement membrane
- CD
cluster of differentiation
- DG
dystroglycan
- Dll
Delta-like ligand
- EC
endothelial cell
- FBS
fetal bovine serum
- HAEC
human aortic endothelial cell
- PFA
paraformaldehyde
- POMGNT
protein O-mannose β-1,2-N-acetylglucosaminyltransferase
- qPCR
quantitative PCR
- VEGFR
VEGF receptor
- WGA
wheat germ agglutinin
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Biswas and J. Watters designed and performed research, analyzed the data, and wrote the manuscript; G. Bachay and S. Varshney performed the research; D. D. Hunter designed the research and critiqued the data analysis; H. Hu designed the research and provided key reagents; W. J. Brunken designed the research, analyzed the data, wrote the manuscript, provided key reagents, and obtained funding.
REFERENCES
- 1.Stahl A., Connor K. M., Sapieha P., Chen J., Dennison R. J., Krah N. M., Seaward M. R., Willett K. L., Aderman C. M., Guerin K. I., Hua J., Löfqvist C., Hellström A., Smith L. E. (2010) The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 51, 2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnanaguru G., Bachay G., Biswas S., Pinzón-Duarte G., Hunter D. D., Brunken W. J. (2013) Laminins containing the β2 and γ3 chains regulate astrocyte migration and angiogenesis in the retina. Development 140, 2050–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas S., Bachay G., Chu J., Hunter D. D., Brunken W. J. (2017) Laminin-dependent interaction between astrocytes and microglia: a role in retinal angiogenesis. Am. J. Pathol. 187, 2112–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemura A., Kusuhara S., Katsuta H., Nishikawa S. (2006) Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp. Cell Res. 312, 676–683 [DOI] [PubMed] [Google Scholar]
- 5.Crist A. M., Young C., Meadows S. M. (2017) Characterization of arteriovenous identity in the developing neonate mouse retina. Gene Expr. Patterns 23-24, 22–31 [DOI] [PubMed] [Google Scholar]
- 6.Simons M., Eichmann A. (2015) Molecular controls of arterial morphogenesis. Circ. Res. 116, 1712–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duarte A., Hirashima M., Benedito R., Trindade A., Diniz P., Bekman E., Costa L., Henrique D., Rossant J. (2004) Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18, 2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitsebaomo J., Portbury A. L., Schisler J. C., Patterson C. (2008) Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circ. Res. 103, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristofaro B., Shi Y., Faria M., Suchting S., Leroyer A. S., Trindade A., Duarte A., Zovein A. C., Iruela-Arispe M. L., Nih L. R., Kubis N., Henrion D., Loufrani L., Todiras M., Schleifenbaum J., Gollasch M., Zhuang Z. W., Simons M., Eichmann A., le Noble F. (2013) Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development 140, 1720–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z. J., Shirakawa T., Li Y., Soma A., Oka M., Dotto G. P., Fairman R. M., Velazquez O. C., Herlyn M. (2003) Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell. Biol. 23, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q., Chanthery Y., Liang W. C., Stawicki S., Mak J., Rathore N., Tong R. K., Kowalski J., Yee S. F., Pacheco G., Ross S., Cheng Z., Le Couter J., Plowman G., Peale F., Koch A. W., Wu Y., Bagri A., Tessier-Lavigne M., Watts R. J. (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11, 53–67 [DOI] [PubMed] [Google Scholar]
- 12.Benedito R., Rocha S. F., Woeste M., Zamykal M., Radtke F., Casanovas O., Duarte A., Pytowski B., Adams R. H. (2012) Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484, 110–114 [DOI] [PubMed] [Google Scholar]
- 13.Aumailley M., Bruckner-Tuderman L., Carter W. G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J. C., Kleinman H. K., Marinkovich M. P., Martin G. R., Mayer U., Meneguzzi G., Miner J. H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J. R., Sasaki T., Sekiguchi K., Sorokin L. M., Talts J. F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U. M., Yamada Y., Yurchenco P. D. (2005) A simplified laminin nomenclature. Matrix Biol. 24, 326–332 [DOI] [PubMed] [Google Scholar]
- 14.Macdonald P. R., Lustig A., Steinmetz M. O., Kammerer R. A. (2010) Laminin chain assembly is regulated by specific coiled-coil interactions. J. Struct. Biol. 170, 398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten K. M., Akassoglou K. (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 71, 1018–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenzel D., Franco C. A., Estrach S., Mettouchi A., Sauvaget D., Rosewell I., Schertel A., Armer H., Domogatskaya A., Rodin S., Tryggvason K., Collinson L., Sorokin L., Gerhardt H. (2011) Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12, 1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y. N., Radner S., French M. M., Pinzón-Duarte G., Daly G. H., Burgeson R. E., Koch M., Brunken W. J. (2012) The γ3 chain of laminin is widely but differentially expressed in murine basement membranes: expression and functional studies. Matrix Biol. 31, 120–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobov I. B., Renard R. A., Papadopoulos N., Gale N. W., Thurston G., Yancopoulos G. D., Wiegand S. J. (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellström M., Phng L. K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A. K., Karlsson L., Gaiano N., Yoon K., Rossant J., Iruela-Arispe M. L., Kalén M., Gerhardt H., Betsholtz C. (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 [DOI] [PubMed] [Google Scholar]
- 20.Pinzón-Duarte G., Daly G., Li Y. N., Koch M., Brunken W. J. (2010) Defective formation of the inner limiting membrane in laminin beta2- and gamma3-null mice produces retinal dysplasia. Invest. Ophthalmol. Vis. Sci. 51, 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisanuki Y. Y., Hammer R. E., Miyazaki J., Williams S. C., Richardson J. A., Yanagisawa M. (2001) Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 [DOI] [PubMed] [Google Scholar]
- 22.Cohn R. D., Henry M. D., Michele D. E., Barresi R., Saito F., Moore S. A., Flanagan J. D., Skwarchuk M. W., Robbins M. E., Mendell J. R., Williamson R. A., Campbell K. P. (2002) Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110, 639–648 [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Ball S. L., Yang Y., Mei P., Zhang L., Shi H., Kaminski H. J., Lemmon V. P., Hu H. (2006) A genetic model for muscle-eye-brain disease in mice lacking protein O-mannose 1,2-N-acetylglucosaminyltransferase (POMGnT1). Mech. Dev. 123, 228–240 [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa M., Hojo M., Imayoshi I., Goto M., Ando M., Ohtsuka T., Kageyama R., Miyamoto S. (2013) Hes1 and Hes5 regulate vascular remodeling and arterial specification of endothelial cells in brain vascular development. Mech. Dev. 130, 458–466 [DOI] [PubMed] [Google Scholar]
- 25.Lobov I. B., Cheung E., Wudali R., Cao J., Halasz G., Wei Y., Economides A., Lin H. C., Papadopoulos N., Yancopoulos G. D., Wiegand S. J. (2011) The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 117, 6728–6737 [DOI] [PubMed] [Google Scholar]
- 26.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ido H., Ito S., Taniguchi Y., Hayashi M., Sato-Nishiuchi R., Sanzen N., Hayashi Y., Futaki S., Sekiguchi K. (2008) Laminin isoforms containing the γ3 chain are unable to bind to integrins due to the absence of the glutamic acid residue conserved in the C-terminal regions of the γ1 and γ2 chains. J. Biol. Chem. 283, 28149–28157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condic M. L., Letourneau P. C. (1997) Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature 389, 852–856 [DOI] [PubMed] [Google Scholar]
- 29.Yu M., He Y., Wang K., Zhang P., Zhang S., Hu H. (2013) Adeno-associated viral-mediated LARGE gene therapy rescues the muscular dystrophic phenotype in mouse models of dystroglycanopathy. Hum. Gene Ther. 24, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O., Sorokin L. M. (2001) Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153, 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saint-Geniez M., D’Amore P. A. (2004) Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 48, 1045–1058 [DOI] [PubMed] [Google Scholar]
- 32.Henkind P., Wise G. N. (1974) Retinal neovascularization, collaterals, and vascular shunts. Br. J. Ophthalmol. 58, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck S. D., Zacks D. N., Eibschitz-Tsimhoni M. (2005) Retinal and intracranial arteriovenous malformations: Wyburn-Mason syndrome. J. Neuroophthalmol. 25, 205–208 [DOI] [PubMed] [Google Scholar]
- 34.Stalmans I., Ng Y. S., Rohan R., Fruttiger M., Bouché A., Yuce A., Fujisawa H., Hermans B., Shani M., Jansen S., Hicklin D., Anderson D. J., Gardiner T., Hammes H. P., Moons L., Dewerchin M., Collen D., Carmeliet P., D’Amore P. A. (2002) Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 109, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby R. T., Champliaud M. F., Claudepierre T., Xu Y., Gibbons E. P., Koch M., Burgeson R. E., Hunter D. D., Brunken W. J. (2000) Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J. Neurosci. 20, 6517–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y., Larrivée B., Zhuang Z. W., Atri D., Moraes F., Prahst C., Eichmann A., Simons M. (2013) Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood 121, 3988–3996, S1–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence H. J., Dhillon A. S., James M., Winder S. J. (2004) Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 5, 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oppizzi M. L., Akhavan A., Singh M., Fata J. E., Muschler J. L. (2008) Nuclear translocation of beta-dystroglycan reveals a distinctive trafficking pattern of autoproteolyzed mucins. Traffic 9, 2063–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawaguchi S., Varshney S., Ogawa M., Sakaidani Y., Yagi H., Takeshita K., Murohara T., Kato K., Sundaram S., Stanley P., Okajima T. (2017) O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. eLife 6, 24419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehling M., Adams S., Benedito R., Adams R. H. (2013) Notch controls retinal blood vessel maturation and quiescence. Development 140, 3051–3061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.