Abstract
The formation of an abdominal aortic aneurysm (AAA) is characterized by inflammation, macrophage infiltration, and vascular remodeling. In this study, we tested the hypothesis that mesenchymal stromal cell (MSC)–derived extracellular vesicles (EVs) immunomodulate aortic inflammation, to mitigate AAA formation via modulation of microRNA-147. An elastase-treatment model of AAA was used in male C57BL/6 wild-type (WT) mice. Administration of EVs in elastase-treated WT mice caused a significant attenuation of aortic diameter and mitigated proinflammatory cytokines, inflammatory cell infiltration, an increase in smooth muscle cell α-actin expression, and a decrease in elastic fiber disruption, compared with untreated mice. A 10-fold up-regulation of microRNA (miR)-147, a key mediator of macrophage inflammatory responses, was observed in murine aortic tissue in elastase-treated mice compared with controls on d 14. EVs derived from MSCs transfected with miR-147 mimic, but not with miR-147 inhibitor, attenuated aortic diameter, inflammation, and leukocyte infiltration in elastase-treated mice. In vitro studies of human aortic tissue explants and murine-derived CD11b+ macrophages induced proinflammatory cytokines after elastase treatment, and the expression was attenuated by cocultures with EVs transfected with miR-147 mimic, but not with miR-147 inhibitor. Thus, our findings define a critical role of MSC-derived EVs in attenuation of aortic inflammation and macrophage activation via miR-147 during AAA formation.—Spinosa, M., Lu, G., Su, G., Bontha, S. V., Gehrau, R., Salmon, M. D., Smith, J. R., Weiss, M. L., Mas, V. R., Upchurch, G. R., Sharma, A. K. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147.
Keywords: inflammation, stem cells, exosomes, high mobility group box 1, IL-17
Abdominal aortic aneurysm (AAA) is a multifactorial, complex, inflammatory vascular disease process that can lead to progressive expansion and abrupt rupture, thereby causing an increase in mortality rate in patients (1–5). Histologically, AAAs are characterized by early leukocyte infiltration of the aortic wall, with subsequent destruction of elastin and collagen in the media and adventitia by excessive local production of proinflammatory cytokines, matrix-degrading enzymes, and smooth muscle cell apoptosis, with thinning of the aortic wall (1, 6–8). We have recently demonstrated that CD4+ T-cell–dependent IL-17 and macrophage-dependent high-mobility group box (HMBG)1 production plays a key role in the initiation and progression of experimental murine AAA formation which can be effectively mitigated by administration of human mesenchymal stromal cells (MSC) (9, 10). Furthermore, we recently showed evidence that conditioned medium from murine bone marrow–derived MSCs significantly attenuates experimental AAA formation, similar to MSCs themselves (11).
MSCs are undifferentiated, nonembryonic stromal cells with multilineage potential and immunomodulatory properties (12–14). Although considerable experimental evidence has demonstrated the ability of MSCs in structural and functional improvement of many organs and tissues after injury, challenges remain in the clinical application as they have been reported to promote oncogenic growth and malformations (15). Therefore, it is imperative to harness the protective properties of MSCs by investigating safe and effective alternatives for their desired application. Recent studies have demonstrated that MSCs can release extracellular vesicles (EVs), such as exosomes and microvesicles (MVs), which can elucidate protective paracrine effects by transferring proteins, lipids, and genetic material to target cells (16–20). EVs originating from MSCs are small vesicles that carry membrane and cytoplasmic constituents of the cells from which they originate and have been shown to shuttle mRNA and microRNAs (miRs), thereby playing a critical role in the continuum model of stem cell biology (17, 21–23).
miRs are noncoding, small RNAs (21–23 nt) that bind to the 3′ UTR region of specific mRNA targets, leading to translational suppression or target degradation (24, 25). miRs are potent regulators of diverse biologic and pathophysiologic processes and have been shown to play an important role in human and animal studies of AAA (26–29). However, the role of specific miRNAs in inhibiting formation of an aneurysm and expansion remains unresolved and should be investigated further.
In this study, we focused on the effects of MSC-derived EVs in the attenuation of inflammation, immune cell activation, and proinflammatory cytokine production, and maintenance of smooth muscle cell integrity, leading to a protective phenotype in the elastase treatment model of murine AAA. Moreover, we evaluated in vitro the effects of miR-modified EVs on the release of key regulatory cytokines (CD4+ T-cell–produced IL-17 and macrophage-produced HMGB-1) and aortic smooth muscle cell activation, both of which are known to exert a pivotal signaling cascade in the pathogenesis of AAA formation (9, 30). Our findings demonstrate that derivatives of MSCs (i.e., EVs) can effectively immunomodulate AAA formation. These protective effects of EVs are dependent on miR-147 as the attenuation of aortic inflammation and vascular remodeling during AAA formation is reversed upon treatment with modified MVs transfected with miR-147 inhibitor.
MATERIALS AND METHODS
Human aortic tissue analysis
Collection of human aortic tissue was approved by the University of Virginia’s Institutional Review Board (13178). Consent was obtained from all patients before surgery. AAA tissue from male patients was resected during open surgical AAA repair. Aortic explants from human male patients with AAA were cultured on 25-mm plates on synthetic basement membrane (Matrigel; BD Biosciences, Franklin Lakes, NJ, USA). Aortic explants were transiently treated with type I porcine pancreatic elastase (0.4 U/ml for 5 min; MilliporeSigma, Burlington, MA, USA), with or without coculture with EVs (1 × 105 cells), and culture supernatants were collected after 24 h for further analysis.
Animals
Adult male C57BL/6J mice (8–12 wk of age) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained in a temperature-controlled room (25°C) on a 12-h light/dark cycle with free access to food and water. All animal experimentation was approved by the University of Virginia’s Institutional Animal Care and Use Committee (Protocol 3848).
Elastase treatment model of aneurysm formation
A murine elastase treatment model of AAA formation was used as described by Laser et al. (31). In brief, the infrarenal aorta was treated topically with 30 μl elastase reconstituted with normal saline (5 U/mg protein) or 30 μl heat-inactivated elastase (at 90°C for 30 min) as a control group. The topical application was accomplished by dropping the elastase on the anterior aorta from a 2 cm height for 5 min. Video micrometric measurements of aortic diameters were made in situ before perfusion, after perfusion, and before harvesting the aorta on d 14.
Human mesenchymal stem cell isolation and characterization
Human umbilical cord–derived MSCs were isolated from Wharton’s jelly. MSCs were characterized by flow cytometry confirming a pattern consistent with MSC population showing an expression of cluster of differentiation (CD)90, CD73, CD105, and CD44. Also, MSCs lacked expression of CD45, CD34, CD11b, CD19, and HLA-DR. MSCs were differentiated with differentiation kits for chondrogenesis, adipogenesis, and osteogenesis, according to the protocols included with the kits (StemPro; Thermo Fisher Scientific, Waltham, MA, USA). After randomized assignment, wild-type (WT) mice received 1 × 106 MSCs in 200 μl saline intravenously via tail vein injection on d 1 in the in vivo experiments after treatment with elastase alone or with heat-inactivated elastase.
Isolation and characterization of EVs
EVs were obtained from supernatants of MSCs cultured overnight in RPMI deprived of fetal bovine serum and supplemented with 0.5% bovine serum albumin (MilliporeSigma), as we have recently described (32). The cell viability was 99% for MSCs, as detected by trypan blue exclusion. To obtain EVs, the supernatants from MSCs underwent centrifugation at 10,000 g for 20 min to remove debris, cell-free supernatant was centrifuged at 100,000 g (Optima Ultracentrifuge; Beckman Coulter, Brea, CA, USA) for 1 h at 4°C, washed in serum-free medium with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (MilliporeSigma), and subjected to a second ultracentrifugation under similar conditions. The mean size and particle concentration were evaluated with Nanosight LM10 instrument (Malvern Instruments, Malvern, United Kingdom). Further characterization of EVs was performed by imaging flow cytometry (ImageStreamX Imaging FC) (33) (Millipore, Billerica, MA, USA), using CD90-FITC, CD44-allophycocyanin, CD73-PerCP-Cy5.5 (eBioscience, San Diego, CA, USA), and a lipophilic dye (DilC18; Thermo Fisher Scientific). The protein content of EVs was quantified by the Bradford method (Bio-Rad, Hercules, CA, USA). Total RNA was isolated from EVs with the RNeasy Kit (Qiagen, Germantown, MD USA), and quality and concentration were assessed by the NanoDrop UV spectrophotometer (Thermo Fisher Scientific). The characterization of human umbilical cord–derived MSCs was performed by flow cytometry, which showed a significant increase in the expression of CD105, CD90, CD73, and CD44 (Supplemental Fig. 1A) but no expression was detected for CD34, CD11b, CD19, CD45, and HLA-DR (data not shown). The MSCs also demonstrated multilineage differentiation capability (data not shown).
MSCs were used to successfully isolate and characterize the EVs that were isolated from the conditioned medium. The mean size (164 ± 10.4 nm) and particle concentration of EVs were calculated by Nanoparticle Tracking Analysis (Malvern Panalytical, Malvern, United Kingdom) software (Supplemental Fig. 1B). Protein and total RNA quantities of 50 μl EVs were 54.34 ± 5.99 μg and 35.97 ± 5.4 ng, respectively, released by 5 × 106 MSCs (Supplemental Fig. 1C). This protein concentration falls in the range of previously reported studies using EVs in lung and kidney injury disease models (20, 34). Furthermore, imaging flow cytometry analysis of EVs by ImageStream (MilliporeSigma) confirmed the cell surface marker expression for MSCs (i.e., CD90, CD44, and CD73) (Supplemental Fig. 1D).
Histology
Murine AAA specimens were immediately fixed in 10% formalin. After 24 h, fixed samples were embedded in paraffin, and sections were stained by immunohistochemistry, as reported by Sharma et al. (9). Aortic sections were also stained with hematoxylin and eosin, and Verhoeff-Van Gieson for elastin. For macrophages, neutrophils, and CD3+ T-cell (marker for both CD4+ and CD8+ T cells) immunostaining, the aorta sections (5 µm) were dehydrated and incubated with 1% hydrogen peroxide followed by boiling in 1× unmasking solution (Vector Laboratories, Burlingame, CA, USA) for 15 min and blocked with 10% serum. After microwave antigen retrieval, antibodies were bound and detected with the VectaStain Elite Kit (Vector Laboratories). Antibodies for immunohistochemical staining were anti-rat Mac2 for macrophages (1:10,000; Cedarlane Laboratories, Burlington, ON, Canada), anti-mouse anti-neutrophil (Ly6B.2) for neutrophils (1:10,000; AbD Serotec, Oxford, United Kingdom), anti-goat CD3+ for T cells (1:500; Santa Cruz Biotechnology, Dallas, TX, USA). Visualization color development was completed with diaminobenzidine (Agilent Technologies) to produce a brown precipitate. Sections were then counterstained with hematoxylin. Images were acquired with ×20 magnification by an Olympus microscope equipped with a digital camera (Olympus America, Center Valley, PA, USA) and ImagePro software (Media Cybernetics, Rockville, MD, USA).
Microarray analysis of miR expression
Murine aortic tissue was harvested and collected in RNALater (Thermo Fisher Scientific) solution on d 14 after control or elastase treatment of WT mice and total RNA was isolated using the Trizol reagent and following the manufacturer’s protocol (Thermo Fisher Scientific). RNA purity analysis was performed in accordance with previous established parameters (35). Total RNA was labeled with FlashTag Biotin HSR RNA Labeling Kit (Thermo Fisher Scientific) and used for Affymetrix GeneChip microRNA v.4.0 microarray hybridization. After hybridization, each chip was scanned on an Affymetrix GeneChip Scanner 3000 G7 according to the GeneChip Expression Analysis Technical Manual procedures (Thermo Fisher Scientific). Raw intensities for every probe were stored in electronic files (.dat and .cel formats) by the Affymetrix GeneChip Operating Software (GCOS; Thermo Fisher Scientific). The detection of individual intensity values above background for each probe set from the microarray raw data was performed with the Wilcoxon test. Data normalization and expression summaries were obtained with the robust multiarray analysis (RMA) algorithm. A 2-sample Student’s t test analysis was used for pairwise comparison in the R programming environment. A value of P < 0.01 was considered significant by controlling for a false discovery rate below 10% calculated from estimated q values for each miR probe set ID.
Overexpression of miR-147
Human MSCs were transfected with 25 nM miR-147 inhibitor or mimic (Thermo Fisher Scientific) using Lipofectamine RNAiMax Transfection Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. After 24 h of transfection, EVs were isolated from MSCs as previously described and used for further experiments.
RNA preparation, reverse transcription, and real-time quantitative PCR
RNA was isolated with Total Exosome RNA and Protein Isolation Kit (Thermo Fisher Scientific) from MSC-derived EVs after transfection with miR-147b mimic, inhibitor, or respective controls. Single-stranded cDNA was generated according to the instructions for the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). For miR-147b detection, real-time PCR was performed with the TaqMan PCR Kit (Thermo Fisher Scientific) and Real-Time PCR Detection System (Bio-Rad). The mouse hypoxanthine phosphoribosyltransferase (Mm03024075_m1) was used as the normalization control (Thermo Fisher Scientific).
Purification of primary aortic smooth muscle cells
Primary aortic smooth muscle cells (AoSMCs) were purified from WT mice, as described by Colonnello et al. (36).
Primary cell culture and ELISA
Primary CD4+ T cells and CD11b+ F4/80+ macrophages were purified from mouse spleens by using a Magnetic Bead-Based Cell Isolation Kit according to the manufacturer’s protocol (Miltenyi Biotec, Sunnyvale, CA, USA). In brief, a single cell suspension from mouse spleens was prepared with the gentleMACS dissociator, and CD4+ T cells or CD11b+ macrophages were isolated from the single-cell suspensions with the respective isolation kits. Cells were fluorescently stained with a CD4+ or CD11b+ cell cocktail and analyzed by flow cytometry after cell debris and dead cell exclusion from analysis based on scatter signals and propidium iodide fluorescence. We routinely achieved a relative enrichment of CD4+ T cells and CD11b+ macrophages of >90% by using this selection process. CD4+ T cells or CD11b+ macrophages (both 1 × 105) were plated in 48-well culture dishes, with or without coculturing with EVs. The cultures were then exposed to transient elastase treatment for 5 min followed by washing with PBS and replacement of the medium. After 24 h, cell culture supernatants were collected and analyzed for IL-17A (R&D Systems, Minneapolis, MN, USA) or HMGB-1 (IBL International, Hamburg, Germany) production by ELISA, according to the manufacturer’s protocol.
Cytokine array
Cytokine contents of murine aortic tissue homogenates and cell culture supernatants were quantified with the Bioplex Bead Array technique, with a multiplex cytokine panel assay (Bio-Rad) per the manufacturer’s instructions.
Statistical analysis
Values are means ± sem, and statistical evaluation was performed with Prism 6 software (GraphPad, La Jolla, CA, USA). A 1-way ANOVA after the nonparametric Kruskal-Wallis statistical test was used to determine the differences among multiple groups. An unpaired Student’s t test with nonparametric Mann-Whitney test was also used for pair-wise comparisons of groups. A value of P < 0.05 was considered statistically significant.
RESULTS
EVs attenuate aortic inflammation and AAA formation
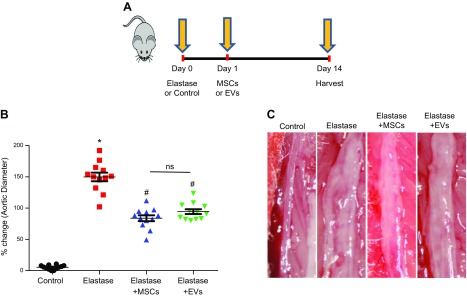
In the elastase treatment model, WT mice underwent elastase or heat-inactivated elastase treatment on d 0. Treatment with MSCs (1 × 106) or EVs (50 μl saline containing ∼1 × 106 EVs with ∼54 μg protein) was administered intravenously on d 1 after elastase treatment, and aortic diameter was measured on d 14 (Fig. 1A). There was a significant increase in mean aortic diameter in elastase-treated mice compared to that in heat-inactivated elastase-treated controls (149.8 ± 6.9% vs. 5.2 ± 0.9%; Fig. 1B). AAA formation was significantly attenuated in elastase-treated mice administered MSCs or EVs compared to mice treated with elastase alone (83.6 ± 4.7% and 94.3 ± 3.8% vs. 149.8 ± 6.9%, respectively; Fig. 1C). There was no significant difference in aortic diameter between controls treated with MSCs or EVs or not (data not shown).
Figure 1.
MSCs and EVs attenuate AAA formation. A) The elastase treatment model. Infrarenal mouse aortas were treated with elastase or heat-inactivated elastase (control) followed by treatment with MSCs or EVs or not. Aortic diameter was measured on d 14, and tissue was harvested for further analysis. B) The aortic diameter of elastase-treated WT mice also treated with MSCs or EVs showed a significant attenuation in AAA formation compared with that in elastase-alone–treated mice. C) Representative example of aortic morphology in the experimental groups. The aortic diameter decreased in mice administered MSCs or EVs after elastase treatment vs. elastase treatment alone (n = 12 mice/group). Ns, not significant. *P < 0.05 vs. control, #P < 0.05 vs. elastase.
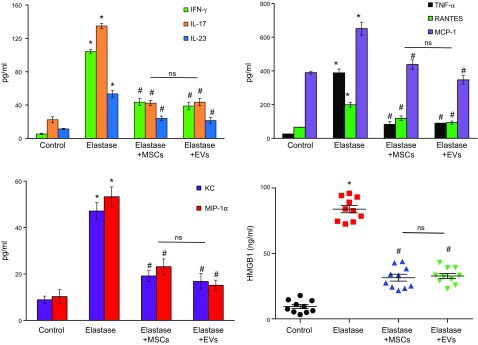
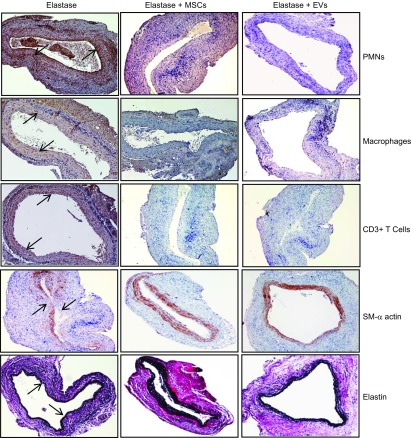
A significant increase in IL-17, IL-23, IFN-γ, regulated on activation normal T-cell expressed and secreted (RANTES), keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-1α, TNF-α, monocyte chemoattractant protein (MCP)-1, and HMGB-1 (Fig. 2) was observed on d 14 in aortic tissue from elastase-treated WT mice compared with controls. Moreover, proinflammatory cytokines were significantly attenuated in aortic tissue of MSC- or EV-treated mice compared with elastase-alone–treated mice. Also, mice treated with MSCs or EVs mitigated immune cell infiltration and protected aortic morphology, as seen by comparative histology and immunostaining of aortic tissue, which revealed a significant attenuation of inflammatory cell (CD3+ T cells, macrophages, and neutrophils) infiltration, increase in α-smooth muscle actin (α-SMA) expression, and decrease in elastic fiber disruption compared to elastase treatment alone (Fig. 3). These results demonstrate that EVs offer efficacy equivalent to that of MSCs in the down-regulation of inflammation, immune cell infiltration, and proinflammatory cytokine levels and lead to a decrease in AAA formation.
Figure 2.
Proinflammatory cytokine production is attenuated by EVs. Aortic tissue from elastase-treated WT mice after administration of MSCs or EVs showed a significant attenuation in production of proinflammatory cytokines and chemokines, especially IL-17 and HMGB-1, compared with WT mice treated with elastase alone (n = 6–10 mice/group). Ns, not significant. *P < 0.05 vs. control, #P < 0.05 vs. elastase.
Figure 3.
Comparative histology performed on d 14 indicates that elastase-treated WT mice administered MSCs or EVs have a marked decrease in polymorphonuclear neutrophil, macrophage (Mac-2), and CD3+ T-cell infiltration; an increase in α-SMA expression; and a decrease in elastic fiber disruption (Verhoeff-Van Gieson staining for elastin) compared with mice treated with elastase alone (n = 5 mice/group). Arrows: areas of immunostaining.
Differentially expressed microRNAs after AAA formation
Pairwise comparison analyses of elastase-treated mice compared with controls identified significantly differentially expressed miR profiles associated with AAA formation on d 14. An intensity value analysis of hybridized microarrays detected 449 miR probe sets in the murine aortic tissue. In brief, 28 miR probe sets were demonstrated to be significantly differentially expressed between study groups in accordance with established statistical parameters (Supplemental Table 1). Of those, 22 miRs were found to be up-regulated, and 6 miRs down-regulated after elastase treatment compared with the control (Fig. 4A). Ontology analysis with IPA (Qiagen) identified 26 miR families. One of these families (miR-466b-3p) was represented by more than 2 different miRs (Supplemental Table 2). The highest up-regulation was observed in miR-147 which showed an ∼10-fold increase in elastase-treated aortic tissue compared to controls on d 14 (Fig. 4B). There was no significant difference in miR-147 expression between elastase-treated and control mice on d 3 and 7. Core analysis with IPA software demonstrated organismal injury and abnormalities, as well as inflammatory responses, to be significant functions associated with miR-147, miR-19, Let-7i (lethal-7i), and miR-34a (Fig. 4C). Furthermore, predicted gene targets involved in functions, such as progression of atherosclerotic lesions (coagulation factor-7, insulin-like growth factor-2), as well as T-lymphocyte development and differentiation (Bruton tyrosine kinase, butyrophilin subfamily 2 member A2, GTPase immune-associated nucleotide-binding protein 2, homeoboxA9, insulin-like growth factor-2, potassium voltage-gated channel subfamily A member 3, and peroxiredoxin-2), were associated with miR-147 (Supplemental Fig. 2).
Figure 4.
Identification of miRs involved in mouse AAA formation. A) Total RNA obtained from abdominal aortic tissue of WT mice on d 14 was analyzed by miR microarray. Hierarchical cluster analysis of elastase-treated aortic tissue vs. controls is shown in by heat map. Each column represents the respective sample, and each row represents a single miR probe. B) A significant increase in miR-147 expression was observed in the aortic tissue from elastase-treated mice compared to controls on d 14, whereas there was no change on d 3 and 7 in the respective groups. C) Analysis of differentially expressed miRs associated with top relevant and significant disease functions on d 14 (n = 5 mice/group). *P < 0.05 vs. control.
EV-mediated protection against vascular inflammation and AAA formation is dependent on miR-147
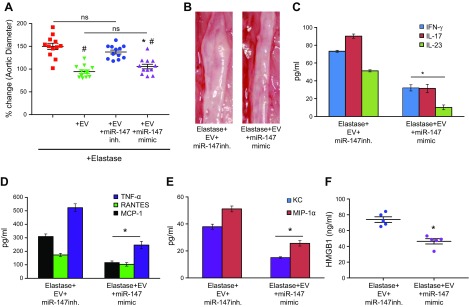
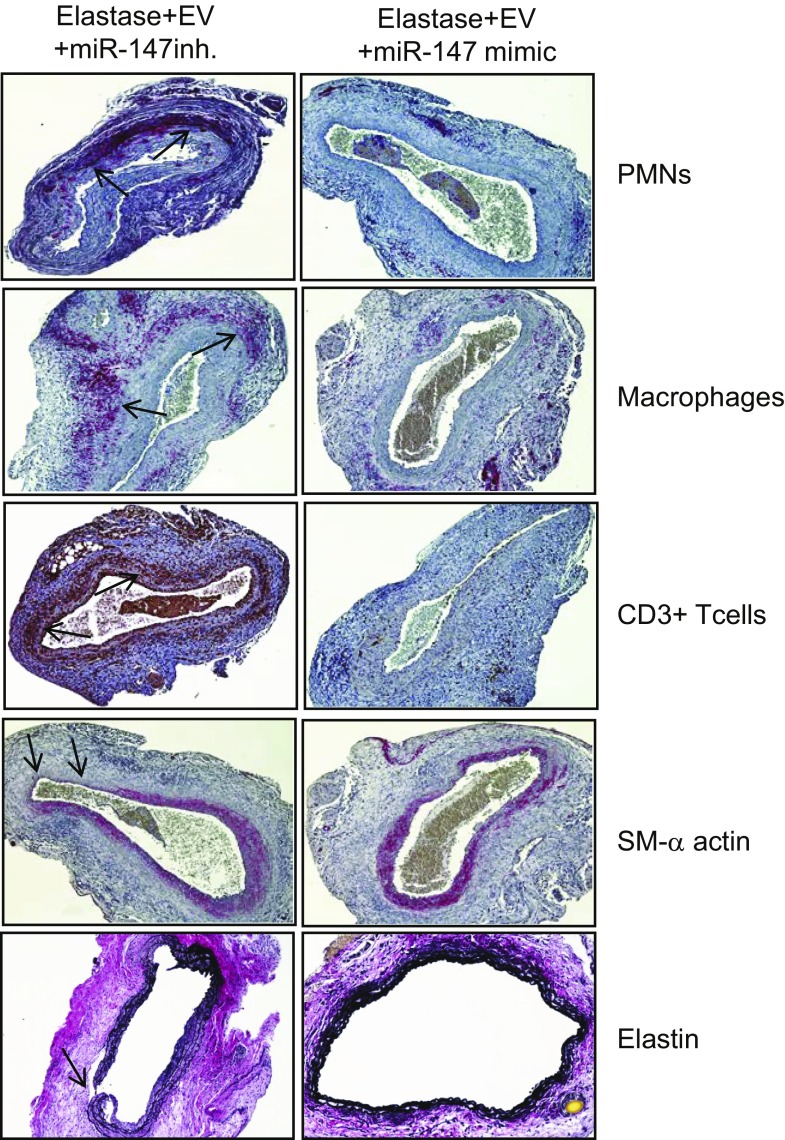
To investigate the role of miRs in EV-mediated protection against AAA formation, we used EVs from MSCs transfected with miR-147 mimic or inhibitor in an in vivo mouse model. First, we analyzed the functional effects of miR-147b mimic and inhibitors by quantifying the expression of miR-147b in EVs by RT-PCR. We observed a significant increase in miR-147b expression in EVs from MSCs transfected with miR-147b mimic compared to mimic controls, as well as a significant decrease in miR-147b expression in EVs from MSCs transfected with miR-147b inhibitor compared to inhibitor controls (Supplemental Fig. 3). Subsequently, we used EVs isolated from transfected MSCs in our in vivo model and observed a significant decrease in aortic diameter of mice treated with EVs isolated from MSCs that were transfected with miR-147 mimic compared with EVs from MSCs transfected with miR-147 inhibitor (105.7 ± 5% vs. 137.5 ± 4.2%) (Fig. 5A, B). There was no significant difference in aortic diameter between elastase-treated mice with and without administration of EVs from MSCs transfected with miR-147 inhibitor (137.5 ± 4.2% vs. 149.8 ± 6.9%), both of which showed AAA formation. Also, we observed no significant differences in aortic diameter in elastase-treated mice that were administered either EVs alone or EVs from MSCs transfected with miR-147 mimic (94.3 ± 3.8% vs. 105.7 ± 5%; Supplemental Fig. 4). Furthermore, we observed no significant differences in the expression of proinflammatory cytokine expression between aortic tissue from mice treated with EVs alone or EVs isolated from MSCs transfected with miR-147 mimic (Supplemental Fig. 4). Moreover a significant attenuation of proinflammatory cytokine expression was observed in elastase-treated mice administered EVs isolated from MSCs transfected with miR-147 mimic, compared with EVs isolated from MSCs transfected with miR-147 inhibitor (Fig. 5C, D). Also, mice treated with EVs from MSCs transfected with miR-147 mimic showed mitigation of immune cell infiltration and protection of aortic morphology, as seen by comparative histology and immunostaining of aortic tissue which revealed a significant attenuation of inflammatory cell (CD3+ T cells, macrophages, and neutrophils) infiltration, increase in α-SMA expression, and decrease in elastic fiber disruption compared to treatment with EVs from MSCs transfected with miR-147 inhibitor (Fig. 6).
Figure 5.
EV-mediated protection in AAA formation is dependent on miR-147. A) Aortic diameter measurement of mice treated with EVs isolated from MSCs transfected with miR-147 mimic showed significant attenuation in AAA formation vs. treatment with EVs isolated from MSCs transfected with miR-147 inhibitor (inh.). There was no significant difference between mice treated with elastase, with or without administration of EVs isolated from MSCs transfected with miR-147 inhibitor. Also, elastase-treated mice after administration of EVs alone or EVs from MSCs with miR-147 mimic transfection demonstrated no significant differences in aortic diameter on d 14. B) Representative example of aortic morphology in the experimental groups. C–F) Aortic tissue from elastase-treated mice after administration of EVs from MSCs transfected with miR-147 mimic showed significant attenuation in expression of proinflammatory cytokines, especially IL-17, TNF-α, and HMGB-1, compared to expression after treatment with EVs from MSCs transfected with miR-147 inhibitor (n = 5–12 mice/group). Ns, not significant. *P < 0.05 vs. elastase+EV+miR-147 inhibitor, #P < 0.05 vs. elastase.
Figure 6.
Immunohistochemistry of aortic tissue demonstrates reduction in polymorphonuclear neutrophil, macrophage (Mac-2), and CD3+ T-cell infiltration; an increase in α-SMA expression; and a decrease in elastic fiber disruption (Verhoeff-Van Gieson staining for elastin) in mice treated with EVs from MSCs transfected with miR-147 mimic compared to EVs from MSCs transfected with miR-147 inhibitor (n = 5 mice/group). Arrows: areas of immunostaining.
EVs down-regulate IL-17 and HMGB-1 expression via miR-147
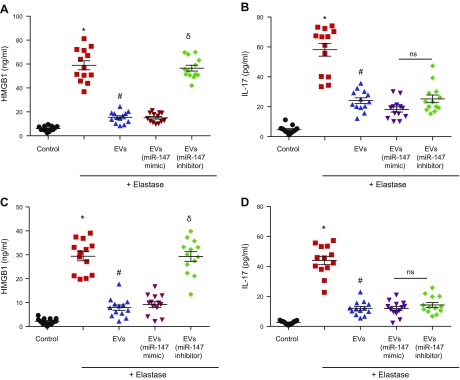
Human aortic explants from male AAA patients were cultured in vitro, with or without EVs, and thereafter were transiently exposed to elastase. After 24 h, a significant increase in HMGB-1 expression was observed in cell culture supernatants after elastase treatment compared with controls and was significantly attenuated by EVs alone, as well as by EVs isolated from MSCs transfected with miR-147 mimic, but not by EVs isolated from MSCs transfected with miR-147 inhibitor (Fig. 7A). EVs, with or without miR-147, mimicked transfection-inhibited, elastase-induced IL-17 expression (Fig. 7B) in a similar manner. However, in contrast to the HMGB-1 regulation, EVs isolated from MSCs transfected with miR-147 inhibitor also attenuated IL-17 expression compared with elastase treatment alone, thereby pointing to an miR-147–independent mechanism of EVs in attenuating IL-17 secretion.
Figure 7.
EV-mediated inhibition of HMGB-1, but not IL-17, production is dependent on miR-147. A) Human aortic explants were cultured and transiently treated with elastase or not. A significant increase in HMGB-1 was observed after elastase treatment vs. control. Aortic explants cocultured with EVs displayed significant attenuation of HMGB-1 expression. Similarly, EVs from MSCs transfected with miR-147 mimic also attenuated HMGB-1 release but EVs from MSCs transfected with miR-147 inhibitor failed to do so. B) IL-17 production was significantly increased after elastase treatment compared to control. Treatments of human aortic explants with EVs, EVs from MSCs transfected with miR-147 mimic, or EVs from MSCs transfected with miR-147 inhibitor attenuated IL-17 production. C) CD11b+ macrophages purified from spleens of WT mice were exposed to transient exposure with elastase (0.4 U/ml for 5 min) and HMGB-1 secretion was measured in cell culture supernatants after 24 h. A multifold increase in HMGB-1 production was observed in macrophages vs. controls and was significantly attenuated by cocultures with EVs. Also, EVs from MSCs transfected with miR-147 mimic, but not EVs from MSCs transfected with miR-147 inhibitor, attenuated HMGB-1 production. D) IL-17 production by CD4+ T cells was significantly increased after elastase treatment compared to control. Treatment of CD4+ T cells with EVs alone, EVs from MSCs transfected with miR-147 mimic, and EVs from MSCs transfected with miR-147 inhibitor attenuated IL-17 production (n = 13/group). Ns, not significant. *P < 0.05 vs. control, #P < 0.05 vs. elastase, δP < 0.05 vs. elastase+EVs (miR-147 mimic).
Furthermore, to specifically decipher the protective mechanism of EV-mediated immune cell activation, primary CD11b+ macrophages and CD4+ T cells were purified from WT mice, grown in culture with or without EVs, and exposed to transient treatment with elastase (0.4 U/ml for 5 min.). The cells were washed with PBS, and fresh medium was added. Macrophage-dependent HMGB-1 and CD4+ T-cell–dependent IL-17 expression was measured in culture supernatants after 24 h. A significant increase in HMGB-1 production was observed in elastase-treated macrophages that was attenuated by coculture with MVs alone, as well as EVs isolated from MSCs transfected with miR-147 mimic, but not by EVs isolated from MSCs transfected with miR-147 inhibitor (Fig. 7C). Also, elastase treatment induced CD4+ T-cell–dependent IL-17 production that was attenuated by EVs alone as well, as well as by EVs transfected with miR-147 mimic. Moreover, EVs from MSCs transfected with miR-147 inhibitor attenuated elastase-induced IL-17 production, suggesting an miR-147–independent mechanism for EV-mediated mitigation of CD4+ T-cell–produced IL-17 (Fig. 7D). These results suggest that EVs attenuate immune cell activation to decrease HMGB-1 and IL-17 expression from macrophages and CD4+ T cells, respectively. Furthermore, the data show that miR-147 plays a role in EV-mediated attenuation of macrophage-dependent HMGB-1 secretion, but not of CD4+ T-cell–dependent IL-17 production.
EVs attenuate aortic smooth muscle cell activation via miR-147
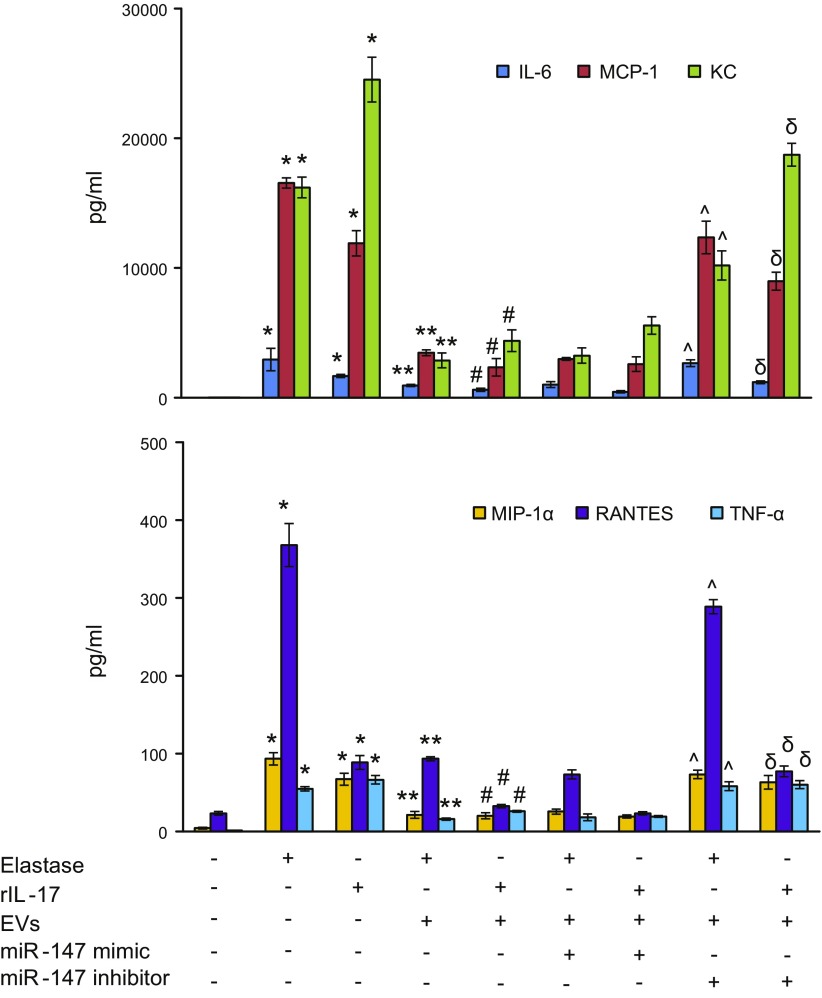
One feature of the pathogenesis of AAA formation is inflammation and vascular remodeling of AoSMCs. To investigate the protective role of EVs in AoSMC activation, smooth muscle cells were separately treated with elastase (0.4 U/ml) or recombinant IL-17 (10 ng/ml) for 24 h, with or without coculturing with EVs. Cytokine production in the supernatants was measured at the endpoints. Elastase or IL-17 treatment of AoSMCs significantly increased the production of IL-6, MCP-1, KC, MIP-1α, TNF-α, and RANTES (Fig. 8) that was attenuated by EVs alone. In addition, EVs from MSCs transfected with miR-147 mimic, but not EVs from MSCs transfected with miR-147 inhibitor, significantly decreased proinflammatory cytokine production induced by elastase or IL-17 treatment of AoSMCs. These results demonstrate that EVs attenuate AoSMC activation in an miR-147–dependent manner.
Figure 8.
IL-17-induced AoSMC activation is inhibited by EVs in an miR-147–dependent manner. Primary AoSMCs, cocultured with or without EVs, were treated with elastase or recombinant I(r)L-17, and cytokine secretion was analyzed in cell culture supernatants after 24 h. A multifold increase in IL-6, MCP-1, KC, MIP-1α, RANTES, and TNF-α production was observed in AoSMCs after elastase- or IL-17-treatment vs. controls and was significantly attenuated by cocultureing with EVs alone. Also, EVs from MSCs transfected with miR-147 mimic attenuated cytokine release in elastase- or IL-17–treated AoSMCs, but EVs from MSCs transfected with miR-147 inhibitor failed to inhibit cytokine production (n = 8/group). *P < 0.05 vs. control, **P < 0.05 vs. elastase; #P < 0.05 vs. rIL-17, ^P < 0.05 vs. elastase+EVs+miR-147 mimic, δP < 0.05 vs. rIL-17+EVs+miR-147 mimic.
DISCUSSION
This study provides insight into the mechanisms of AAA pathogenesis and establishes that EVs derived from MSCs can mitigate experimental AAA formation. The results pinpoint 3 important new findings. First, human MSC-derived EVs inhibited aortic inflammation and AAA formation in an elastase-treated mouse model and were an effective tool for immunomodulating aortic inflammation and vascular remodeling. Second, the EV-mediated mitigation was dependent on modulation of immune cell and aortic smooth muscle cell activation by miR-147. Finally, using human AAA explant tissue, we confirmed that the mechanism and actions by MSCs and EVs in aortic tissue replicated the experimental animal data. These results demonstrate that EVs are protective and immunomodulatory derivatives of MSCs in attenuating AAA formation, and this phenomenon is partly modulated by miR-147. The findings suggest that human MSC-derived EVs have therapeutic value in mitigating AAA formation at the onset of inflammatory damage.
We and other researchers have established that MSCs can effectively attenuate formation of aneurysms in various experimental animal models (9, 11, 37–40). However, the precise mechanisms of MSC-mediated immunomodulation of aortic inflammation and protection against AAA formation remain to be identified. This is the first study to demonstrate that MSC-derived EVs are as effective as MSCs in improving aortic inflammation and AAA formation. The paracrine effects of MSCs have been postulated to play an important role in inhibition of vascular remodeling, and we have determined that conditioned medium from MSCs is as beneficial in attenuating aneurysm formation as MSCs alone (11, 19). The present study identifies EVs as the source of MSC-mediated paracrine effects in substantially down-regulating inflammation by targeting immune cells and AoSMCs to protect the aortic morphology.
Recent studies in other disease processes have identified derivatives of MSCs, such as exosomes and MVs, as potent inhibitors of injury and inflammation in various organs and tissues (20, 22, 34, 41). Furthermore, the genetic content of exosomes and MVs has been shown to contain mRNA and miRs capable of regulating transcription and modulating angiogenesis, atherogenesis, and other pathways in recipient cells (21, 42, 43). These novel mediators of intercellular communication have the ability to deliver complex biologic messages between different cells (44, 45). These EVs can be classified as exosomes (originating from multivesicular bodies, 10–100 nm) or MVs (derived from plasma membrane, 100–1000 nm) and carry specific markers, such as CD44, β1-integrins, CD73, and CD81, in addition to surface markers of their cells of origin (46, 47). The miR cargo of MSC-derived EVs has been characterized as mediating prosurvival and antiapoptotic effects in injury models (21, 23). We also showed evidence that EVs exert their anti-inflammatory protective effects via miR-147, which has been reported to regulate the macrophage inflammatory response (48), and therefore may act as a vehicle to deliver specific antagomiRs (antisense miRs) or protectomiRs (overexpressing miRs; i.e., miR-147) to target AoSMCs and immune cell activation. It is also plausible that EVs themselves contain biologically relevant miRs (i.e., miR-147, Let-7i, miR-19a and miR-98) to mediate antiproliferative immunomodulation of aortic tissue in AAA. Previous studies have illustrated that specific miRs are expressed in MSC-derived EVs, along with specific growth factors, mitochondria, and anti-inflammatory molecules, including angiopoietin-1, β-defensin, IL-1 receptor antagonist, and IL-10 mRNA, which are postulated to exert an immunomodulatory role in inhibiting vascular inflammation (44, 49–51). Recent studies have also shown the functional RNA component in EVs to be microRNAs, by detailed profile analysis via high-throughput screening (12, 52). Thus these studies provide confirmatory evidence of the presence of specific miRs in MSC-derived EVs. Moreover, the uptake of EVs by the inflamed tissue depends upon glycoproteins and cell surface markers (i.e., CD44 and β1-integrins) that are critical for the internalization and incorporation of EVs into the target cells (41, 53). EVs can also get incorporated into aortic endothelium via cell surface tetraspanin-8, thereby demonstrating the ability to penetrate the vessel wall (54). Further studies are needed to determine the fate and pharmacokinetics of MSC-derived EVs, as the route of administration can determine the biodistribution of these nanoparticles (55).
Other studies have indicated an important role of various miRs (i.e., miR-29b, miR-24 and miR-712) in the pathogenesis of AAA (27–29). The benefit of targeting specific microRNAs with antagomiR-712 or -205 has been shown to reduce expansion of the aortic wall (29). In vivo administration of anti-miR-29b led to increased collagen expression in the aortic wall, leading to a significant reduction in AAA formation (28). These studies show the protective effects of targeting single miRs in regulating numerous gene networks in a coordinated fashion. However, the potential challenges of using anti-miR administration limit its clinical utility for long-term administration in chronic diseases, such as AAA, and could make necessary multiple, invasive procedures to deliver this treatment to the site of the desired target. The use of lentiviral vectors could be a useful tool for delivering miRs to the injured aorta, but the homologous recombination of these vectors remains a safety concern. Moreover, the use of single antagomiR or protectomiR is likely to have limited benefit in a complex, multifactorial disease process and may have unintended off-target effects. Therefore, we chose to administer EVs, which can inhibit inflammatory signaling pathways via its multiple inherent properties involving keratinocyte , vascular endothelial, and human growth factors (56, 57), prostaglandin E2 (58), and indoleamine-2,3-dioxygenase (59), and act as a unique platform to deliver specific miRs. This approach of using modified EVs is a multifaceted therapeutic strategy that combines the beneficial properties of MSCs and acts as delivery platform for specific antagomiRs (miR-98, Let-7i) or protectomiRs (miR-147) to effectively engage multiple immune cells and aortic smooth muscle cells, leading to a protective phenotype against aneurysm.
Although the present study identifies MSC-derived EVs as a potential mechanism for inhibiting inflammatory signaling, a few shortcomings of this study should be noted. First, we used a single administration of EVs on d 1, which may have limited the extent of protection against AAA formation. A more clinically relevant approach would be to administer multiple doses on subsequent days to optimize the maximal therapeutic effect of EVs. Second, although EVs were administered after elastase treatment on d 1, it would be more clinically relevant to assess their protective effect on preformed aneurysms. Therefore, our future studies will investigate whether EVs can be useful for regeneration or repair of an existing AAA. The third limitation is that the process of ultracentrifugation inadvertently contains a heterogeneous population of EVs, including MVs and exosomes with varied contents. The relative contribution of these microparticles and their contents should be further delineated in research. Finally, we have deciphered only the role of miR-147 in this study, but there are other relevant miRs that may also contribute to the pathogenesis of AAA. It is plausible that the use of modified EVs with a combination of specific antagomiRs and protectomiRs suppresses aortic inflammation and vascular remodeling, offering enhanced protection against AAA formation. Harnessing the regulatory transcriptional effects via multiple miRs may provide a useful tool via dampening proinflammatory mechanistic pathways via HMGB-1 and IL-17, as well as up-regulating anti-inflammatory signaling pathways involving IL-10 and TGF-β through regulatory T cells and M2 macrophages.
In summary, in our study EVs were as effective as MSCs in inhibiting experimental AAA formation and down-regulated immune cell and AoSMC activation in an miR-147–dependent manner. The data provide insight into the mechanistic capabilities of EVs derived from MSCs, with respect to their potential to modulate several pathways in the recipient cells. These observations provide a molecular framework for the clinical application of MSC-derived EVs for aortic tissue repair and remodeling.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Rupande Tripathi (University of Virginia) for isolation of primary aortic smooth muscle cells; Kimberly Kelly (University of Virginia) for assistance with isolation of EVs and use of the Nanosight instrument; Joanne Lannigan (Flow Core Facility, University of Virginia) for help with the ImageStream experiments; and anonymous donors for providing umbilical cords for research and the nurses at the Via Christi Birthing Center (Wichita, KS, USA) for supplying discarded umbilical cord tissue. This study was supported by American Heart Association Grant-in-Aid 17GRNT33370027 (to A.K.S.), and U.S. National Institutes of Health, National Heart, Lung and Blood Institute Grant R01 HL081629 (to G.R.U.). The authors declare no conflicts of interest.
Glossary
- AAA
abdominal aortic aneurysm
- α-SMA
α-smooth muscle actin
- AoSMC
aortic smooth muscle cell
- CD
cluster of differentiation
- EV
extracellular vesicle
- HMGB
high-mobility group box
- IPA
Ingenuity Pathway Analysis
- Let-7i
lethal-7i
- miR
microRNA
- MSC
mesenchymal stromal cell
- MV
microvesicle
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. R. Upchurch Jr., and A. K. Sharma designed the research; M. Spinosa, G. Lu, G. Su, S. V. Bontha, R. Gehrau, M. D. Salmon, and A. K. Sharma performed the research; J. R. Smith and M. L. Weiss contributed new reagents; V. R. Mas, G. R. Upchurch, Jr., and A. K. Sharma analyzed the data; A. K. Sharma wrote the manuscript with contributions from all authors; and all authors reviewed and approved the final manuscript.
REFERENCES
- 1.Ailawadi G., Eliason J. L., Upchurch G. R., Jr (2003) Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 38, 584–588 [DOI] [PubMed] [Google Scholar]
- 2.Bengtsson H., Sonesson B., Bergqvist D. (1996) Incidence and prevalence of abdominal aortic aneurysms, estimated by necropsy studies and population screening by ultrasound. Ann. N. Y. Acad. Sci. 800, 1–24 [DOI] [PubMed] [Google Scholar]
- 3.Dimick J. B., Stanley J. C., Axelrod D. A., Kazmers A., Henke P. K., Jacobs L. A., Wakefield T. W., Greenfield L. J., Upchurch G. R., Jr (2002) Variation in death rate after abdominal aortic aneurysmectomy in the United States: impact of hospital volume, gender, and age. Ann. Surg. 235, 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston K. W., Rutherford R. B., Tilson M. D., Shah D. M., Hollier L., Stanley J. C. (1991) Suggested standards for reporting on arterial aneurysms: Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 13, 452–458 [DOI] [PubMed] [Google Scholar]
- 5.Weintraub N. L. (2009) Understanding abdominal aortic aneurysm. N. Engl. J. Med. 361, 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anidjar S., Dobrin P. B., Eichorst M., Graham G. P., Chejfec G. (1992) Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J. Vasc. Surg. 16, 139–147 [DOI] [PubMed] [Google Scholar]
- 7.Aziz F., Kuivaniemi H. (2007) Role of matrix metalloproteinase inhibitors in preventing abdominal aortic aneurysm. Ann. Vasc. Surg. 21, 392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freestone T., Turner R. J., Coady A., Higman D. J., Greenhalgh R. M., Powell J. T. (1995) Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 15, 1145–1151 [DOI] [PubMed] [Google Scholar]
- 9.Sharma A. K., Lu G., Jester A., Johnston W. F., Zhao Y., Hajzus V. A., Saadatzadeh M. R., Su G., Bhamidipati C. M., Mehta G. S., Kron I. L., Laubach V. E., Murphy M. P., Ailawadi G., Upchurch G. R., Jr (2012) Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation 126(11 Suppl 1), S38–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A. K., Salmon M. D., Lu G., Su G., Pope N. H., Smith J. R., Weiss M. L., Upchurch G. R., Jr (2016) Mesenchymal stem cells attenuate NADPH oxidase-dependent high mobility group box 1 production and inhibit abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 36, 908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J. P., Salmon M., Pope N. H., Lu G., Su G., Sharma A. K., Ailawadi G., Upchurch G. R., Jr (2015) Attenuation of aortic aneurysms with stem cells from different genders. J. Surg. Res. 199, 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan C. G., Zhang Q. J., Zhou J. R. (2011) Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 7, 195–207 [DOI] [PubMed] [Google Scholar]
- 13.Singer N. G., Caplan A. I. (2011) Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 6, 457–478 [DOI] [PubMed] [Google Scholar]
- 14.Charbord P. (2010) Bone marrow mesenchymal stem cells: historical overview and concepts. Hum. Gene Ther. 21, 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squillaro T., Peluso G., Galderisi U. (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848 [DOI] [PubMed] [Google Scholar]
- 16.Heldring N., Mäger I., Wood M. J., Le Blanc K., Andaloussi S. E. (2015) Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene Ther. 26, 506–517 [DOI] [PubMed] [Google Scholar]
- 17.Gatti S., Bruno S., Deregibus M. C., Sordi A., Cantaluppi V., Tetta C., Camussi G. (2011) Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 26, 1474–1483 [DOI] [PubMed] [Google Scholar]
- 18.Camussi G., Deregibus M. C., Bruno S., Cantaluppi V., Biancone L. (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 78, 838–848 [DOI] [PubMed] [Google Scholar]
- 19.Camussi G., Deregibus M. C., Cantaluppi V. (2013) Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem. Soc. Trans. 41, 283–287 [DOI] [PubMed] [Google Scholar]
- 20.Bruno S., Grange C., Collino F., Deregibus M. C., Cantaluppi V., Biancone L., Tetta C., Camussi G. (2012) Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 7, e33115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eirin A., Riester S. M., Zhu X. Y., Tang H., Evans J. M., O’Brien D., van Wijnen A. J., Lerman L. O. (2014) MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551, 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loyer X., Vion A. C., Tedgui A., Boulanger C. M. (2014) Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 114, 345–353 [DOI] [PubMed] [Google Scholar]
- 23.Yu B., Gong M., Wang Y., Millard R. W., Pasha Z., Yang Y., Ashraf M., Xu M. (2013) Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PLoS One 8, e73304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C. (2008) MicroRNAs: role in cardiovascular biology and disease. Clin. Sci. (Lond.) 114, 699–706 [DOI] [PubMed] [Google Scholar]
- 25.Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 26.Adam M., Raaz U., Spin J. M., Tsao P. S. (2015) MicroRNAs in abdominal aortic aneurysm. Curr. Vasc. Pharmacol. 13, 280–290 [DOI] [PubMed] [Google Scholar]
- 27.Maegdefessel L., Spin J. M., Raaz U., Eken S. M., Toh R., Azuma J., Adam M., Nakagami F., Heymann H. M., Chernogubova E., Jin H., Roy J., Hultgren R., Caidahl K., Schrepfer S., Hamsten A., Eriksson P., McConnell M. V., Dalman R. L., Tsao P. S. (2014) miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 5, 5214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maegdefessel L., Azuma J., Toh R., Merk D. R., Deng A., Chin J. T., Raaz U., Schoelmerich A. M., Raiesdana A., Leeper N. J., McConnell M. V., Dalman R. L., Spin J. M., Tsao P. S. (2012) Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Invest. 122, 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C. W., Kumar S., Son D. J., Jang I. H., Griendling K. K., Jo H. (2014) Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler. Thromb. Vasc. Biol. 34, 1412–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohno T., Anzai T., Kaneko H., Sugano Y., Shimizu H., Shimoda M., Miyasho T., Okamoto M., Yokota H., Yamada S., Yoshikawa T., Okada Y., Yozu R., Ogawa S., Fukuda K. (2012) High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 59, 299–306 [DOI] [PubMed] [Google Scholar]
- 31.Laser A., Lu G., Ghosh A., Roelofs K., McEvoy B., DiMusto P., Bhamidipati C. M., Su G., Zhao Y., Lau C. L., Ailawadi G., Eliason J. L., Henke P. K., Upchurch G. R., Jr (2012) Differential gender- and species-specific formation of aneurysms using a novel method of inducing abdominal aortic aneurysms. J. Surg. Res. 178, 1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone M. L., Zhao Y., Robert Smith J., Weiss M. L., Kron I. L., Laubach V. E., Sharma A. K. (2017) Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 18, 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdbrügger U., Rudy C. K., Etter M. E., Dryden K. A., Yeager M., Klibanov A. L., Lannigan J. (2014) Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytometry A 85, 756–770 [DOI] [PubMed] [Google Scholar]
- 34.Lee C., Mitsialis S. A., Aslam M., Vitali S. H., Vergadi E., Konstantinou G., Sdrimas K., Fernandez-Gonzalez A., Kourembanas S. (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126, 2601–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mas V. R., Maluf D. G., Archer K. J., Yanek K., Kong X., Kulik L., Freise C. E., Olthoff K. M., Ghobrial R. M., McIver P., Fisher R. (2009) Genes involved in viral carcinogenesis and tumor initiation in hepatitis C virus-induced hepatocellular carcinoma. Mol. Med. 15, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colonnello J. S., Hance K. A., Shames M. L., Wyble C. W., Ziporin S. J., Leidenfrost J. E., Ennis T. L., Upchurch G. R., Jr., Thompson R. W. (2003) Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J. Vasc. Surg. 38, 138–146 [DOI] [PubMed] [Google Scholar]
- 37.Hashizume R., Yamawaki-Ogata A., Ueda Y., Wagner W. R., Narita Y. (2011) Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J. Vasc. Surg. 54, 1743–1752 [DOI] [PubMed] [Google Scholar]
- 38.Parvizi M., Harmsen M. C. (2015) Therapeutic prospect of adipose-derived stromal cells for the treatment of abdominal aortic aneurysm. Stem Cells Dev. 24, 1493–1505 [DOI] [PubMed] [Google Scholar]
- 39.Yamawaki-Ogata A., Fu X., Hashizume R., Fujimoto K. L., Araki Y., Oshima H., Narita Y., Usui A. (2014) Therapeutic potential of bone marrow-derived mesenchymal stem cells in formed aortic aneurysms of a mouse model. Eur. J. Cardiothorac. Surg. 45, e156–e165 [DOI] [PubMed] [Google Scholar]
- 40.Fu X. M., Yamawaki-Ogata A., Oshima H., Ueda Y., Usui A., Narita Y. (2013) Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J. Transl. Med. 11, 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruno S., Grange C., Deregibus M. C., Calogero R. A., Saviozzi S., Collino F., Morando L., Busca A., Falda M., Bussolati B., Tetta C., Camussi G. (2009) Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collino F., Deregibus M. C., Bruno S., Sterpone L., Aghemo G., Viltono L., Tetta C., Camussi G. (2010) Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 5, e11803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bobis-Wozowicz S., Kmiotek K., Sekula M., Kedracka-Krok S., Kamycka E., Adamiak M., Jankowska U., Madetko-Talowska A., Sarna M., Bik-Multanowski M., Kolcz J., Boruczkowski D., Madeja Z., Dawn B., Zuba-Surma E. K. (2015) Human induced pluripotent stem cell-derived microvesicles transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells 33, 2748–2761 [DOI] [PubMed] [Google Scholar]
- 44.Phinney D. G., Di Giuseppe M., Njah J., Sala E., Shiva S., St Croix C. M., Stolz D. B., Watkins S. C., Di Y. P., Leikauf G. D., Kolls J., Riches D. W., Deiuliis G., Kaminski N., Boregowda S. V., McKenna D. H., Ortiz L. A. (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 6, 8472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monsel A., Zhu Y. G., Gennai S., Hao Q., Hu S., Rouby J. J., Rosenzwajg M., Matthay M. A., Lee J. W. (2015) Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 192, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathivanan S., Ji H., Simpson R. J. (2010) Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920 [DOI] [PubMed] [Google Scholar]
- 47.Corrado C., Raimondo S., Chiesi A., Ciccia F., De Leo G., Alessandro R. (2013) Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int. J. Mol. Sci. 14, 5338–5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G., Friggeri A., Yang Y., Park Y. J., Tsuruta Y., Abraham E. (2009) miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc. Natl. Acad. Sci. USA 106, 15819–15824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao L., Zhang Y., Lan B., Wang J., Zhang Z., Zhang L., Xiao P., Meng Q., Geng Y. J., Yu X. Y., Li Y. (2017) MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Res. Int. 2017, 4150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsuda T., Ochiya T. (2015) Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 6, 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan A., Farber E. L., Rapoport A. L., Tejada D., Deniskin R., Akhmedov N. B., Farber D. B. (2009) Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One 4, e4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander M., Hu R., Runtsch M. C., Kagele D. A., Mosbruger T. L., Tolmachova T., Seabra M. C., Round J. L., Ward D. M., O’Connell R. M. (2015) Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 6, 7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulcahy L. A., Pink R. C., Carter D. R. (2014) Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, v3.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nazarenko I., Rana S., Baumann A., McAlear J., Hellwig A., Trendelenburg M., Lochnit G., Preissner K. T., Zöller M. (2010) Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 70, 1668–1678 [DOI] [PubMed] [Google Scholar]
- 55.Di Rocco G., Baldari S., Toietta G. (2016) Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. 2016, 5029619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y. G., Feng X. M., Abbott J., Fang X. H., Hao Q., Monsel A., Qu J. M., Matthay M. A., Lee J. W. (2014) Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 32, 116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deuse T., Peter C., Fedak P. W., Doyle T., Reichenspurner H., Zimmermann W. H., Eschenhagen T., Stein W., Wu J. C., Robbins R. C., Schrepfer S. (2009) Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation 120(11 Suppl), S247–S254 [DOI] [PubMed] [Google Scholar]
- 58.Cho K. S., Lee J. H., Park M. K., Park H. K., Yu H. S., Roh H. J. (2015) Prostaglandin E2 and transforming growth factor-β play a critical role in suppression of allergic airway inflammation by adipose-derived stem cells. PLoS One 10, e0131813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Y., Zhou S., Liu H., Shen B., Zhao H., Peng K., Wu X. (2015) Indoleamine 2, 3-dioxgenase transfected mesenchymal stem cells induce kidney allograft tolerance by increasing the production and function of regulatory T cells. Transplantation 99, 1829–1838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.