Abstract
Melanocortin 2 receptor accessory protein (MRAP) is a single transmembrane domain accessory protein and a critical component of the hypothamo-pituitary-adrenal axis. MRAP is highly expressed in the adrenal gland and is essential for adrenocorticotropin hormone (ACTH) receptor expression and function. Human loss-of-function mutations in MRAP cause familial glucocorticoid (GC) deficiency (FGD) type 2 (FGD2), whereby the adrenal gland fails to respond to ACTH and to produce cortisol. In this study, we generated Mrap-null mice to study the function of MRAP in vivo. We found that the vast majority of Mrap−/− mice died at birth but could be rescued by administration of corticosterone to pregnant dams. Surviving Mrap−/− mice developed isolated GC deficiency with normal mineralocorticoid and catecholamine production, recapitulating FGD2. The adrenal glands of adult Mrap−/− mice were small, with grossly impaired adrenal capsular morphology and cortex zonation. Progenitor cell differentiation was significantly impaired, with dysregulation of WNT4/β-catenin and sonic hedgehog pathways. These data demonstrate the roles of MRAP in both steroidogenesis and the regulation of adrenal cortex zonation. This is the first mouse model of isolated GC deficiency and reveals the role of MRAP in adrenal progenitor cell regulation and cortex zonation.—Novoselova, T. V., Hussain, M., King, P. J., Guasti, L., Metherell, L. A., Charalambous, M., Clark, A. J. L., Chan, L. F. MRAP deficiency impairs adrenal progenitor cell differentiation and gland zonation.
Keywords: ACTH, accessory protein, melanocortin, cell fate, familial glucocorticoid deficiency
Melanocortin receptor accessory proteins (MRAPs) are a class of single-pass transmembrane domain accessory protein (1–3). MRAP and MRAP2 have been shown to interact in vitro with the melanocortin receptors (MCRs), a family of GPCRs with diverse physiological functions that are stimulated by pro-opiomelanocortin–derived peptide agonists. MRAP2 is implicated in metabolic regulation via its interaction with MC4R, although other mechanisms may be involved (2, 4–7).
MRAP is essential for the function of the adrenocorticotropin hormone (ACTH) receptor/melanocortin 2 receptor (MC2R) and is highly expressed in adrenal glands (1). MRAP has been shown to form unique antiparallel homodimers that complex with, and assist, MC2R trafficking and signaling (8–10). The MRAP/MC2R complex on the surface of adrenocortical cells is essential to mediate the action of pituitary ACTH and subsequent steroidogenesis. Mutations in MC2R account for up to 25% of cases of familial glucocorticoid (GC) deficiency (FGD) type 1 (FGD1; OMIM:202200) (11, 12). FGD manifests in early childhood with hypoglycemia, lethargy, and overwhelming infection due to isolated GC deficiency while maintaining normal aldosterone levels. Mutations in MRAP are responsible for ∼20% of cases of FGD type 2 (FGD2; OMIM: 609196) and cause earlier disease onset compared with FGD1 (13).
Hormones secreted from the three concentric zones of the adrenal cortex are vital for life and general well-being. Mineralocorticoids (aldosterone) from the outer zona glomerulosa (ZG) regulate electrolyte levels and blood pressure. The inner zona fasciculata (ZF) is responsible for GC production (cortisol in humans and corticosterone in the majority of rodents, except the spiny mouse that produces cortisol) and is important for the stress response and for metabolic and immune function. The most medial zona reticularis (ZR) in humans secretes adrenal androgens that control adrenarche in puberty. Unlike in humans, the innermost zone in mice is called the X zone. This distinct zone is the remaining remnants of the fetal zone, which regresses during the first pregnancy in female mice and at puberty in male mice (14). The mechanisms controlling adrenal cortex zonation and cell renewal are under increasing scrutiny and have been implicated in adrenal diseases, tumorigenesis, and aging (15, 16). In particular, the adrenal capsule, a thin covering of cells adjacent to the ZG, and the subscapular region are thought to house the pool of adrenal stem/progenitor cells capable of dividing, migrating centripetally, and differentiating into mature steroid-producing cell types (15–17). A number of paracrine and endocrine factors have been implicated in adrenal zonation and renewal, including sonic hedgehog (SHH) signaling, WNT/β-catenin pathways, and, more recently, ACTH-mediated PKA activation (15, 18, 19). Our previous work, localizing MRAP to the undifferentiated zone of the rat adrenal gland, allowed us to question the importance of MRAP in adrenal cell differentiation and maintenance (20). In this study, we generated Mrap−/− mice to investigate the function of MRAP in FGD pathophysiology and its role in adrenocortical development and maintenance.
MATERIALS AND METHODS
Generation of Mrap−/− mice
Design of targeting vector and generation of Mrap−/− mice was performed by Genoway (Lyon, France). The strategy is illustrated in Fig. 1A. Briefly, the genomic region of interest was amplified by PCR and cloned into the targeting vector containing a neomycin selection cassette flanked by flippase recognition target sites and loxP flanking sites. All PCR fragments were validated by DNA sequencing. The targeting vector was electroporated into C57Bl/6 ES cells. G418-resistant clones were harvested and screened by PCR and Southern blot analysis. The recombined ES cell clones were microinjected into C57BL/6J blastocysts. To generate global Mrap−/− mice, male mice with the Mrap recombined locus were bred with female mice expressing Flp recombinase to remove the selectable marker. Animals were bred with germline Cre delete mice to excise exon 1 of Mrap.
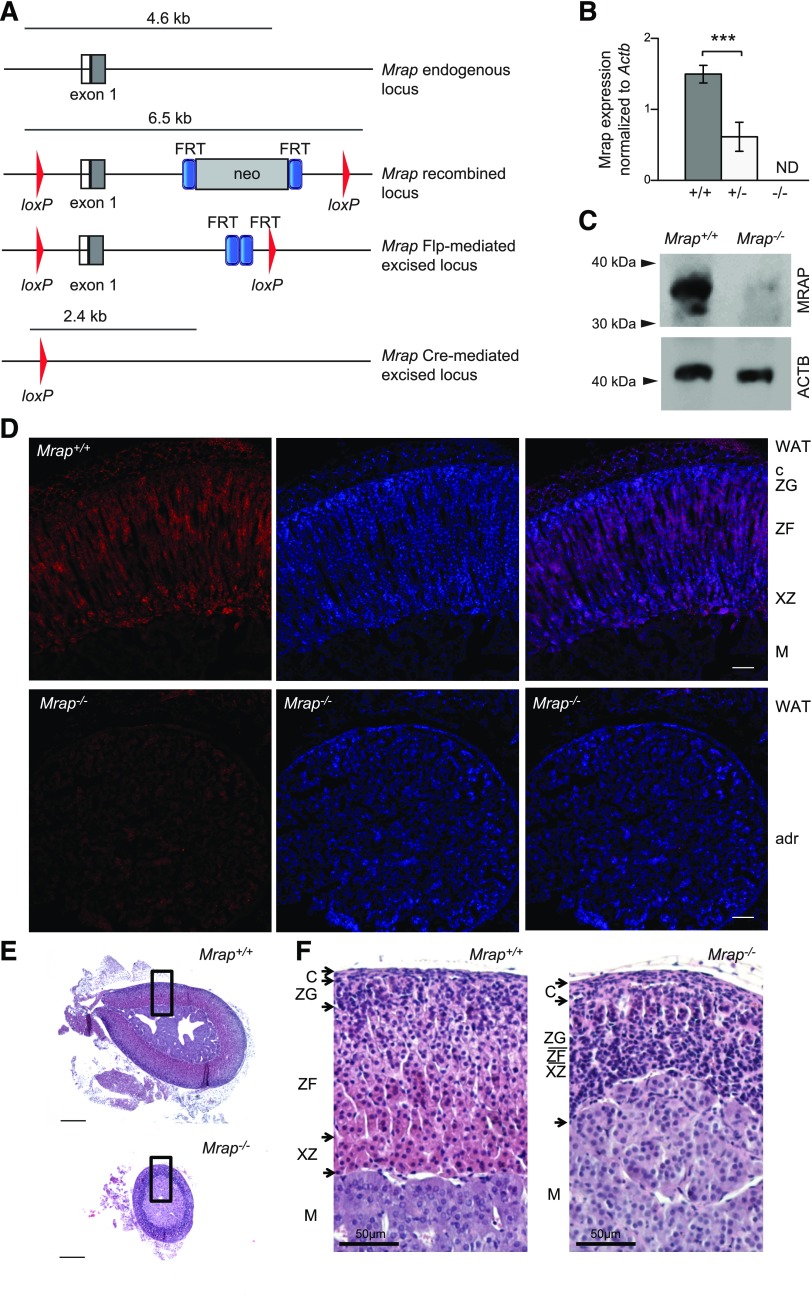
Figure 1.
Generation of Mrap−/− mice. A) Schematic representation of targeting of the exon 1 of Mrap mouse for the line production. B) qRT-PCR analysis of Mrap expression in the adrenal gland of Mrap+/+ (n = 7), Mrap+/− (n = 7), and Mrap−/− (n = 4) mice showing that Mrap+/− mice express approximately half of the Mrap transcript found in the wild-type adrenals, whereas no transcript was detected in Mrap−/−adrenals. ***P < 0.0005, Student’s t-test. C) Immunoblotting of adrenal gland lysates from Mrap+/+ and Mrap−/− mice with anti-MRAP and anti–actin b (ACTB) antibodies. The arrows on the left indicate the approximate MW position. The position of the main band in Mrap+/+ corresponds to the anticipated size of a mature MRAP dimer, with the lower band representing the nonglycosylated form. D) Immunostaining of MRAP (red) in the adrenal gland of Mrap+/+ (top panel) and Mrap−/− mice (bottom panel). The nuclei are stained with DAPI (blue). The adrenal zonation is marked in the wild-type gland as follows: C, capsule; M, medulla; WAT, white adipose tissue; XZ, X-zone. Scale bars, 100 μm. E) Representative images of the adrenals from the Mrap+/+ and Mrap−/− mice sectioned though the middle of the organ, demonstrating the reduction in the gland’s size. The black boxes indicate the where the higher-magnification images were obtained. Scale bars, 200 μm. F) Adrenal gland zonation is impaired in Mrap−/− mice (right panel) compared with Mrap+/+ mice (left panel) as shown by the H&E staining. Panel images are from female mice, although similar adrenal gland zonation impairment is seen in male mice (data not shown). Adrenal gland zonation in male mice: C, capsule; M, medulla; XZ, X zone. The zonation in the Mrap−/− adrenal gland is not defined. Scale bars, 50 µm.
Animal husbandry, procedures, and hormone analysis
The care and use of all animals were carried out in accordance with the United Kingdom Home Office Regulations, United Kingdom Animals (Scientific Procedures). Mice were kept under standard 12-h light/dark cycle with food and water ad libitum unless stated otherwise. Corticosterone hormone replacement was performed by administering 50 μg/ml in drinking water to breeder cages starting embryonic day (E)17.5; drinking water was changed daily. The treatment was stopped at weaning [postnatal day (P)21] or continued until 8 wk of lifetime corticosterone replacement. An ACTH stimulation test was performed in the morning by intraperitoneal injection of 10 ng/g synacthen (Alliance Pharmaceutical, Chippenham, United Kingdom). Mice were euthanized by exsanguination 1 h after injection. For ACTH measurement, the animals were fasted for 16 h followed by exsanguination. Twenty-four–hour urine was collected using metabolic cages. Heparinized plasma was subject to ELISA for corticosterone and aldosterone (KGE009 and KGE016, respectively; R&D Systems, Abingdon, United Kingdom). ACTH was measured using a 6-Plex Luminex Assay (Thermo Fisher Scientific, Cheshire, United Kingdom). Urine catecholamines and creatinine were assessed by HPLC.
Histology and histochemistry
Tissues were fixed in ice-cold 4% paraformaldehyde in PBS followed by paraffin embedding for hematoxylin and eosin (H&E) staining. For immunohistochemistry, tissues were cryoprotected with 20% sucrose in PBS after fixation followed by cryosectioning and heat-mediated antigen retrieval with citric buffer (10 mM, pH 6.0) as previously described (18). The primary antibodies used in this study were rabbit anti-MRAP 1/20 (10); mouse anti-CYP11B1 and rabbit anti-CYP11B2, both 1/50 (kind gift of Dr. C. E. Gomez-Sanches, University of Mississippi, Jackson, MI, USA); rabbit anti-PNMT 1/250 (Abcam, Cambridge, United Kingdom); mouse anti–β-catenin 1/300 (MilliporeSigma, Cambridge, United Kingdom); rabbit anti-WNT4 1/20 (Abcam); mouse anti-StAR 1/400 (Abcam), rabbit anti-20αHSD (1/5000, kind gift from Y. Weinstein, Israel), rabbit anti-DAB2 1:200 (Santa Cruz Biotechnology, Dallas, TX, USA), and rabbit anti-LEF1 1/100 (Abcam). In situ hybridization was performed as described by Guasti et al. (21). The Shh sequence used for RNA probe synthesis was derived from adult mouse adrenal cDNA using the primers forward, 5′-ATGCTGCTGCTGCTGGCCAGATG-3′ and reverse, 5′-GGGCCCCGAGTCGTTGTGCGGCG-3′. The 849 bp fragment was then ligated into pGEM-Teasy (Promega, Madison, WI, USA) and confirmed by Sanger sequencing. T7 and SP6 (Roche, Basel, Switzerland) were used for in vitro translation to produce the riboprobe. Capsule counts were performed using at least eight H&E-stained sections cut approximately through the middle, and capsule thickness (μm) was measured using the average of at least three measurements for each section analyzed. The images were analyzed and processed using Leica LAS AF (Leica Microsystems, Wetzlar, Germany), Adobe Photoshop, and Adobe Illustrator (San Jose, CA, USA).
Western blotting
Adrenal glands were lysed in 2× Laemmli sample buffer, sonicated for 10 s, and centrifuged at 13,000 g for 15 min. The samples were then denatured for 1 h at 37°C and loaded onto 4–12% Bis-Tris Novex Acrylamide Gel (Thermo Fisher Scientific). Western blotting was performed per standard protocol and immunostained using rabbit anti-MRAP antibody 1/500 and mouse anti-actin b 1/10,000 (Abcam), anti-rabbit Odyssey Li-Cor 800, and anti-mouse Odyssey Li-Cor 680 secondary antibodies and visualized using the Odyssey Fc Imaging System (Li-Cor Biosciences, Lincoln, NE, USA).
Quantitative RT-PCR
The tissue was dissected and immediately placed in liquid nitrogen. RNA was then extracted using the RNAsy Kit (Qiagen, Hilden, Germany), and 1 μg of RNA was used for cDNA synthesis using SuperScript IV RT (Thermo Fisher Scientific). TaqMan predesigned assays (Thermo Fisher Scientific, listed in Supplemental Data, and Supplemental Table 1) were then performed according to the manufacturer’s protocol, including no template or reverse transcription control, and analyzed using the ΔCt method.
Statistical analysis
All values were calculated as means ± sem. Comparisons of two groups were analyzed by Student’s t test. For more than 2 groups, 1-way ANOVA was performed followed by Bonferroni multiple comparison test. In all analyses, a 2-tailed probability of P < 0.05 was considered statistically significant.
RESULTS
Generation and production of Mrap−/− mice
Mrap−/− mice were produced by deletion of exon 1 of Mrap (Fig. 1A). No Mrap transcript was detected in the knock-out animals, whereas Mrap+/− mice produced approximately half of the transcript level found in the wild-type littermates (Fig. 1B). The lack of protein expression in Mrap−/− mice was confirmed using an anti-MRAP antibody. Immunoblotting demonstrated a stable homodimer of MRAP, with electrophoretic mobility of ∼35 kDa in the adrenal gland of Mrap+/+ mice but not in the mutant (Fig. 1C). Immunohistochemistry showed that MRAP is detected mostly in the ZF in the wild-type adrenal gland with some staining in the X zone (Fig. 1D and Supplemental Fig. 1). This staining was not observed in the adrenal gland of Mrap−/− mice, consistent with the absence of MRAP protein production (Fig. 1D).
Mrap deficiency was associated with neonatal lethality, which was prevented with corticosterone replacement
Of the 325 mice generated from Mrap+/− intercrosses, only three Mrap−/− mice survived until weaning without corticosterone replacement. Mrap−/− mice were usually found dead before P0.5 without milk in their stomach. Excised lungs from deceased neonatal Mrap−/− mice sank when submerged in water/saline, suggesting a failure of lung inflation. It is known that fetal GCs play an important role in fetal preparation for neonatal life by facilitating lung maturation and hepatic glycogen accumulation. We examined E19.5 embryos and found that the lungs of the mutant mice resembled immature lungs prior to terminal differentiation of the alveoli (Supplemental Fig. 2A). Mrap mRNA was not detected in wild-type fetal (at E15.5; Supplemental Fig. S2B) or adult lungs (Supplemental Fig. 2C) by quantitative RT-PCR (qRT-PCR), and fetal lungs stained negatively for Mrap expression by in situ hybridization at E14.5 (Supplemental Fig. 2D). Glycogen storage in the liver of Mrap−/− E19.5 preterm embryos was reduced compared with Mrap+/+ littermate controls (Supplemental Fig. 2E). These findings suggested that the pups probably died of failure to breathe and/or defective neonatal nutritional adaptation due to GC deficiency. Therefore, to produce Mrap−/− mice and matched Mrap+/+ littermate controls, we intercrossed Mrap+/− mice and administered corticosterone in the drinking water (50 μg/ml) of pregnant dams from E17.5 until weaning of the pups on P21. With this strategy, the survival of Mrap−/− mice at weaning was close to the expected rate [61 (24.7%) Mrap−/−, 115 (46.7%) Mrap+/−, and 70 (28%) Mrap+/+]. Mrap−/− mice were visually indistinguishable from their wild-type, sex-matched littermates who had also received corticosterone from E17.5 until weaning. After weaning, the mice no longer received corticosterone in the drinking water. No adult mortality was noted in the surviving Mrap−/− mice, and both female and male Mrap−/− mice were fertile.
Surviving Mrap−/− mice develop isolated GC deficiency
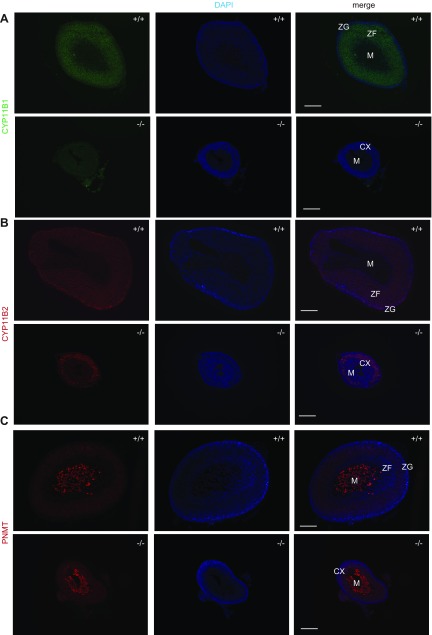
Adrenal glands in Mrap−/− mice were macroscopically detectable but significantly reduced in size at 8 wk (Fig. 1E). This is in contrast to the relatively similar adrenal gland sizes between fetal Mrap−/− and Mrap+/+ mice at E19.5 (Supplemental Fig. 3). Histologic analysis of adult adrenal glands revealed grossly abnormal zonation of the adrenal cortex in both male and female Mrap−/− mice (Fig. 1F). Immunohistochemistry demonstrated that the adrenal glands in Mrap−/− mice were negative for CYP11B1, the key terminal enzyme essential for corticosterone production, suggesting the absence of GC-producing cells (Fig. 2A). Expression of CYP11B2, the key enzyme for aldosterone production, was restricted to the ZG in the wild-type mice, whereas in the Mrap−/− adrenals CYP11B2 stained a larger proportion of cells, with positive cells seemingly taking over large parts of the cortex (Fig. 2B). The adrenal medulla appeared to be unaffected as assessed by PNMT staining (Fig. 2C).
Figure 2.
Impaired zonation in the adrenal cortex of the Mrap−/− mice. A) Severely reduced immunostaining of CYP11B1 (green) in Mrap−/− adrenal glands. B) CYP11B2 (red) localization in Mrap+/+ and Mrap−/− adrenal glands. C) PNMT (red) in the adrenal medulla of Mrap+/+ and Mrap−/− mice. The cell nuclei are stained with DAPI (blue). CX, cortex; M, medulla. Scale bars, 200 µm.
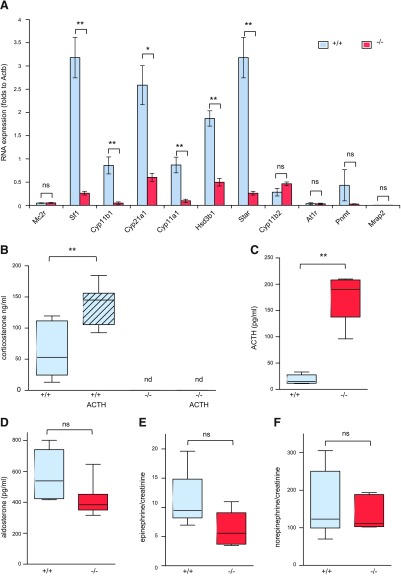
Studying adrenal gland steroidogenic enzyme expression more broadly, RNA levels of Cyp11a1, Hsd3b1, Cyp21a1, and Cyp11b1 as well as Sf-1 and Star (steroidogenic acute regulatory protein) were significantly decreased in the adrenals of Mrap−/− mice (Fig. 3A) compared with wild-type, sex-matched littermates. The expression of the key enzyme of the aldosterone synthesis pathway, Cyp11b2, was not affected. These findings, together with histologic staining of CYP11B2-positive cells, suggest that this pathway is likely to remain functional. There were no changes of Mc2r expression in Mrap−/− mice. Low Mrap2 transcript levels detected in Mrap+/+ mice adrenals were unchanged in Mrap−/− mice (Fig. 3A), indicating that MRAP’s only ortholog, Mrap2, is unlikely to compensate for the loss of Mrap expression at the RNA level. This is in keeping with previous in vitro data that much higher concentrations of Mrap2 are required for the functional expression of MC2R (20).
Figure 3.
ACTH resistance and isolated GC deficiency of Mrap−/− mice. A) qRT-PCR of the adrenal gland of male Mrap+/+ and Mrap−/− mice (n = 4 for each group) shows down-regulation of the steroidogenic pathway. B) Plasma corticosterone levels (ng/ml) at the basal level and in response to ACTH stimulation in male Mrap+/+ (n = 7) and Mrap−/− (n = 7) mice. C) ACTH levels (pg/ml) in male Mrap+/+ (n = 6) and Mrap−/− (n = 5) mice. D) Comparison of plasma aldosterone levels (pg/ml) between the genotypes in male mice (Mrap+/+, n = 7; Mrap−/−, n = 7). E, F) Epinephrine (E) and norepinephrine (F) levels in the 24-h urine of male Mrap+/+ (n = 6) and Mrap−/− (n = 5) mice assessed as the ratio to creatinine to adjust for the kidney function. Student’s t test used for comparison of 2 groups. *P < 0.05, **P < 0.005, ***P < 0.0005. 1-way ANOVA for more than 2 groups. *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant.
To investigate the physiological effects of Mrap gene deletion, we examined the response of mutant mice to exogenously administered ACTH. We could not detect corticosterone in Mrap−/− mice either basally or after ACTH stimulation, whereas ACTH response in wild-type mice was present in both genders (Fig. 3B and Supplemental Fig. 3A). ACTH response in both male and female Mrap+/− mice was intact and similar to Mrap+/+ littermates (Supplemental Fig. 3C, D). The levels of plasma ACTH were greatly increased in Mrap−/− mice compared with Mrap+/+ mice (Fig. 3C). Thus, Mrap−/− mice demonstrated ACTH resistance.
Aldosterone levels were unaffected in mutant mice of both genders (Fig. 3D and Supplemental Fig. 3B), as were urinary catecholamine concentrations (Fig. 3E, F). This Mrap−/− mouse phenotype differs from Mc2r−/− mice, which have been reported to have partial aldosterone and catecholamine deficiency in addition to GC deficiency (22). The Mrap−/− mouse therefore closely replicates the human FGD phenotype of isolated GC deficiency.
Mrap−/− adrenal glands have a thickened capsular layer, which was partially rescued with GC treatment
The adrenal capsule of 8-wk-old Mrap−/− mice was thickened compared with the wild-type littermates in absolute measurement (in micrometers) (Fig. 4A). The capsule thickness was due to an increase in cell number rather than extracellular matrix expansion (Fig. 4A and Supplemental Fig. 4A). This capsular phenotype is established prior to birth (Supplemental Fig. 3E). Studies have demonstrated the importance of the adrenal capsule in the control of adrenocortical cell renewal and zonation (15, 18). More recently, PKA and ACTH signaling have been implicated in the regulation of adrenal zonation determining the ZG/ZF border (23).
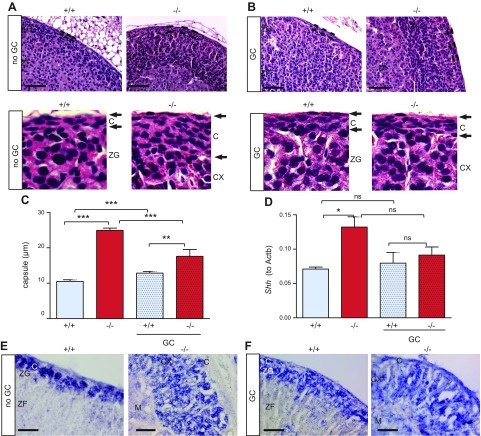
Figure 4.
Capsular layer is thickened in Mrap−/− mice. A, B) Representative images of Mrap−/− adrenal glands and of wild-type littermates showing increased capsule thickness (A; highlighted with black bars) and in response to lifetime GC treatment (B). The bottom panels show digital magnification of the adrenal gland at the capsular layer. C) Capsular layer measurements (micrometers) of the adrenals of the Mrap−/− mice compared with the wild-type mice and in response to lifetime corticosterone replacement. D) Shh expression in the adrenal gland of male Mrap+/+ (n = 6) and Mrap−/− (n = 6) mice and of the animals after lifetime corticosterone replacement (GC) (Mrap+/+, n = 4; Mrap−/−, n = 5) shows an increase in Shh expression in Mrap−/− mice. E, F) In situ hybridization demonstrated expression of Shh outside ZG in the adrenals of Mrap−/− mice (E) and in the animals after lifetime corticosterone replacement (GC) (F). CX, cortex; M, medulla. Scale bars, 50 µm. One-way ANOVA with Bonferroni multiple comparison testing. *P < 0.05, **P < 0.01, ***P < 0.001. Ns, not significant.
To elucidate whether the adrenal capsular phenotype was due to the lack of ACTH signaling or the absence of GCs, we performed corticosterone replacement of Mrap−/− mice and sex-matched, wild-type littermates after weaning. In this case, drinking water was supplemented with corticosterone starting at E17.5 until 8 wk of age when the phenotype was assessed. With this strategy, which we call “lifetime corticosterone treatment,” corticosterone levels between the wild-type and Mrap−/− littermates were normalized (Supplemental Fig. 4B). Examination of the capsule of Mrap−/− mice that had received lifetime corticosterone treatment revealed that chronic GC administration partially reduced the thickness of the capsular layer, suggesting that GC depletion partly contributed to the capsular phenotype independent of ACTH signaling (Fig. 4B, C). The absence of ACTH signaling is likely to further contribute to capsule thickness, as demonstrated by an increase in thickness in corticosterone-treated, wild-type mice, with normalized corticosterone levels and low ACTH (Fig. 4C). Furthermore, capsule thickness in Mrap−/− GC-treated mice remains significantly greater compared with Mrap+/+ GC-treated mice, which would suggest an MRAP-independent effect (Fig. 4C).
Shh signaling is altered in Mrap−/− adrenal glands and is not normalized by treatment with GCs
SHH signaling is one of the key pathways regulating adrenal zonation and identifies progenitors of steroidogenic lineages (18, 21). The expression of Shh was quantitatively increased in the adrenal glands of Mrap−/− mice compared with the wild-type littermates (Fig. 4D). In situ hybridization demonstrated Shh-positive cells throughout the adrenal cortex in Mrap−/− mice, as opposed to the expected subcapsular localization observed in wild-type adrenals (Fig. 4E). These data suggest that loss of MRAP-mediated ACTH signaling and/or GC depletion could be the cause of SHH progenitor accumulation. Lifetime corticosterone replacement did not reverse this phenotype, indicating that the Shh pathway is likely to depend on MRAP-mediated ACTH signaling rather than on GC levels (Fig. 4F). Shh expression measured by qRT-PCR after GC was also statistically unchanged in Mrap−/− mice. Expression of Gli1, a transcriptional target of SHH signaling in the adrenal gland (18), was unaffected in the adrenals of Mrap−/− mice (Supplemental Fig. 4C). The expression of other GLI family members (Gli2 and Gli3) was also unchanged (Supplemental Fig. 4D, E).
β-Catenin signaling is dysregulated in the adrenal cortex of Mrap−/− mice
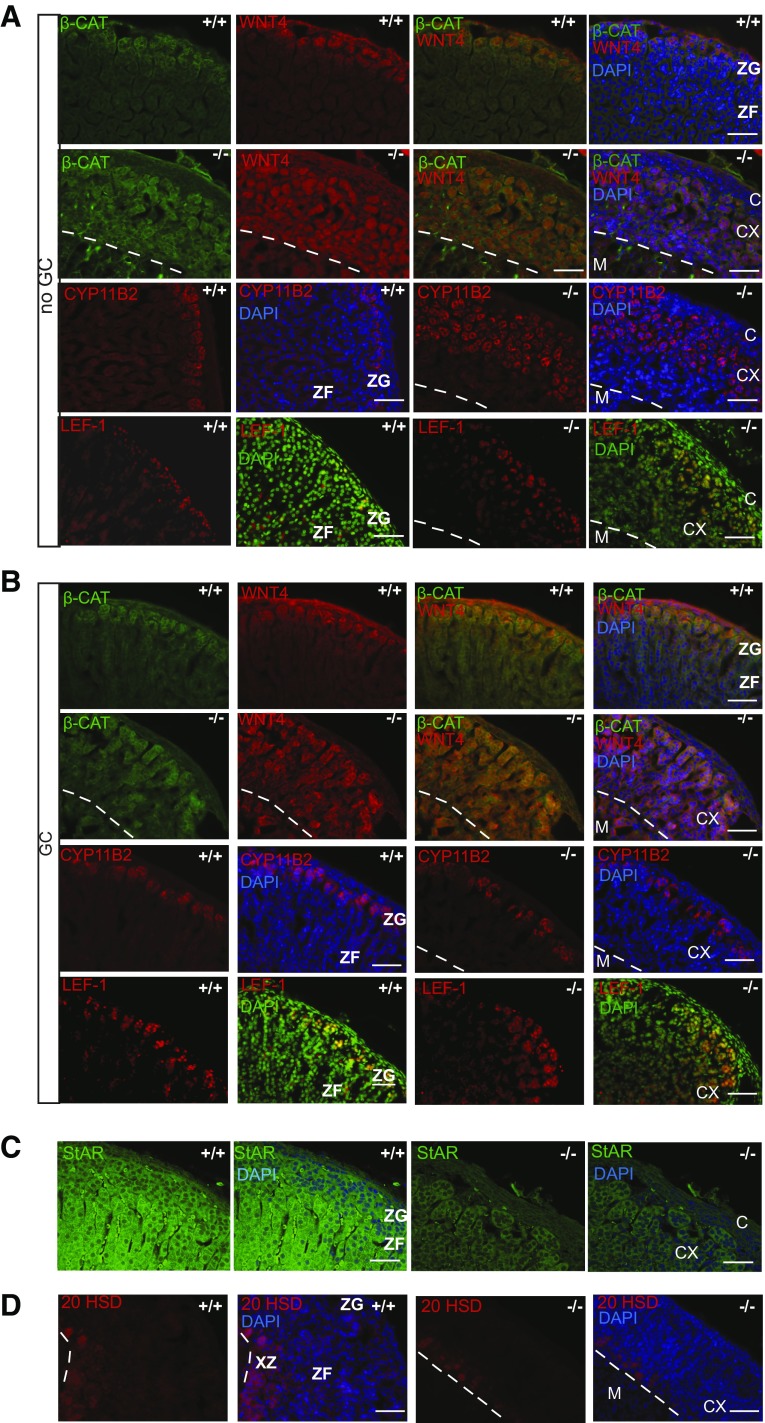
Recent studies implicated canonical wnt/β-catenin signaling in adrenal gland zonation (23–25). Therefore, we tested the function of this pathway in the adrenals of Mrap−/− mice. Immunostaining for WNT4 and β-catenin demonstrated expression in the ZG in wild-type, mice as previously reported (Fig. 5A). Mrap−/− mice demonstrated WNT4 and β-catenin staining throughout the adrenal cortex in both lifetime corticosterone-replaced and nonreplaced animals (Fig. 5A, B).
Figure 5.
Dysregulation of WNT4/β-catenin signaling in Mrap−/− mice. A) Immunohistochemistry showing localization of β-catenin (β-CAT), WNT4, CYP11B2, and LEF1 in the adrenal glands of Mrap+/+ and Mrap−/− mice. B) Immunohistochemistry showing β-CAT, WNT4, CYP11B2, and LEF1 in response to lifetime corticosterone replacement. Cell nuclei are stained with DAPI as blue, with the exception if LEF1 images where the nuclei are stained green to highlight colocalization of LEF1 with nuclei. CX, cortex; M, medulla. Scale bar, 50 µm. C) Immunohistochemistry with anti-StAR antibody shows impairment of steroidogenic potential of the cells in the adrenal cortex of Mrap−/− mice. D) Immunostaining of the adrenal glands with anti-20αHSD antibody shows staining surrounding the medulla in both wild-type and mutant mice. CX, cortex; M, medulla; XZ, X zone. Dashed line indicates medullary boundary. Scale bars, 50 µm.
Expression of WNT4 and β-catenin is associated with ZG cell identity (26, 27). Therefore, we examined the expression of CYP11B2 in these cells. It appeared that some of the cells that were positive for WNT4 and β-catenin expression were negative for CYP11B2 and hence were not aldosterone producing (Fig. 5A and Supplemental Fig. 4F). These cells were also negative for DAB2, another ZG-specific marker (Supplemental Fig. 4G). Expression of Lef1, a downstream component of the β-catenin pathway, was not affected in Mrap−/− mice (Fig. 5A). The level of Lef1 mRNA expression was increased in Mrap−/− adrenals after lifetime GC treatment (Supplemental Fig. 4H). In wild-type mice, immunohistochemistry showed that LEF1 was localized to the nuclei of the ZG (Fig. 5A) as previously reported (28). LEF1-positive cells in Mrap−/− adrenal glands were also found to be expressed more widely in the cortex compared with wild-type mice, although LEF1-positive cells did not penetrate as deeply into the cortex compared with WNT4 and β-catenin cells (Fig. 5A). There was no effect in response to chronic GC administration on LEF1 distribution, suggesting GCs are unlikely to regulate this signaling pathway (Fig. 5B).
AXIN-2 is another transcriptional target of the canonical β-catenin pathway. Consistent with previous reports (14), expression of Axin2 was increased upon chronic GC administration (Supplemental Fig. 4I). Similar to Lef1 expression, Axin2 levels in untreated Mrap−/− mice were similar to the wild-type littermates, and mutant mice responded to GC treatment by an increase in Axin2 expression; however, this response was less prominent compared with that observed in wild-type mice (Fig. 5A, B). These findings suggest that, although WNT4 and β-catenin are accumulated in the vast majority of cells throughout the cortex of the Mrap−/− adrenals, their transcription targets LEF1, AXIN-2, and CYP11B2 are activated only in a proportion of these cells. The adrenal cortical cells in the Mrap−/− mice, including those negative for CYP11B2 and LEF1, showed impairment of the steroidogenic potential as assessed by StAR immunostaining (Fig. 5C). The WNT4 and β-catenin–positive but CYP11B2-negative cells were also negative for 20α-hydroxysteroid dehydrogenase (20-αHSD), suggesting that they were not fetal X-zone cells (29) (Fig. 5D). Furthermore, lifetime treatment with GCs was capable of normalizing the CYP11B2 phenotype in Mrap−/− mice without changing the LEF1 or AXIN-2 phenotypes (Fig. 5A, B).
DISCUSSION
We previously identified mutations in MRAP as a cause of FGD2. Here we describe the first Mrap−/− mouse model of ACTH resistance with isolated GC deficiency, representative of patients with FGD2. This study demonstrates the importance of MRAP in adrenal physiology. Furthermore, we show the importance of MRAP in adrenal progenitor cell differentiation and adrenal cortex zonation. In view of the relative postnatal reduction in adrenal sizes in Mrap−/− mice, MRAP is likely to also play a role in adrenal gland maintenance and self-renewal.
Patients with MRAP mutations are diagnosed earlier in the neonatal period compared with those with MC2R mutations (13); this correlates with the higher neonatal death rates observed in Mrap−/− mice. Mrap−/− pups died due to impaired maturation of the lungs, rescued by corticosterone administration, indicating that this phenotype is due to corticosterone depletion rather than a direct role of Mrap deficiency. Mc2r−/− pups born from homozygous parents died before P0.5 due to lung failure, suggesting that fetal or maternal corticosterone is important for lung maturation (30). Mrap+/− breeders with normal adrenal function and ACTH responsiveness delivered Mrap−/− pups with uninflated lungs. This finding highlights the importance of fetal rather than maternal glucocorticoids in prepartum lung maturation. We also observed depleted glycogen stores in prebirth embryos, which would lead to compromised nutritional adaptation, severe hypoglycemia, and neonatal death, as described for Mc2r−/− mice (22). Furthermore, we were able to rescue neonatal Mrap−/− lethality by the administration of corticosterone in drinking water from E19.5 until weaning. After weaning, Mrap−/− mice with undetectable corticosterone levels survived.
Adult Mrap−/− mice exhibited gross morphologic changes in the adrenal cortex and disturbances of the steroidogenic pathway, with total absence of circulating corticosterone. Despite this severe phenotype, aldosterone levels were similar to those in the wild-type littermates. The lack of StAR staining by immunohistochemisty of Mrap−/−-derived adrenals may represent the inability of the StAR antibody to detect low levels of StAR, measurable by qRT-PCR, that enable normal aldosterone production in picogram per milliliter concentrations. The adrenal medulla, histologically and functionally, appeared to be unaffected. PMNT, an enzyme responsible for conversion of noradrenaline to adrenaline, is expressed in the medulla of corticosterone-deficient Mrap−/− mice. This is surprising because the expression of PNMT is known to be dependent on GCs (31, 32). There is suggestion of a reduction in PNMT mRNA levels in Mrap−/− mice by qRT-PCR, although this was not statistically significant. Furthermore, PNMT activity is clearly adequate for the production of adrenaline in Mrap−/− mice, which was statistically indifferent to the adrenaline levels in Mrap+/+ littermates at 8 wk of age. However, our mice (both genotypes) received relatively high doses of GCs between E17.5 until weaning; hence, it is unclear if this is sufficient for medullary PMNT expression in the first instance. The absence of mineralocorticoid and the catecholamine deficiency in Mrap−/− mice make this model uniquely different from the previously reported Mc2r−/− mice, which exhibited low aldosterone and catecholamines levels (22).
The capsule of Mrap−/− adrenals was thickened with increased cell number; a similar change is seen in Mc2r−/− adrenal glands (22), suggesting that this phenotype is likely due to a lack of ACTH signaling and/or GC production. Our data demonstrate the importance of both GCs and ACTH in capsule thickness. Lifetime GC replacement in Mrap−/− mice partially normalized the thickened adrenal capsule of Mrap−/− mice, whereas ACTH deficiency in lifetime GC-treated wild-type animals resulted in an increase in adrenal capsule thickness. Moreover, our data suggest that MRAP may independently affect capsule thickness. The adrenal capsule gives rise to steroidogenic cells and is implicated in the regulation of adrenal cell differentiation and zonation. One of the key pathways regulating this process is SHH signaling (18). King et al. (18) demonstrated that removing Shh signaling reduced the adrenal capsule thickness to a single layer and suggested that SHH acted either as a capsule cell mitogen/chemoattractant for noncapsule mesenchyme or to maintain capsule progenitors. In keeping with this suggestion, we found that Mrap−/− mice had up-regulated SHH expression and thickening of the capsule. However, we also found ectopic SHH expression throughout the cortex, which is not rescued by lifetime GC replacement. Despite this failure to rescue, the capsule thickness was reduced with GC treatment. This indicates that SHH up-regulation was likely to be due to MRAP-mediated ACTH resistance and not GC depletion. Furthermore, there was dissociation between SHH signaling and capsule thickness. This dissociation could be explained by the lack of change in Gli1 expression levels from Mrap−/− adrenal glands, perhaps pointing to a lack of increase in canonical hedgehog signaling in the cortex of these animals. A thickened capsule is also seen in FgfrIIIb−/− mice, although in this case Gli1 expression in the capsule is increased (33). How the FGF, SHH, and ACTH signaling co-ordinates the regulation of capsule thickness and progenitor cell differentiation is subject to future investigations.
Another key signaling pathway implicated in the adrenal gland zonation regulation, the WNT4/β-catenin pathway, was dysregulated in the adrenals of Mrap−/− mice. The canonical WNT4/β-catenin pathway is a driver of ZG identity and is normally restricted to the subcapsular region, where it is coexpressed with SHH and CYP11B2-positive cells (26, 27, 34). Mrap−/− mice demonstrated ectopic accumulation of WNT4/β-catenin throughout the cortex, mirroring the SHH expression. This is consistent with the hypothesis that ACTH signaling via PKA inhibits the WNT4/β-catenin pathway (15). However, a proportion of such cells were negative for the downstream target of the pathway LEF1, DAB2, and CYP11B2, suggesting that, despite WNT4/β-catenin accumulation, such cells do not contribute to aldosterone production. This suggests that canonical WNT signaling is not active in the inner cortical CYP11B2-negative cells in the adrenal glands of Mrap−/− mice.
In summary, MRAP deficiency results in isolated GC deficiency, making this a unique model replicating the human FGD phenotype. We showed that the key pathways regulating adrenal zonation were dysregulated in the adrenal glands of Mrap−/− mice, demonstrating the role of MRAP-mediated ACTH signaling in the regulation of adrenal progenitor cell differentiation (15, 23). Contrary to current views, in this study we show that canonical β-catenin through LEF1 does not always drive ZG identity. Our mouse model may point to noncanonical WNT signaling pathways in adrenal gland zonation. Moreover, GC replacement did not rescue the SHH or WNT4/β-catenin pathway deregulation but decreased the thickness of the adrenal capsule of Mrap−/− mice, indicating a potential role for long-term GC treatment in regulating the stem cell niche physiology in ACTH resistance syndromes.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by The Medical Research Council (MRC) UK/Academy of Medical Sciences Clinician Scientist Fellowship Grant G0802796 (to L.F.C., supporting T.V.N.), a Society for Endocrinology Early Career award (to T.V.N.), MRC Collaborative Awards in Science and Engineering (CASE) Studentship Grant MR/J006394/1 (to A.J.L.C. and L.F.C. supporting M.H.), Biotechnology and Biological Sciences Research Council Award BB/L00267/1 and funds from the Rosetrees Trust (to L.G.), MRC Grant UK MR/K020455/1 (to L.M.), and MRC Grant MR/L002345/1 (to M.C.). The authors thank Dr. C. E. Gomez-Sanches (University of Mississippi, Jackson, MI, USA) for the kind gift of CYP11B1 and CYP11B2 antibody, Dr. Ed Laufer (Columbia University, NY, USA) for technical advice, Prof. Yacob Weinstein (Ben-Gurion University of the Negev, Israel) for the 20-αHSD antibody, Anthony Price and the staff at the Biological Services Unit at Queen Mary University of London for technical assistance, Dr. Anthony Coll and Sir/Prof. Steven O’Rahilly (Institute of Metabolic Sciences, Cambridge, Uninted Kingdom) for help with conceptualization and support of the project, Keith Burling and Peter Barker [Cambridge University Hospitals National Health Service (NHS) Foundation Trust] for the provision of laboratory services, and David Jackson (QMUL) for the critical reading of the manuscript. The authors declare no conflicts of interest.
Glossary
- ACTH
adrenocorticotropin hormone
- FGD
familial glucocorticoid deficiency
- GC
glucocorticoid
- H&E
hematoxylin and eosin
- MC2R
melanocortin 2 receptor
- MRAP
melanocortin 2 receptor accessory protein
- qRT-PCR
quantitative RT-PCR
- SHH
sonic hedgehog
- ZF
zona fasciculata
- ZG
zona glomerulosa
- ZR
zona reticularis
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. V. Novoselova and L. F. Chan designed the experiments; T. V. Novoselova, M. Hussain, and L. F. Chan performed the experiments; L. F. Chan conceived the study; T. V. Novoselova P. J. King, L. Guasti, L. A. Metherell, M. Charalambous, A. J. L. Clark, and L. F. Chan analyzed the data; L. F. Chan supervised the study; and T. V. Novoselova, P. J. King, L. Guasti, L. A. Metherell, M. Charalambous, A. J. L. Clark, and L. F. Chan wrote the manuscript.
REFERENCES
- 1.Metherell L. A., Chapple J. P., Cooray S., David A., Becker C., Rüschendorf F., Naville D., Begeot M., Khoo B., Nürnberg P., Huebner A., Cheetham M. E., Clark A. J. (2005) Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 [DOI] [PubMed] [Google Scholar]
- 2.Chan L. F., Webb T. R., Chung T. T., Meimaridou E., Cooray S. N., Guasti L., Chapple J. P., Egertová M., Elphick M. R., Cheetham M. E., Metherell L. A., Clark A. J. (2009) MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc. Natl. Acad. Sci. USA 106, 6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novoselova T. V., Jackson D., Campbell D. C., Clark A. J., Chan L. F. (2013) Melanocortin receptor accessory proteins in adrenal gland physiology and beyond. J. Endocrinol. 217, R1–R11 [DOI] [PubMed] [Google Scholar]
- 4.Asai M., Ramachandrappa S., Joachim M., Shen Y., Zhang R., Nuthalapati N., Ramanathan V., Strochlic D. E., Ferket P., Linhart K., Ho C., Novoselova T. V., Garg S., Ridderstråle M., Marcus C., Hirschhorn J. N., Keogh J. M., O’Rahilly S., Chan L. F., Clark A. J., Farooqi I. S., Majzoub J. A. (2013) Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novoselova T. V., Larder R., Rimmington D., Lelliott C., Wynn E. H., Gorrigan R. J., Tate P. H., Guasti L., O’Rahilly S., Clark A. J., Logan D. W., Coll A. P., Chan L. F.; Sanger Mouse Genetics Project (2016) Loss of Mrap2 is associated with Sim1 deficiency and increased circulating cholesterol. J. Endocrinol. 230, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaly A. L., Srisai D., Gardner E. E., Sebag J. A. (2016) The melanocortin receptor accessory protein 2 promotes food intake through inhibition of the prokineticin receptor-1. eLife 5, pii: e12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark A. J., Chan L. F. (2017) Promiscuity among the MRAPs. J. Mol. Endocrinol. 58, F1–F4 [DOI] [PubMed] [Google Scholar]
- 8.Webb T. R., Chan L., Cooray S. N., Cheetham M. E., Chapple J. P., Clark A. J. (2009) Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 150, 720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebag J. A., Hinkle P. M. (2007) Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. USA 104, 20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooray S. N., Almiro Do Vale I., Leung K. Y., Webb T. R., Chapple J. P., Egertová M., Cheetham M. E., Elphick M. R., Clark A. J. (2008) The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology 149, 1935–1941 [DOI] [PubMed] [Google Scholar]
- 11.Clark A. J., McLoughlin L., Grossman A. (1993) Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341, 461–462 [DOI] [PubMed] [Google Scholar]
- 12.Weber A., Clark A. J. (1994) Mutations of the ACTH receptor gene are only one cause of familial glucocorticoid deficiency. Hum. Mol. Genet. 3, 585–588 [DOI] [PubMed] [Google Scholar]
- 13.Chung T. T., Chan L. F., Metherell L. A., Clark A. J. (2010) Phenotypic characteristics of familial glucocorticoid deficiency (FGD) type 1 and 2. Clin. Endocrinol. (Oxf.) 72, 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes P. V., Dickson A. D. (1971) The effect on the mouse adrenal X-zone of blastocysts transplanted under the kidney capsule. J. Reprod. Fertil. 25, 111–113 [DOI] [PubMed] [Google Scholar]
- 15.Drelon C., Berthon A., Sahut-Barnola I., Mathieu M., Dumontet T., Rodriguez S., Batisse-Lignier M., Tabbal H., Tauveron I., Lefrançois-Martinez A. M., Pointud J. C., Gomez-Sanchez C. E., Vainio S., Shan J., Sacco S., Schedl A., Stratakis C. A., Martinez A., Val P. (2016) PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat. Commun. 7, 12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerario A. M., Finco I., LaPensee C., Hammer G. D. (2017) Molecular mechanisms of stem/progenitor cell maintenance in the adrenal cortex. Front. Endocrinol. (Lausanne) 8, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman B. D., Kempna P. B., Carlone D. L., Shah M., Guagliardo N. A., Barrett P. Q., Gomez-Sanchez C. E., Majzoub J. A., Breault D. T. (2013) Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King P., Paul A., Laufer E. (2009) Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc. Natl. Acad. Sci. USA 106, 21185–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penny M. K., Finco I., Hammer G. D. (2017) Cell signaling pathways in the adrenal cortex: links to stem/progenitor biology and neoplasia. Mol. Cell. Endocrinol. 445, 42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorrigan R. J., Guasti L., King P., Clark A. J., Chan L. F. (2011) Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J. Mol. Endocrinol. 46, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guasti L., Cavlan D., Cogger K., Banu Z., Shakur A., Latif S., King P. J. (2013) Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of β1 integrin and ERK1/2. Endocrinology 154, 4675–4684 [DOI] [PubMed] [Google Scholar]
- 22.Chida D., Nakagawa S., Nagai S., Sagara H., Katsumata H., Imaki T., Suzuki H., Mitani F., Ogishima T., Shimizu C., Kotaki H., Kakuta S., Sudo K., Koike T., Kubo M., Iwakura Y. (2007) Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc. Natl. Acad. Sci. USA 104, 18205–18210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal V., Sacco S., Rocha A. S., da Silva F., Panzolini C., Dumontet T., Doan T. M., Shan J., Rak-Raszewska A., Bird T., Vainio S., Martinez A., Schedl A. (2016) The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev. 30, 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim A. C., Reuter A. L., Zubair M., Else T., Serecky K., Bingham N. C., Lavery G. G., Parker K. L., Hammer G. D. (2008) Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593–2602 [DOI] [PubMed] [Google Scholar]
- 25.Drelon C., Berthon A., Mathieu M., Martinez A., Val P. (2015) Adrenal cortex tissue homeostasis and zonation: a WNT perspective. Mol. Cell. Endocrinol. 408, 156–164 [DOI] [PubMed] [Google Scholar]
- 26.Berthon A., Sahut-Barnola I., Lambert-Langlais S., de Joussineau C., Damon-Soubeyrand C., Louiset E., Taketo M. M., Tissier F., Bertherat J., Lefrançois-Martinez A. M., Martinez A., Val P. (2010) Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum. Mol. Genet. 19, 1561–1576 [DOI] [PubMed] [Google Scholar]
- 27.Berthon A., Drelon C., Ragazzon B., Boulkroun S., Tissier F., Amar L., Samson-Couterie B., Zennaro M. C., Plouin P. F., Skah S., Plateroti M., Lefèbvre H., Sahut-Barnola I., Batisse-Lignier M., Assié G., Lefrançois-Martinez A. M., Bertherat J., Martinez A., Val P. (2014) WNT/β-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum. Mol. Genet. 23, 889–905 [DOI] [PubMed] [Google Scholar]
- 28.Behrens J., von Kries J. P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382, 638–642 [DOI] [PubMed] [Google Scholar]
- 29.Hershkovitz L., Beuschlein F., Klammer S., Krup M., Weinstein Y. (2007) Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology 148, 976–988 [DOI] [PubMed] [Google Scholar]
- 30.Chida D., Sato T., Sato Y., Kubo M., Yoda T., Suzuki H., Iwakura Y. (2009) Characterization of mice deficient in melanocortin 2 receptor on a B6/Balbc mix background. Mol. Cell. Endocrinol. 300, 32–36 [DOI] [PubMed] [Google Scholar]
- 31.Bornstein S. R., Tajima T., Eisenhofer G., Haidan A., Aguilera G. (1999) Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J. 13, 1185–1194 [DOI] [PubMed] [Google Scholar]
- 32.Jeong K. H., Jacobson L., Pacák K., Widmaier E. P., Goldstein D. S., Majzoub J. A. (2000) Impaired basal and restraint-induced epinephrine secretion in corticotropin-releasing hormone-deficient mice. Endocrinology 141, 1142–1150 [DOI] [PubMed] [Google Scholar]
- 33.Guasti L., Candy Sze W. C., McKay T., Grose R., King P. J. (2013) FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol. Cell. Endocrinol. 371, 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walczak E. M., Kuick R., Finco I., Bohin N., Hrycaj S. M., Wellik D. M., Hammer G. D. (2014) Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 28, 1471–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.