Abstract
Retention in care and viral suppression are critical to delaying HIV progression and reducing transmission. Neighborhood socioeconomic context (NSEC) may affect HIV care receipt. We therefore assessed NSEC’s impact on RIC and VS in a diverse HIV clinical cohort.
HIV-positive adults with ≥1 visit at the Vanderbilt Comprehensive Care Clinic and 5-digit ZIP code tabulation area (ZCTA) information between 2008 and 2012 contributed. NSEC z-score indices used neighborhood-level socioeconomic indicators for poverty, education, labor-force participation, proportion of males, median age, and proportion of residents of black race by ZCTA. Retention was defined as ≥2 HIV care visits per calendar year, >90 days apart. Viral suppression was defined as an HIV-1 RNA <200 copies/mL at last measurement per calendar year. Modified Poisson regression was used to estimate risk ratios (RR) and 95% confidence intervals (CI).
Among 2,272 and 2,541 adults included for retention and viral suppression analyses, respectively, median age and CD4 count at enrollment were approximately 38 (1st and 3rd quartile: 30, 44) years and 351 (176, 540) cells/μL, respectively, while 24% were female, and 39% were black. Across 243 ZCTAs, median NSEC z-score was 0.09 (−0.66, 0.48). Overall, 79% of person-time contributed was retained and 74% was virally suppressed. In adjusted models, NSEC was not associated with retention, though being in the 4th vs. 1st NSEC quartile was associated with lack of viral suppression (RR=0.88; 95% CI: 0.80–0.97).
Residing in the most adverse NSEC was associated with lack of viral suppression. Future studies are needed to confirm this finding.
Background:
The HIV care continuum characterizes the movement of persons living with HIV/AIDS (PLWHA) from diagnosis, to linkage to care, to retention in care, to use of antiretroviral therapy (ART), and ultimately, to HIV RNA suppression (Ford & Spicer, 2012; Gardner, McLees, Steiner, Del Rio, & Burman, 2011). Multiple domestic public health agencies have enunciated the need for improvement in the participation of PLWHA across the continuum, including narrowing disparities by demographic, behavioral, and social factors (Centers for Disease Control and Prevention et al., 2014; White House Office of National AIDS Policy, 2010; White House Office of National AIDS Policy, 2015). Such improvement may be particularly critical in the southern United States (US).
For example, the South lags behind all other regions of the country with slower improvements in HIV outcomes, higher HIV and AIDS incidence, and higher AIDS-specific mortality (Adimora, Ramirez, Schoenbach, & Cohen, 2014; Centers for Disease Control and Prevention, 2013; Hanna, Selik, Tang, & Gange, 2012; Reif et al., 2014). To date, however, there has been a greater focus on identifying demographic and behavioral contributors to these patterns, while neglecting the role of environmental factors such as adverse neighborhood socioeconomic contexts, which are disproportionately present in the South (Buot et al., 2014; Dean & Fenton, 2010; Reif, Sullivan, Wilson, Berger, & McAllaster, 2016; Wimberley & Morris, 2003).
Aspects of neighborhood context that may contribute to adverse HIV outcomes include structural poverty and disorder, lack of access to transportation, rurality, and housing instability. Specifically, some of the aforementioned neighborhood-level factors have been shown in some studies to negatively influence retention in care, ART adherence, and viral suppression (Chakraborty et al., 2015; Eberhart et al., 2015; Surratt, Kurtz, Levi-Minzi, & Chen, 2015; Nelson Kinder, Johnson, Hall, Hu, Sweet, & Harris, 2016). However, these prior studies focused either on individuals residing within single cities (Eberhart et al., 2015; Surrat et al., 2015), lacked multiple measures of context (Chakraborty et al., 2015; Nelson et al., 2016), or largely assessed individuals only immediately following linkage to care or ART initiation in cross-sectional analyses (Chakraborty et al., 2015; Eberhart et al., 2015; Nelson et al., 2016; Surrat et al., 2015).
The abovementioned neighborhood-level factors have also been associated with increased substance use, mental health issues, and HIV risk behaviors (Bowleg et al., 2014; Fletcher, Kisler, & Reback, 2014; Tobin, Latkin, & Curriero, 2014). Associations between neighborhood context and adverse HIV outcomes may therefore be operating at least in part through mental health and behavioral factors that have been shown to impact the HIV care continuum (American Psychological Association, 2015; Bowleg et al., 2014; Friedman et al., 2009; Latkin, German, Vlahov, & Galea, 2013; Reif et al., 2016; Royal et al., 2009). Thus, to add to the evidence base regarding the role of neighborhood context in adverse HIV outcomes while addressing some of the limitations of prior studies (Chakraborty et al., 2015; Eberhart et al., 2015; Nelson et al., 2016; Surrat et al., 2015), we used cohort data from a well-characterized southern clinic-based population of PLWHA with greater geographic diversity than prior work (Eberhart et al., 2015; Surrat et al., 2015) to quantify the influence of neighborhood socioeconomic context on HIV care continuum stages (i.e., retention in care and viral suppression).
Methods:
Study population:
HIV-positive individuals ≥18 years old with ≥1 clinic visit at the Vanderbilt Comprehensive Care Clinic (VCCC) and residing in 5-digit ZIP code tabulation areas (ZCTAs) with available census-derived socioeconomic information during the study period, 1 January 2008 to 31 December 2012, were included in the study population. Data from the VCCC include encounter and demographic information, vital status, ART use, diagnoses, and laboratory values (e.g. CD4+ lymphocyte count (CD4) and plasma HIV-1 RNA viral load (VL)). The VCCC cohort has been described previously (McGowan et al., 2011).
Outcomes and follow-up:
The HIV care continuum stages of retention in care and viral suppression were assessed. Outcomes were anchored to calendar time in line with common programmatic reporting requirements, such as those for Ryan White client-level data reports (U.S. Department of Health and Human Services Health Resources and Services Administration, 2015). Retention in care was defined as ≥2 visits, >90 days apart, in each year of follow up (Ford & Spicer, 2012; White House Office of National AIDS Policy, 2010). This measure has been associated with other retention measures and multiple HIV disease outcomes and all-cause mortality (Ulett et al., 2009; Yehia et al., 2012). Viral suppression was defined as a VL <200 copies/mL at last measurement in the year, among those with ≥1 clinic visit and a measured VL in the year (Valdiserri, Forsyth, Yakovchenko, & Koh, 2013).
Patients with missing VL data constituted <2% of individuals who had the requisite clinic visits to otherwise qualify for inclusion per calendar year. Patients who did not have the requisite clinic visits to be included in the viral suppression denominator constituted between 4% and 9% of the study sample per calendar year. As these were <10% of the eligible patient population in any calendar year, the outlined exclusions from the viral suppression denominator were considered to be a negligible potential source of selection bias. Furthermore, because the exposure and covariates (described later) were not time-updated, our analysis was not vulnerable to missing exposure or covariate information during gaps in care.
For retention in care, participants were followed from their first visit during the study period until death or end of the study period. However, if enrollment occurred within the last 3 months of a given calendar year during the study period, then person-time between enrollment and the end of that calendar year was excluded because the retention in care definition could not be met during the person-time available.
For viral suppression, participants were followed from their first visit during the study period until death or the end of the study period. To maximize the number of VL assessments, individual patient follow-up was not censored because of missing VL data or the occurrence of a calendar year without the requisite number of clinic visits. Instead, these patients were not included in the viral suppression denominator in the pertinent calendar year.
Neighborhood socioeconomic context:
Neighborhood context characteristics were determined based on geocoded patient residential data from the first visit during the study period. Publicly available US Decennial Census (2010) and American Community Survey (2008–2012) data at the 5-digit ZIP code tabulation area (ZCTA) level were used to derive neighborhood context (American Psychological Association, 2015; Latkin et al., 2013). Specifically, for each ZCTA, the neighborhood socioeconomic contextual indicators included: the percentage of the population of black race, the median age, the percentage with male sex assigned at birth (sex), the percentage living below twice the Federal Poverty Level, per capita income, percentage with less than a high school education, and percentage not participating in the labor force. The aforementioned selected indicators were informed by prior work conducted by Arnold, Hsu, Pipkin, McFarland, & Rutherford GW (2009), and subsequently supported by others, in which these indicators were directly or indirectly linked to poverty and socioeconomic context (Arnold et al., 2009; Dean & Fenton, 2010; McKenzie & Rapino, 2011; Wilson et al., 2011).
In line with the methods of Arnold et al. (2009), Z-scores for each indicator were calculated across ZCTAs. The resulting Z-scores for each ZCTA were assigned to each individual according to their residential ZCTA; these Z-scores were then summed across indicators for each individual to create a neighborhood socioeconomic context (NSEC) index score for each individual (Supplemental Figure 1) (Arnold et al., 2009; Geronimus & Bound, 1998; Soobader, LeClere, Hadden, & Maury, 2001). The final NSEC index score was modeled by quartile. A higher score (and therefore higher quartile) represented more extreme positive scores on constituent factors, representing more adverse overall NSEC.
Covariates:
Dates of clinic enrollment, HIV healthcare provider visits, and death were used to establish follow-up over the study period. Covariates such as year of birth, sex (i.e., male or female), race/ethnicity (i.e., categorized as white Non-Hispanic (white), black Non-Hispanic (black), Hispanic, and other/unknown), and HIV risk factor (i.e., categorized as male-to-male sexual contact (MSM), injection drug use (IDU), heterosexual contact (Hetero), or other/unknown-including perinatal infection) were collected at clinic enrollment and did not vary over the study period. Laboratory values at baseline such as CD4 and VL did not vary after baseline either and were used as covariates to describe the study population at baseline.
Time since enrollment in HIV care (in years) at the start of follow-up was used as a covariate that did not vary over the study period. There were no missing covariate values in the study sample.
Statistical analysis:
Modified Poisson regression was used to estimate relative risks (RR) and 95% confidence intervals (95% CI) for outcomes by quartile of the NSEC index score (Zou, 2004). The time scale was time since the first visit during the study period. Generalized estimating equations (GEE) were used to account for potentially correlated outcomes within ZCTAs and individual over time (Miglioretti & Heagerty, 2007; Zeger, Liang, & Albert, 1988). Adjusted models accounted for individual-level factors including year of birth, sex, race/ethnicity, and time since enrollment in HIV care (years). Covariates were modeled using restricted cubic splines or categorical indicators. Trends in RRs were tested using orthogonal polynomials in adjusted regression models (Hubert, 1973).
Conditional probabilities of the outcomes were extracted from linear combinations of predictors included in the adjusted model, set to mean covariate values. All tests were two-tailed and considered statistically significant with p<0.05. Analyses were conducted using Stata 12 (StataCorp, College Station, TX).
The Vanderbilt University School of Medicine Institutional Review Board approved all study activities. All procedures performed were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent was not required.
Results:
Among 2,272 adults contributing 8,439 person-years for the retention analysis, median age at enrollment was 38 (1st quartile, 3rd quartile: 30, 44) years and median CD4 was 351 (176, 540) cells/μL, while 24% were female, 39% were black, and 8% had IDU as HIV risk factor (Table 1a). Among 2,541 adults contributing 8,972 person-years for the viral suppression analysis, median age at enrollment was 37 (29, 44) years and median CD4 was 437 (266, 630) cells/μL, while 24% were female, 39% were black, and 8% had IDU as HIV risk factor (Table 1b).
Tables 1 a, b.
Sample size at Enrollment and Person-years Contributed during the Study Period Stratified by Characteristics among Vanderbilt Comprehensive Care Clinic Participants, across Retention in Care (a.) and Viral Suppression (b.) status, 2008–2012
| a. | ||||||
|---|---|---|---|---|---|---|
| Sample Size at Enrollment |
Retention in Care | |||||
| (Unless Specified Otherwise) |
Not Retained | Retained | ||||
| Characteristic | Number | Col. % | Person- Years |
Row % | Person- Years |
Row % |
| Total | 2,272 | 100% | 1,740 | 20.6% | 6,699 | 79.4% |
| Neighborhood Risk Score (Quartile) |
||||||
| 1st | 617 | 27.2% | 430 | 18.7% | 1,871 | 81.3% |
| 2nd | 610 | 26.9% | 416 | 18.6% | 1,827 | 81.5% |
| 3rd | 499 | 21.9% | 380 | 20.8% | 1,448 | 79.2% |
| 4th | 546 | 24.0% | 514 | 24.9% | 1,553 | 75.1% |
| Median Age at Enrollment, years (1st quartile, 3rd quartile) |
38 (30, 44) | |||||
| Age at Enrollment (years) | ||||||
| 18–20 | 44 | 1.9% | 44 | 33.1% | 89 | 66.9% |
| 20–29 | 515 | 22.7% | 470 | 26.1% | 1,329 | 73.9% |
| 30–39 | 748 | 32.9% | 686 | 23.5% | 2,237 | 76.5% |
| 40–49 | 719 | 31.7% | 429 | 15.6% | 2,315 | 84.4% |
| 50–59 | 208 | 9.2% | 101 | 14.3% | 604 | 85.7% |
| 60–69 | 33 | 1.5% | 8 | 6.7% | 111 | 93.3% |
| 70–81 | 5 | 0.2% | 2 | 12.5% | 14 | 87.5% |
| Sex Assigned at Birth | ||||||
| Male | 1,718 | 75.6% | 1,338 | 21.2% | 4,979 | 78.8% |
| Female | 554 | 24.4% | 402 | 18.9% | 1,720 | 81.1% |
| Race/Ethnicity | ||||||
| White, Non-Hispanic | 1,149 | 50.6% | 800 | 18.4% | 3,555 | 81.6% |
| Black, Non-Hispanic | 897 | 39.5% | 819 | 24.9% | 2,468 | 75.1% |
| Hispanic | 103 | 4.5% | 62 | 16.6% | 312 | 83.4% |
| Other/Unknown | 123 | 5.4% | 59 | 13.9% | 364 | 86.1% |
| HIV Transmission Risk | ||||||
| MSM | 1,205 | 53.0% | 845 | 19.0% | 3,601 | 81.0% |
| IDU | 188 | 8.3% | 198 | 26.8% | 541 | 73.2% |
| Hetero | 793 | 34.9% | 638 | 21.0% | 2,393 | 79.0% |
| Other/Unknown | 86 | 3.8% | 59 | 26.5% | 164 | 73.5% |
| Median CD4 at Enrollment, cells/mm3 (1st quartile, 3rd quartile) |
351 (176, 540) | |||||
| Years Since Enrollment (at start of follow-up) |
||||||
| 0–5 | 1,561 | 68.7% | 437 | 20.4% | 1,700 | 79.6% |
| 5–9 | 624 | 27.5% | 772 | 20.5% | 2,992 | 79.5% |
| 10 | 87 | 3.8% | 531 | 20.9% | 2,007 | 79.1% |
| b. | ||||||
|---|---|---|---|---|---|---|
| Sample Size at Enrollment |
Viral Suppression | |||||
| (Unless Specified Otherwise) |
Not Suppressed | Suppressed | ||||
| Characteristic | Number | Col. % | Person- Years |
Row % | Person- Years |
Row % |
| Total | 2,541 | 100% | 2,349 | 26.2% | 6,623 | 73.8% |
| Neighborhood Risk Score (Quartile) |
||||||
| 1st | 681 | 26.8% | 515 | 20.9% | 1,943 | 79.1% |
| 2nd | 682 | 26.8% | 631 | 25.9% | 1,806 | 74.1% |
| 3rd | 579 | 22.8% | 502 | 25.5% | 1,466 | 74.5% |
| 4th | 599 | 23.6% | 701 | 33.2% | 1,408 | 66.8% |
| Median Age at Enrollment, years (1st quartile, 3rd quartile) |
37 (29, 44) | |||||
| Age at Enrollment (years) | ||||||
| <20 | 51 | 2.0% | 63 | 40.4% | 93 | 59.6% |
| 20–29 | 602 | 23.7% | 749 | 38.2% | 1,213 | 61.8% |
| 30–39 | 817 | 32.2% | 722 | 24.3% | 2,245 | 75.7% |
| 40–49 | 784 | 30.9% | 625 | 21.3% | 2,307 | 78.7% |
| 50–59 | 240 | 9.5% | 167 | 20.9% | 633 | 79.1% |
| 60–69 | 41 | 1.6% | 16 | 11.8% | 120 | 88.2% |
| 70–81 | 6 | 0.2% | 7 | 36.8% | 12 | 63.2% |
| Sex | ||||||
| Male | 1,934 | 76.1% | 1,640 | 24.3% | 5,107 | 75.7% |
| Female | 607 | 23.9% | 709 | 31.9% | 1,516 | 68.1% |
| Race/Ethnicity | ||||||
| White, Non-Hispanic | 1,299 | 51.1% | 998 | 21.5% | 3,638 | 78.5% |
| Black, Non-Hispanic | 999 | 39.3% | 1,136 | 32.9% | 2,313 | 67.1% |
| Hispanic | 112 | 4.4% | 98 | 24.0% | 310 | 76.0% |
| Other/Unknown | 131 | 5.2% | 117 | 24.4% | 362 | 75.6% |
| HIV Transmission Risk | ||||||
| MSM | 1,330 | 52.3% | 1,104 | 23.2% | 3,664 | 76.8% |
| IDU | 202 | 8.0% | 252 | 34.1% | 488 | 65.9% |
| Hetero | 851 | 33.5% | 886 | 28.3% | 2,246 | 71.7% |
| Other/Unknown | 158 | 6.2% | 107 | 32.2% | 225 | 67.8% |
| Median CD4+ at Enrollment, cells/mm3 (1st quartile, 3rd quartile ) |
355 (176, 546) | |||||
| Years Since Enrollment (at start of follow-up) |
||||||
| 0–5 | 1,830 | 72.0% | 1,062 | 33.1% | 2,144 | 66.9% |
| 5–9 | 624 | 24.6% | 781 | 22.6% | 2,669 | 77.4% |
| 10 | 87 | 3.4% | 506 | 21.8% | 1,810 | 78.2% |
Percentages may not sum to 100%, due to rounding
Col. % uses the total sample size of individuals (n=2,541) as the denominator; Row % uses the total person-years within a given row, summed across “Not Retained” and “Retained” (in Table 1a) or else across “Not Suppressed” and “Suppressed” (in Table 1b), as the denominator
Neighborhood Risk Score: z-score index for each individual based on % with income less than twice the poverty level, per-capita income, % less than high school educated, % non-participation in labor force, median age, % male sex, and % black race within a given 5-digit ZCTA
Ref.: reference value; MSM: men who have sex with men; IDU: history of injection drug use; Hetero: heterosexual contact
Patients in the VCCC resided in 243 5-digit ZCTAs during the study period, providing adequate heterogeneity in exposure. Approximately 58% of the study population resided in the Nashville area (21 ZCTAs within Davidson county), 18% in the Nashville suburbs (25 ZCTAs in four counties surrounding Davidson county), and 24% beyond Nashville (197 remaining ZCTAs); 88% of the study population lived in an urban ZCTA (i.e., a ZCTA with census-derived proportion urban ≥50%). Across these 243 ZCTAs, median NSEC z-score was 0.09 (−0.66, 0.48) (Figure 1, Supplemental Figure 1). Overall, 79% of person-time contributed was retained and 74% was virally suppressed. Younger individuals were more poorly retained and less likely to be virally suppressed while older individuals achieved better outcomes (Tables 1 a, b). Female patients were retained in similar proportions to males, but were virally suppressed in lower proportions (Tables 1 a, b). Furthermore, both black and IDU patients had the poorest outcomes (Tables 1 a, b).
Figure 1.
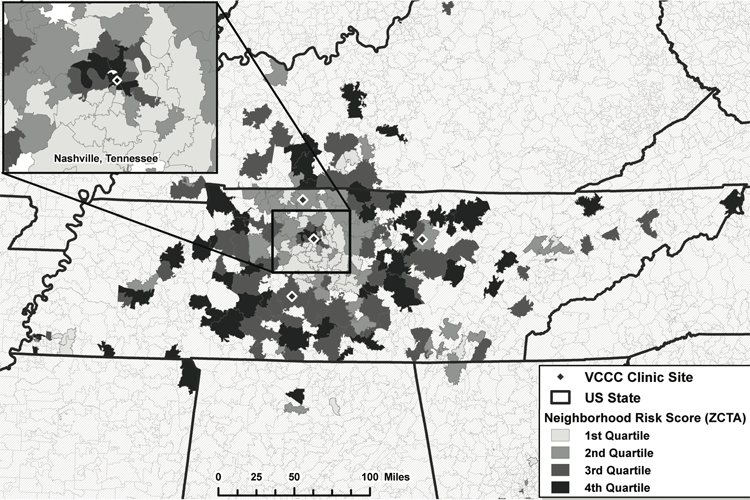
Distribution of Neighborhood Socioeconomic Context Quartiles by Residential 5-digit ZIP-Code Tabulation Areas (ZCTAs) of Vanderbilt Comprehensive Care Clinic Participants, 2008–2012.
The observed percentage of person-time retained was generally lower in more adverse neighborhoods (75% in the 4th vs. 81% in the 1st NSEC quartile; Table 1a). However, in unadjusted models and adjusted models accounting for individual year of birth, sex, race/ethnicity, and time since enrollment in HIV care, more adverse socioeconomic context was not significantly associated with poorer retention. In contrast, more adverse socioeconomic context was significantly associated with lack of viral suppression for the 4th vs. the 1st NSEC quartile (RR=0.88; 95% CI: 0.80–0.97). Neither predicted probabilities of retention nor viral suppression demonstrated statistically significant dose-response relationships by NSEC quartile (Figure 2), though point estimates were indicative of a trend, with the 4th NSEC quartile experiencing worse outcomes than lower quartiles (which indicate better NSEC) (Figure 2, Table 2).
Figure 2.
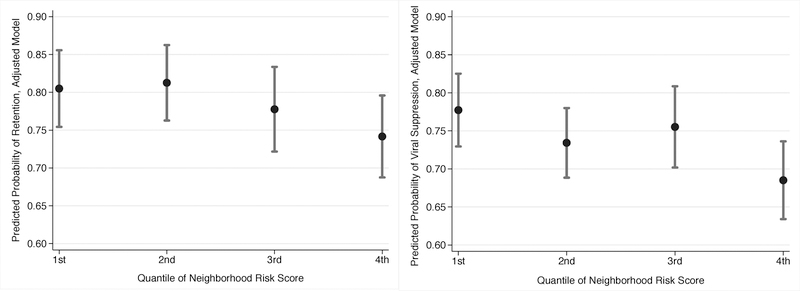
Predicted Conditional Probability of Retention in Care (a.) and Viral Suppression (b.) by Neighborhood Socioeconomic Context Risk Score Quartile, from Adjusted Model Accounting for Year of Birth, Sex, Race/ethnicity, and Time since Enrollment in HIV care, among those with ≥1 visit at the Vanderbilt Comprehensive Care Clinic, 2008–2012
Table 2.
Retention in Care and Viral Suppression by Neighborhood Socioeconomic Context Risk Score Quartile among those with ≥1 Visit at the Vanderbilt Comprehensive Care Clinic, 2008–2012
| Retention | Viral Suppression | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | RR | (95% CI) | aRR | (95% CI) | RR | (95% CI) | aRR | (95% CI) |
| Neighborhood Risk Score (Quartile) |
||||||||
| 1st | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 2nd | 1.01 | (0.93–1.10) | 1.01 | (0.92–1.10) | 0.93 | (0.85–1.02) | 0.94 | (0.87–1.03) |
| 3rd | 0.97 | (0.89–1.06) | 0.97 | (0.88–1.06) | 0.96 | (0.87–1.06) | 0.97 | (0.88–1.06) |
| 4th | 0.92 | (0.84–1.01) | 0.92 | (0.84–1.02) | 0.87 | (0.79–0.97) | 0.88 | (0.80–0.97) |
RR: unadjusted Risk Ratio
aRR: adjusted Risk Ratio
95% CI: 95% Confidence Interval
Discussion:
In this analysis more adverse neighborhood socioeconomic context was found not to be significantly related to retention. However, the poorest socioeconomic context score was found to be associated with lack of viral suppression compared to the best score. These findings persisted even after accounting for demographics and secular trends. It may also be noteworthy that higher NSEC score did significantly predict lack of retention and viral suppression when assuming independence between individuals within ZCTAs (results not shown). However, we assert this is not a reasonable assumption.
It is possible that, because this particular study population had high levels of retention in care and viral suppression and access to robust safety-net services, differences in access to care, which may be expected to vary according to neighborhood socioeconomic context, were mitigated (Doshi, Milberg, Isenberg, Matthews, Malitz, Matosky, & Cheever, 2015). However, it is unlikely our null findings resulted from insufficient variability in indicators across quartiles of NSEC risk score. In quantile regression clustered at the ZIP code level, there were indeed significant differences across the quartiles of NSEC risk score in the socioeconomic contextual indicators that were used to develop the NSEC score (p<0.01 each).
Other studies in various US settings have obtained mixed results. In a largely urban cohort in Philadelphia, Eberhart et al. found lower economic deprivation areas associated with a lower probability of retention. In the same study, however, the authors found higher economic deprivation areas associated with a lower probability of viral suppression (Eberhart et al. 2015). Nelson et al. found both rural and urban residents had lower retention and viral suppression compared to residents of metropolitan areas (≥500,000 residents) using CDC data from 28 jurisdictions in 2012, though they did not control for demographic or socioeconomic determinants in their analysis (Nelson et al., 2016). In another analysis spanning a large, geographically diverse cohort of PLWHA across the US over a decade, Rebeiro et al. showed only decreased median age and increased proportion of residents of black race, as opposed to neighborhood economic indicators, were associated with adverse retention at the 3-digit ZCTA level (Rebeiro, Gange, Horberg, Abraham, Napravnik, Samji, & Curriero, 2016). However, these studies were either cross-sectional (Eberhart et al., 2015; Nelson et al. 2016), or did not examine neighborhood risk using an index similar to our own (Rebeiro et al., 2016).
Meanwhile, studies in Chicago, St. Louis, and Florida found no neighborhood characteristic associations with viral suppression (Burke-Miller et al., 2016; Shacham, Lian, Onen, Donovan, & Overton, 2013; Shacham, Lopez, Onen, & Overton, 2017; Sheehan, Fennie, Mauck, Maddox, Lieb, & Trepka, 2015). These more recent analyses generally agree with our own study findings, though only Sheehan et al. explicitly evaluated retention in care, finding a null association for that outcome as well.
Our own analysis had important limitations. First, we did not have complete data on factors that may influence residential location (e.g., individual socioeconomic position) that may also influence outcomes of interest. Therefore, our results may be subject to unmeasured confounding. Second, we may have misclassified retention due to our inability to track care visits not occurring at the VCCC.
Despite these potential pitfalls, this study had several strengths. First, this study addresses an urgent and growing concern in HIV research among PLWHA residing in the highest-incidence, highest-burden, and poorest HIV outcome region of the US (namely, the South). Second, we sought to address this concern and the limitations of prior work (Burke-Miller et al., 2016; Chakraborty et al., 2015; Eberhart et al., 2015; Nelson et al., 2016; Shacham et al., 2013; Shacham et al., 2017; Surrat et al., 2015; Taylor, Leibowitz, Simon, & Grusky 2006) by using high-quality, validated clinical data, with fidelity to its longitudinal nature in our analytic methods rather than cross-sectional analyses as in prior work (Shacham et al., 2013; Shacham et al., 2017; Surrat et al., 2015). The longitudinal study design also allowed participants to be followed beyond linkage to care or initiation of ART allowing for a more clinically diverse study population than other studies (Eberhart et al., 2015). This study also included individuals from across a broad region, both rural and urban, allowing for a more geographically diverse study population than several other studies (Burke-Miller et al., 2016; Eberhart et al., 2015; Shacham et al., 2013; Shacham et al., 2017; Surrat et al., 2015; Taylor et al., 2006).
Although not entirely supported by the present study findings, contemporary qualitative work in similar populations has helped establish low income, poor job prospects, limited healthcare access, social stigma, and the presence of illicit substances, as major impediments affecting health-seeking behavior and healthcare decision-making. These factors including at the neighborhood level may therefore demarcate populations who face difficulties in fully participating in all aspects of HIV care. They may also operate in a synergistic fashion with each other as well as with other factors (e.g., awareness of opportunities for drug or insurance assistance programs) as drivers of suboptimal HIV outcomes (Kahana et al., 2016; Walcott, Kempf, Merlin, & Turan, 2016).
Therefore, drawing attention to and quantifying the risk of poor continuum engagement as a function of contextual, structural factors is critical to an improved understanding of the mechanisms leading to so-called leaks in the care continuum. Improved understanding may lead to more effective intervention design including identifying populations most likely to benefit from interventions. Documenting these disparities also remains important given the possibility that our study population, though geographically and demographically diverse, may not have been representative of all populations at risk for poor HIV outcomes within the region.
Documenting potential neighborhood-based disparities is also critical to evaluating the impact of interventions aimed at reducing neighborhood-based disparities. Thus, additional research must be conducted to reconcile the presently contradictory findings regarding the impact of adverse neighborhood contexts on HIV care continuum outcomes.
Supplementary Material
Acknowledgements:
We thank Dr. Akilah Dulin-Keita for her guidance in constructing the neighborhood socioeconomic context score.
Funding:
This work was supported by National Institute of Allergy and Infectious Diseases: [Grant Number K01-AI131895, P30-AI110527, U01-AI069923]. This work was supported in part by the National Institutes of Health (Tennessee Center for AIDS Research, P30-AI110527; Rebeiro, K01-AI131895). This work was also supported by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA)(U01-AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Mental Health (NIMH), and the Office Of The Director, National Institutes Of Health (OD).
References:
- Adimora AA, Ramirez C, Schoenbach VJ, & Cohen MS (2014). Policies and politics that promote HIV infection in the Southern United States. AIDS, 28(10),1393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. (2015). National HIV/AIDS Strategy: APA Recommendations for Prioritizing Mental and Behavioral Health in Federal Implementation Efforts. https://www.apa.org/pi/aids/resources/policy/nhas-policy-response.pdf. Accessed 10 May 2015.
- Arnold M, Hsu L, Pipkin S, McFarland W, & Rutherford GW (2009). Race, place and AIDS: the role of socioeconomic context on racial disparities in treatment and survival in San Francisco. Soc Sci Med, (1), 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L, Neilands TB, Tabb LP, Burkholder GJ, Malebranche DJ, & Tschann JM (2014). Neighborhood context and Black heterosexual men’s sexual HIV risk behaviors. AIDS Behav, 18(11), 2207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buot ML, Docena JP, Ratemo BK, Bittner MJ, Burlew JT, Nuritdinov AR, Robbins JR (2014). Beyond race and place: distal sociological determinants of HIV disparities. PLoS One, 9(4), e91711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke-Miller JK, Weber K, Cohn SE, Hershow RC, Sha BE, French AL, Cohen MH (2016). Neighborhood community characteristics associated with HIV disease outcomes in a cohort of urban women living with HIV. AIDS Care, 28(10),1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). HIV Surveillance Report; vol. 25. [Google Scholar]
- Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, the National Minority AIDS Council, … Urban Coalition for HIV/AIDS Prevention Services. (2014). Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014 http://stacks.cdc.gov/view/cdc/26062. Accessed 12 September 2017.
- Chakraborty H, Iyer M, Duffus WA, Samantapudi AV, Albrecht H, & Weissman S (2015). Disparities in viral load and CD4 count trends among HIV-infected adults in South Carolina. AIDS Patient Care STDS, 29(1), 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean HD, & Fenton KA (2010). Addressing social determinants of health in the prevention and control of HIV/AIDS, viral hepatitis, sexually transmitted infections, and tuberculosis. Public Health Rep, 125(Suppl 4),1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi RK, Milberg J, Isenberg D, Matthews T, Malitz F, Matosky M, … Cheever LW (2015). High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis, 60(1), 117–25. [DOI] [PubMed] [Google Scholar]
- Eberhart MG, Yehia BR, Hillier A, Voytek CD, Fiore DJ, Blank M, … Brady KA (2015). Individual and community factors associated with geographic clusters of poor HIV care retention and poor viral suppression. J Acquir Immune Defic Syndr, 69(Suppl 1), S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JB, Kisler KA, & Reback CJ (2014). Housing status and HIV risk behaviors among transgender women in Los Angeles. Arch Sex Behav, 43(8), 1651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MA, & Spicer CM (2012). Monitoring HIV care in the United States: indicators and data systems National Academies Press. [PubMed] [Google Scholar]
- Friedman MS, Marshal MP, Stall R, Kidder DP, Henny KD, Courtenay-Quirk C, … Holtgrave DR (2009). Associations between substance use, sexual risk taking and HIV treatment adherence among homeless people living with HIV. AIDS Care, 21(6), 692–700. [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, & Burman WJ (2011). The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis, 52(6), 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, & Bound J (1998). Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol, 148(5), 475–86. [DOI] [PubMed] [Google Scholar]
- Hanna DB, Selik RM, Tang T, & Gange SJ (2012). Disparities among US states in HIV-related mortality in persons with HIV infection, 2001–2007. AIDS, 26(1), 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert LJ (1973). The use of orthogonal polynomials for trend analysis. American Educational Research Journal, 10(3), 241–244. [Google Scholar]
- Kahana SY, Jenkins RA, Bruce D, Fernandez MI, Hightow-Weidman LB, Bauermeister JA; Adolescent Medicine Trials Network for HIV/AIDS Interventions. (2016). Structural Determinants of Antiretroviral Therapy Use, HIV Care Attendance, and Viral Suppression among Adolescents and Young Adults Living with HIV. PLoS One, 11(4), e0151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA, German D, Vlahov D, & Galea S (2013). Neighborhoods and HIV: A social ecological approach to prevention and care. American Psychologist. American Psychological Association, 68(4), 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, Rebeiro PF, … Hulgan T (2011). Drug use and receipt of highly active antiretroviral therapy among HIV-infected persons in two U.S. clinic cohorts. PLoS One, 6(4), e18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie B, & Rapino M (2011). Commuting in the United States: 2009. American Community Survey Reports, ACS-15 Washington, DC. [Google Scholar]
- Miglioretti DL, & Heagerty PJ (2007). Marginal modeling of nonnested multilevel data using standard software. Am J Epidemiol, 165(4), 453–63. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Kinder A, Johnson AS, Hall HI, Hu X, Sweet D, … Harris J (2016). Differences in Selected HIV Care Continuum Outcomes Among People Residing in Rural, Urban, and Metropolitan Areas-28 US Jurisdictions. J Rural Health [Epub ahead of print] [DOI] [PubMed]
- Rebeiro PF, Gange SJ, Horberg MA, Abraham AG, Napravnik S, Samji H, … Curriero FC, for the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) (2016). Geographic Variations in Retention in Care among HIV-Infected Adults in the United States. PLOS ONE, 11(1):e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif SS, Whetten K, Wilson ER, McAllaster C, Pence BW, Legrand S, Gong W (2014). HIV/AIDS in the Southern USA: a disproportionate epidemic. AIDS Care, 26(3), 351–9. [DOI] [PubMed] [Google Scholar]
- Reif SS, Sullivan K, Wilson E, Berger M, & McAllaster C (2016). HIV/AIDS Care and Prevention Infrastructure in the US Deep South. SouthernAIDSStrategy.org. Accessed 12 September 2017.
- Royal SW, Kidder DP, Patrabansh S, Wolitski RJ, Holtgrave DR, Aidala A, … Stall R (2009). Factors associated with adherence to highly active antiretroviral therapy in homeless or unstably housed adults living with HIV. AIDS Care, 21(4), 448–55. [DOI] [PubMed] [Google Scholar]
- Shacham E, Lian M, Onen NF, Donovan M, Overton ET (2013). Are neighborhood conditions associated with HIV management? HIV Medicine, 14(10), 624–632. [DOI] [PubMed] [Google Scholar]
- Shacham E, Lopez JD, Onen NF, Overton ET (2017). The Relationship of Social Support and Neighborhood Perceptions among Individuals with HIV. Journal of the International Association of Providers of AIDS Care, 16(5), 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DM, Fennie KP, Mauck DE, Maddox LM, Lieb S, Trepka MJ (2017). Retention in HIV Care and Viral Suppression: Individual-and Neighborhood-Level Predictors of Racial/Ethnic Differences, Florida, 2015. AIDS Patient Care STDS, 31(4):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soobader M, LeClere FB, Hadden W, & Maury B (2001). Using aggregate geographic data to proxy individual socioeconomic status: does size matter? Am J Public Health, 91(4), 632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt HL, Kurtz SP, Levi-Minzi MA, & Chen M (2015). Environmental Influences on HIV Medication Adherence: The Role of Neighborhood Disorder. Am J Public Health, 105(8), 1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SL, Leibowitz A, Simon PA, & Grusky O (2006). ZIP code correlates of HIV-testing: a multi-level analysis in Los Angeles. AIDS and Behavior, 10(5), 579–586. [DOI] [PubMed] [Google Scholar]
- Tobin KE, Latkin CA, & Curriero FC (2014). An examination of places where African American men who have sex with men (MSM) use drugs/drink alcohol: a focus on social and spatial characteristics. Int J Drug Policy, 25(3), 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Health Resources and Services Administration, HIV/AIDS Bureau (2015). Ryan White HIV/AIDS Program Part B Manual – Revised 2015 IV, Chapter 7, 41. [Google Scholar]
- Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, … Mugavero MJ (2009). The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS, 23(1), 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiserri RO, Forsyth AD, Yakovchenko V, & Koh HK (2013). Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep, 128(5), 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott M, Kempf MC, Merlin JS, & Turan JM (2016). Structural community factors and sub-optimal engagement in HIV care among low-income women in the Deep South of the USA. Cult Health Sex, 18(6), 682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White House Office of National AIDS Policy. (2010). National HIV/AIDS strategy for the United States. White House Office of National AIDS Policy from: http://purl.access.gpo.gov/GPO/LPS124282. Accessed 12 September 2017.
- White House Office of National AIDS Policy. (2015). National HIV/AIDS strategy for the United States: Updated to 2020. White House Office of National AIDS Policy from: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. Accessed 12 September 2017.
- Wilson LE, Korthuis T, Fleishman JA, Conviser R, Lawrence PB, Moore RD, Gebo KA (2011). HIV-related medical service use by rural/urban residents: a multistate perspective. AIDS Care, 23(8), 971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberley RC, & Morris LV (2003). US poverty in space and time: Its persistence in the South. Sociation Today, 1(2), 1. [Google Scholar]
- Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, … Gebo KA; HIV Research Network. (2012). Comparing different measures of retention in outpatient HIV care. AIDS, 26(9), 1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, & Albert PS (1988). Models for longitudinal data: a generalized estimating equation approach. Biometrics, 44(4), 1049–60. [PubMed] [Google Scholar]
- Zou G (2004). A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol, 159(7), 702–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


