Abstract
Calcium pyrophosphate crystals are related to a variety of articular manifestations known as calcium pyrophosphate deposition (CPPD) arthritis. Acute CPPD arthritis is commonly known as pseudogout, but there are many other presentations. Diverse endocrine and metabolic diseases may be related to CPPD arthritis. Septic arthritis is in the differential diagnosis of acute CPPD arthritis. The treatment options for CPPD arthritis include non-steroidal anti-inflammatories and steroids.
Introduction
The most recognized crystalline arthritis is gout, caused by monosodium urate crystals, but other crystals cause musculoskeletal symptoms as well. For example, basic calcium phosphate crystals cause acute calcific tendinitis and the Milwaukee shoulder syndrome. More significantly, calcium pyrophosphate crystals are related to a variety of articular manifestations known as calcium pyrophosphate deposition (CPPD) arthritis. CPPD arthritis is very common and its incidence increases with age. Acute CPPD arthritis is known as pseudogout but there are other presentations less familiar to clinicians, including asymptomatic calcium pyrophosphate dihydrate crystal deposition in fibrocartilage and hyaline cartilage (chondrocalcinosis).1 This review will focus on the different clinical manifestations, diagnosis, and treatment of CPPD arthritis.
Pathogenesis
The most important risk factor for the development of CPPD arthritis is aging. Up to 36% of patients older than 75 years have radiographic evidence of CPPD deposition (mainly chondrocalcinosis).2 Male and female patients are affected equally. Osteoarthritis, joint trauma, and joint surgery are also risk factors.
An important aspect of CPPD arthritis is its association with metabolic and endocrine disorders (See Table 1). There are clear associations with hemochromatosis, primary hyperparathyroidism, and hypomagnesemia but the pathogenic mechanisms remain obscure. Patients with these diseases have an increased risk of developing CPPD arthritis. Screening for these conditions should be considered in all patients but the yield is highest in young patients and in those with polyarticular disease.3 It is particularly important to evaluate for hemochromatosis because early treatment will prevent cirrhosis even though it will not reverse the arthritis. Other conditions thought to be related to CPPD arthritis but with no proven association include hypothyroidism, gout, and Wilson’s disease. Familial forms of CPPD arthritis are rare and involve a mutation of a membrane protein involved in phosphorus transport.
Table 1.
Disease Associations and Screening Tests
| Disease | Association | Screening tests |
|---|---|---|
| Hemochromatosis | Definite | Transferrin saturation, ferritin |
| Hyperparathyroidism | Definite | Intact parathyroid hormone (PTHi) |
| Hypophosphatasia | Definite | Serum phosphorous |
| Hypomagnesemia | Definite | Serum magnesium |
| Gitleman’s syndrome | Definite | Thiazide testing |
| Hypothyroidism | Probable | TSH and Free T4 |
| Gout | Possible | Serum Uric acid |
The precise mechanism of calcium pyrophosphate crystal deposition in cartilage is unknown. Shedding of these crystals into the joint may be caused by trauma, sepsis, or joint lavage. When the crystals are released they are taken up by phagocytic cells in the joint that release proinflammatory cytokines, and activate inflammatory cells and cause leukocyte and mononuclear cell migration. Microtrauma and usual wear-and-tear degeneration may explain the high prevalence of CPPD arthritis in the elderly.
Clinical Manifestations
CPPD arthritis includes several distinct presentations (See Table 2). These differ in the number and pattern of joints affected and in the intensity of the inflammation, ranging from acute CPPD arthritis (known as pseudogout) to asymptomatic radiographic findings.4
Table 2.
Presentations of CPPD Arthritis
| Common |
| Pseudogout |
| Pseudo-rheumatoid arthritis |
| Pseudo-osteoarthritis |
| Asymptomatic (often with chondrocalcinosis on radiographs) |
| Less Common |
| Pseudo-neuropathic arthropathy |
| Pseudo-hemarthrosis |
| Pseudo-septic arthritis |
Pseudogout is characterized by acute arthritis usually of one joint. It may be precipitated by an injury, severe illness, or major surgery. The patient is often an adult male with severe joint pain and swelling that reaches maximum intensity within hours. The knee is most commonly affected but any joint may be involved, including the first metatarsal joint (pseudopodagra.) The joint appears swollen, red, warm and tender and may have a large effusion. Fever is not uncommon. Acute CPPD arthritis usually resolves spontaneously in one or two weeks and leaves no residual swelling or deformity. Some patients develop pseudogout in multiple joints accompanied by fever and occasionally mental status changes, particularly in the elderly.
Chronic polyarticular CPPD arthritis can mimic rheumatoid arthritis (pseudo-rheumatoid arthritis.) The typical patient is an elderly female with a several month history of pain and swelling in multiple joints. The wrists are characteristically affected with tenderness and synovial thickening (synovitis). This may result in decreased flexion and extension. Bilateral shoulder and knee involvement are also common and may be accompanied by muscle atrophy in longstanding cases. Patients may have prominent inflammatory symptoms of morning stiffness and fatigue, but fever is rare.
Another form of CPPD arthritis occurs when multiple joints are affected but inflammatory symptoms (such as morning stiffness) and inflammatory signs (synovitis) are lacking. This condition has been named pseudo-osteoarthritis. It can produce slowly progressive joint degeneration which may be indistinguishable from osteoarthritis (OA), with bilateral involvement of many different joints including wrists, hips, knees, shoulders and elbows.
Asymptomatic arthritis is the fourth common form of CPPD arthritis. Most patients are diagnosed when radiographs reveal chondrocalcinosis or other typical findings of CPPD arthritis. The prevalence of asymptomatic CPPD arthritis increases significantly with age and may coexist with OA and other arthropathies.
Less common forms of CPPD arthritis include pseudo-neuropathic joint disease with severe degenerative and destructive arthritis identical to that seen in diabetes and tabes dorsalis. Spinal involvement with calcium pyrophosphate deposition along the ligamentum flavum may cause symptoms of cord compression. In rare cases joint aspiration reveals bloody fluid (pseudo-hemarthrosis) or purulent material (pseudo-septic arthritis).
Diagnosis
History and Physical Examination
The practitioner must keep in mind the diverse presentations of CPPD arthritis in order to diagnose it correctly. The history and physical examination are key and should focus on: which joints are involved, the onset and duration of symptoms and the presence of articular swelling, warmth, tenderness and effusion.5, 6 CPPD arthritis should be suspected in the following patient presentations:
Acute mono- or oligoarthritis (pseudogout)
Chronic polyarthritis with synovial swelling in joints typically involved in rheumatoid arthritis (pseudo-rheumatoid arthritis)
Chronic pain of joints not commonly involved in OA (pseudo-osteoarthritis)
Laboratory Assessment
The erythrocyte sedimentation rate and the C-reactive protein level may be elevated in patients with pseudogout but do not distinguish CPPD arthritis from other causes of acute arthritis. Uric acid levels are normal, unless there is coexisting hyperuricemia and gouty arthritis.
Associated metabolic and endocrine disorders are not commonly seen, screening may have a higher yield in patients who are young or have polyarticular disease. Calcium, phosphorus and magnesium levels and intact parathyroid hormone levels (PTHi) are adequate screening for endocrinopathies. Iron studies (serum ferritin, and transferrin saturation) will detect hereditary hemochromatosis.7
An acutely swollen joint is a medical emergency and aspiration must be performed urgently in order to rule out septic arthritis which can lead to rapid joint destruction if not treated. Aspiration and synovial fluid analysis are essential to confirm the diagnosis of CPPD arthritis and exclude other conditions such as gout and infection. In acute CPPD disease white blood cell counts typically range from 5000 to 25,000 cells/microL. Lower leukocyte counts are seen in patients with chronic polyarthritis, and very high counts (of up to 100,000 white blood cells/microL) are seen with pseudo-septic presentations. All patients with acute monoarthritis should have synovial fluid gram stains and cultures performed to exclude infection. Crystals are often found in septic joint fluid, and therefore do not rule out infection. In addition, synovial fluid leukocyte counts are variable in patients with septic arthritis. A conservative rule of thumb suggests all synovial fluids with over 2,000 white blood cells/microL should be considered septic until proven otherwise.8
Crystal analysis with a polarizing microscope shows calcium pyrophosphate dihydrate crystals as rhomboidal and positively birefringent (in contrast to the needle-shaped, negatively birefringent urate crystals of gout.)9 (See Figure 1). This analysis is performed in most commercial and institutional laboratories but even in the best of hands the results may be false negative (too few crystals) or false positive (artifacts or debris may mimic the crystals.) Therefore, even if no crystals are identified, CPPD arthritis is possible, especially in an elderly patient with typical clinical and radiographic findings.
Figure 1.
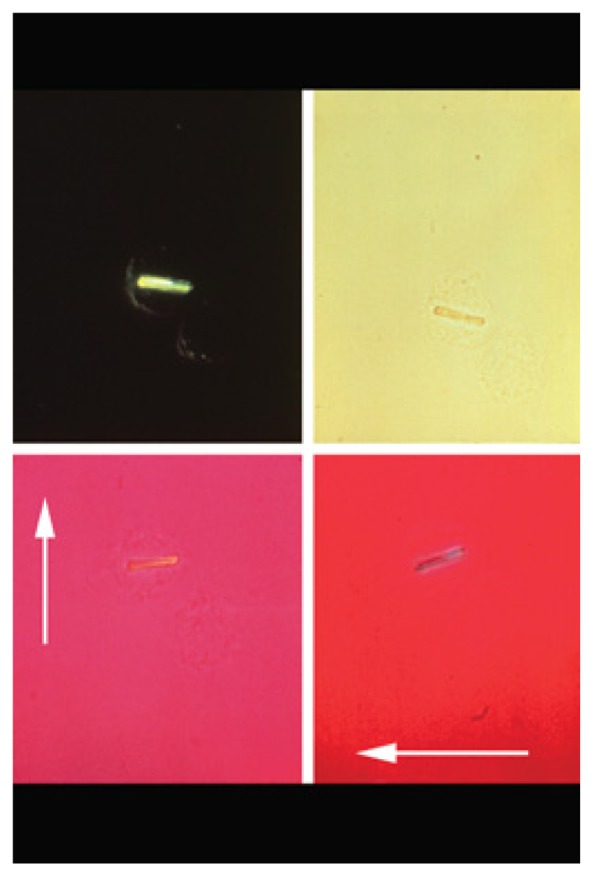
Crystal analysis with a polarizing microscope shows calcium pyrophosphate dihydrate crystals as rhomboidal and positively birefringent (in contrast to the needle-shaped, negatively birefringent urate crystals of gout.)
(c) 2012 American College of Rheumatology. Used with permission.
Imaging Studies
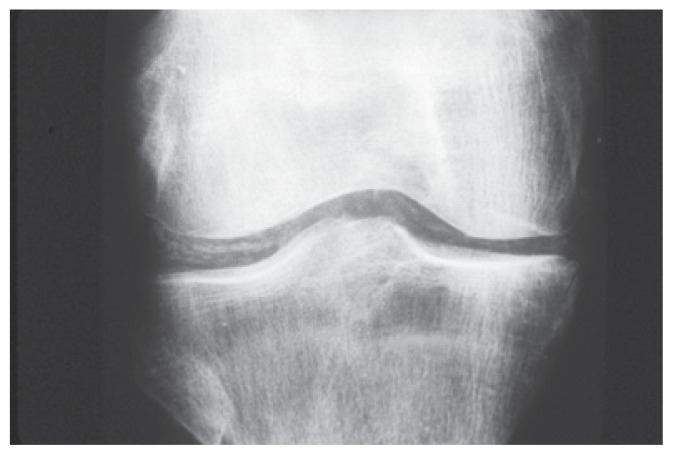
Conventional radiographs demonstrate typical abnormalities in most patients with CPPD arthritis and are usually sufficient to confirm the clinical impression. The findings in CPPD arthritis are similar to the findings in primary OA with joint space narrowing, osteophytes and subchondral cysts, however there are differences.10 The most characteristic abnormality is chondrocalcinosis seen as a fine radio-opaque line (of calcium pyrophosphate dihydrate crystals) within the cartilage. (See Figure 2). In addition, CPPD arthritis characteristically affects joints that are not affected by primary OA including metacarpophalangeal, wrist, elbow, glenohumeral and patello-femoral joints. When a radiograph report describes osteoarthritis in those joints, CPPD arthritis must be suspected. Neither gout nor rheumatoid arthritis demonstrates chondrocalcinosis. Computed tomography and magnetic resonance imaging are not usually necessary. Criteria for diagnosis by ultrasound have been developed but are rarely used.11
Figure 2.
The most characteristic abnormality is chondrocalcinosis seen as a fine radio-opaque line (of calcium pyrophosphate dihydrate crystals) within the cartilage.
(c) 2012 American College of Rheumatology. Used with permission.
Differential Diagnosis
CPPD arthritis may present as acute monoarthritis, polyarticular or oligoarticular arthritis, neuropathic joint disease, or as an asymptomatic finding on radiographs. Its differential diagnosis can be extensive including trauma, bleeding, and infection as well as other crystal-associated diseases (e.g., gout), or other inflammatory joint diseases.
Acute monoarticular attacks of pseudogout are often misdiagnosed as gout. Patients with acute CPPD arthritis may not have the usual risk factors for hyperuricemia (obesity, hypertension, excessive alcohol intake) and may not have the family history common in gout. Tophi are not found with CPPD arthritis. Crystal fluid analysis is definitive but does not exclude a septic joint. Therefore, a gram stain and cultures of joint fluid and blood are necessary to rule out an infectious etiology.
Polyarticular or oligoarticular inflammatory presentations can mimic rheumatoid arthritis (RA) and other inflammatory joint diseases. A considerable proportion of healthy elderly individuals can have a positive rheumatoid factor (RF) complicating the diagnosis. Pseudo-rheumatoid arthritis can lead to a diagnostic dilemma when joint inflammation is symmetrical and when RF is present. High titers of RF with synovitis involving the hands and feet and characteristic radiographic erosions suggest true RA rather than pseudo-rheumatoid arthritis. The presence of anti-cyclic citrullinated peptide (anti-CCP) antibodies also suggests RA.
Pseudo-osteoarthritis is another presentation of pseudogout which can be difficult to distinguish from primary or post-traumatic OA. CPPD crystal deposition is often associated with chondrocalcinosis and exuberant osteophyte formation on radiographs. CPPD should be considered when radiographic features of OA occur in joints not commonly affected in primary OA. Diagnosis can be difficult when the pattern of distribution of osteoarthritic changes is similar to that of typical OA. However, some distinctions can be made. In the knees for example, the medial compartment is more commonly involved in primary OA, resulting in varus changes. Pseudo-osteoarthritis is more likely to affect the lateral compartment, causing valgus changes. Isolated patellofemoral osteoarthritis is also a common presentation. It is important to remember, however, that CPPD arthritis and OA may coexist.
Pseudo-neuropathic joint disease can resemble neuropathic arthropathy with characteristic joint destruction. Neuropathic arthropathy (Charcot joint) is commonly associated with diabetes mellitus, tabes dorsalis, syringomyelia, and spinal cord injuries. True neuropathic arthropathy has impaired pain sensation including vibration and proprioception. Patients with CPPD pseudo-neuropathic joint disease have normal pain perception. This is an important distinction as patients with pseudo-neuropathic joints are offered joint replacement while those with true neuropathic joints are not.
Treatment
Treatment of CPPD disease includes treatment of acute attacks, prophylaxis against recurrent acute attacks, and management of chronic symptoms12 (See Table 3). This treatment includes both pharmacological and non-pharmacological options. If a disease associated with CPPD deposition is present, specific therapy directed at the underlying disorder should be initiated although it usually does not reverse CPPD arthritis.
Table 3.
Treatment of CPPD Crystal Deposition Disease
| Treatments | Dosing | Side-effects |
|---|---|---|
| Oral Non-steroidal anti-inflammatory drugs | Varies | Gastrointestinal distress, liver, renal toxicity, tinnitus |
| Oral Colchicine | 0.6 mg twice daily | Diarrhea, abdominal pain |
| Intraarticular corticosteroid preparation | Varies. For large joints: triamcinolone acetonide 40–80 mg mixed with 1 or 2 mL of 1% lidocaine. Less for smaller joints. | Skin atrophy, local depigmentation, and telangiectasia, infections |
| Oral glucocorticoid preparation | Varies | Weight gain, hypertension, glucose intolerance, osteoporosis, cataracts |
| Methotrexate | 7.5–20 mg once weekly | Liver function test abnormalities, stomatitis, infections, cytopenia |
| Hydroxychloroquine | 200–400 mg daily | Abnormal color vision, abnormal retinal pigmentation, retinal atrophy |
Acute Management
Several approaches are available for the treatment of acute monoarticular pseudogout attacks and include removal of crystals by joint aspiration, administration of nonsteroidal anti-inflammatory drugs (NSAID) or colchicine, intra-articular injections of a glucocorticoid preparation, cold packs, and joint immobilization. When more than two joints are inflamed, joint injection is impractical. In this setting oral NSAIDs or oral colchicine are the preferred treatments. If NSAIDs or colchicine are contraindicated, systemic glucocorticoids can be given.
Prophylaxis against Recurrent Attacks
In patients suffering recurrent pseudogout attacks, colchicine may be effective as a prophylactic agent at a dosage of 0.6 mg twice daily. Prophylactic therapy may be indicated in patients with three or more attacks annually. If side-effects of abdominal distress or diarrhea occur, a reduction in dose to 0.6 mg once daily, or every other day, may be effective. Alternatively, oral NSAIDs with gastroprotective treatment can be tried. Both NSAIDs and colchicine should be used with caution in patients with renal insufficiency and liver disease.
Chronic Disease Management
In contrast to gouty arthritis, no therapy is available to prevent the deposition of calcium pyrophosphate crystals or to remove the calcium pyrophosphate deposits already present. Patients with chronic arthropathy from CPPD in the form of pseudo-osteoarthritis or pseudo-rheumatoid pattern are managed with physical therapy, analgesics or NSAIDs. Some individuals with persistent chronic inflammation may benefit from low dose corticosteroids, methotrexate or hydroxychloroquine.
In patients with associated endocrine or metabolic disorders, such as hemochromatosis, hyperparathyroidism, or hypothyroidism, successful treatment of the underlying disorder has not resulted in reversal of cartilage calcification. In fact, new calcifications can develop in some individuals. Asymptomatic CPPD deposition is typically discovered when chondrocalcinosis is incidentally noted on radiographs. Specific treatment is not required.
Conclusion
CPPD arthritis is very common and its incidence increases with age. The most common presentation is acute monoarthritis but patients may present mimicking OA and rheumatoid arthritis. Metabolic and endocrine disorders can be associated with CPPD arthritis. There are many treatment options for acute arthritis but no medications prevent calcium crystal deposition.
Biography
Dedri Ivory, MD, is a Fellow, and Celso R. Velazquez, MD, is an Assistant Professor of Clinical Medicine. Both are at the University of Missouri School of Medicine, Division of Immunology and Rheumatology.
Contact: velazquezc@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Rosenthal A, Ryan L. Arthritis and Allied Conditions. 15th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. Calcium pyrophosphate crystal deposition disease, pseudogout, and articular chondrocalcinosis; pp. 2373–2396. [Google Scholar]
- 2.Felson D, Anderson J, Naimark A, Kannel W, Meenan J. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. Journal of Rheumatology. 1989;16:1241–1245. [PubMed] [Google Scholar]
- 3.Zhang W, Doherty M, Bardin T, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Annals of the Rheumatic Diseases. 2011;70(4):563–570. doi: 10.1136/ard.2010.139105. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Dieppe Paul. Clinical Aspects of Calcium Pyrophosphate Dihydrate Crystal Deposition. Rheumatic Disease Clinics of North America. 1988;14(2):395–414. [PubMed] [Google Scholar]
- 5.Pinals R. Polyarthritis and fever. New England Journal of Medicine. 1994;330:769–774. doi: 10.1056/NEJM199403173301108. [DOI] [PubMed] [Google Scholar]
- 6.Baker D, Schumacher H. Acute monoarthritis. New England Journal of Medicine. 1993;329:1013–1020. doi: 10.1056/NEJM199309303291407. [DOI] [PubMed] [Google Scholar]
- 7.Qaseem A, Aronson M, Fitterman N, et al. Screening for Hereditary Hemochromatosis: A Clinical Practice Guideline from the American College of Physicians. Annals of Internal Medicine. 2005;143(7):517–521. doi: 10.7326/0003-4819-143-7-200510040-00010. [DOI] [PubMed] [Google Scholar]
- 8.Shmerling RH, Delbanco TL, Tosteson ANA, Trentham DE. Synovial Fluid Tests. JAMA: The Journal of the American Medical Association. 1990;264(8):1009–1014. [PubMed] [Google Scholar]
- 9.Pascual E, Sivera F, Andres M. Synovial fluid analysis for crystals. Current Opinion in Rheumatology. 2011;23:161–169. doi: 10.1097/BOR.0b013e328343e458. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach L. Calcium pyrophosphate dihydrate and calcium hydroxyapatite crystal deposition diseases: imaging perspectives. Radiologic Clinics of North America. 2004;42(1):185–205. doi: 10.1016/S0033-8389(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 11.Frediani B, Filippou G, Falsetti P, et al. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Annals of the Rheumatic Diseases. 2005;64(4):638–640. doi: 10.1136/ard.2004.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: Management. Annals of the Rheumatic Diseases. 2011;70(4):571–575. doi: 10.1136/ard.2010.139360. [DOI] [PubMed] [Google Scholar]