Abstract
The spondyloarthropathies (SpA) are a group of inflammatory rheumatic diseases affecting the spine, peripheral joints and nonarticular structures. Often referred to as “seronegative” due to the absence of rheumatoid factor, SpA include ankylosing spondylitis (AS), reactive arthritis (ReA), enteropathic (IBD) associated arthritis, psoriatic arthritis (PsA), as well as undifferentiated, and juvenile SpA. A broad and overlapping spectrum of disease presentations creates difficulties in determining an initial diagnosis. In the last 10 years treatment options have expanded.
Introduction
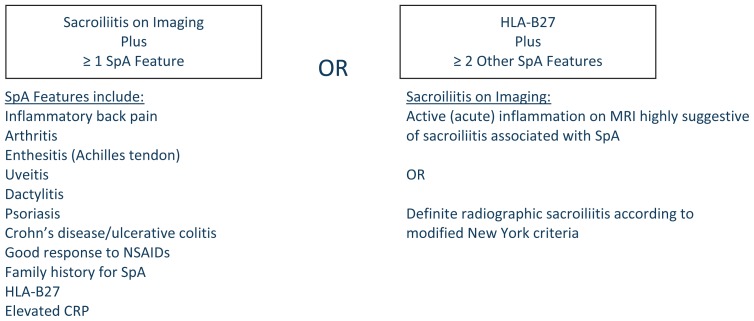
Spondyloarthropathies (SpA) are a group of inflammatory rheumatic diseases affecting the spine, peripheral joints, and nonarticular structures. The articular manifestations often include swelling, warmth and stiffness which improve with activity.. Several organizations including the European Spondyloarthropathy Study Group and the Assessment of Spondyloarthritis International Society (ASAS) developed criteria to aid in the diagnosis of these diseases (See Figure 1). Genetics (such as HLA-B27), inflammatory cytokines (such as TNFα) and more recently the IL-23/IL-17 cytokine pathway have links to pathogenesis. Biologic agents, particularly tumor necrosis factor (TNF) blockers, are primary treatment options and have been shown to reduce symptomatic inflammation.
Figure 1.
ASAS Classification Criteria for Axial SpA (in patients with back pain ≥ 3 months and age at onset < 45 years)2
Ankylosing Spondylitis
Considered the most common and typical SpA, ankylosing spondylitis (AS) affects men more than women (3:1) and typically presents during late adolescence or in the 20s.1–4 As defined by the modified New York criteria (See Table 1), the estimated prevalence of AS in the United States is 197 per 100,000 with an incidence of 7.3 per 100,000 person-years. 5, 6 In people who are HLA-B27 positive, a family history of AS confers an increased risk of developing the disease.5
Table 1.
Modified New York criteria for Ankylosing Spondylitis 5
A) Clinical criteria
|
B) Radiological criteria
|
Definite AS: Radiological criterion with at least one clinical criterion.
Clinical Presentation and Diagnosis
Patients often present with symptomatic sacroiliitis (low back or buttock pain) associated with morning stiffness that improves with exercise and worsens with rest. The broad differential of back pain can lead to a delay in diagnosis by five-six years. This necessitates an accurate distinction between inflammatory back pain and mechanical back pain.4 Inflammatory back pain often begins before age 40, has an insidious onset, improves with activity, does not improve with rest, and can manifest as nighttime discomfort.7 Axial skeleton findings include marginal syndesmophytes and squaring of vertebrae. Progression and extension of the disease can lead to ankylosis, known as a “bamboo spine.” Bilateral sacroiliac (SI) imaging may reveal widened, irregular borders with erosions, or joint fusion in late disease.
Peripheral large joints including shoulder, hip, and knee as well as temporomandibular joints may be affected. Dactylitis (sausage digits) is also reported. Enthesopathy (inflammation at a tendon insertion site) can be seen at the Achilles tendon insertion, plantar fascia, as well as the costochondral (CC) junctions. CC involvement can produce chest pain and occasionally, limited chest wall excursion.
Extraskeletal manifestations are common. Acute anterior uveitis occurs as a presenting symptom in 25–40% of patients4. Cardiac involvement may be silent, but can include aortitis, aortic valve incompetence, conduction abnormalities, cardiomegaly and pericarditis.1–4, 7 Lung involvement, a rare and late manifestation (often 20 years after disease onset) is characterized by slowly progressing fibrosis of the upper lobes of the lungs, and is often found with CT imaging.9 Cough, dyspnea and hemoptysis are typical symptoms. Neurological involvement may include atlantoaxial subluxation, atlantooccipital subluxation, and upward subluxation of the axis due to the inflammatory process.10 Cauda equina may be a late complication. Spinal osteoporosis is frequently observed and reflects the inflammatory cytokines effect on trabecular bone.11
The diagnosis of AS is predominantly clinical, but sacroiliitis is an essential feature of the disease. Radiographic evidence of sacroiliitis has been incorporated into diagnostic criteria including the ASAS criteria for axial SpA (See Figure 1) and the modified New York criteria for AS.3 Early sacroilliac (SI) inflammatory changes can be missed with plain radiographs; therefore MRI imaging is useful in identifying inflammation that precedes radiographic changes.2
Etiopathogenesis and Genetics
The association of HLA-B27 and AS was first noted in the 1970s. HLA-B27 is present in over 90% of AS patients and up to 50% with other SpA. However, less than 5% of HLA-B27 positive individuals in the general population develop AS. The contribution of HLA-B27 to AS is estimated to be between 16–30% based on linkage and association studies with the MHC region.12 The role of genetic factors is highlighted by demonstrating disease concordance in 75% of monozygotic twins compared to 13% of non-identical twins.13 The pathogenic role of HLA-B27 is not yet clear.14, 15, 16 Some hypotheses suggest HLA-B27 is the antigen, or HLA-B27 is permissive for continued infection and inflammation, or HLA-B27 presents antigen leading to the disease process.14, 15, 16 The importance of the IL23/IL17 cytokine axis is emerging in SpA and AS (discussion in IBD and PsA sections).
Treatment
The treatment goal for AS is to reduce the pain and stiffness, increase well-being and function, and delay ankylosis. Unfortunately, no treatment has been proven to prevent bony progression, and treatment is aimed at addressing individual disease manifestations. Physiotherapy and non-steroidal anti-inflammatory drugs (NSAIDs) are early treatment interventions. Sulfasalazine (SSZ) and methotrexate (MTX) have little to no benefit on spinal disease but demonstrate some benefits for peripheral arthritis and enthesial disease.1,3 The use of biologic agents has revolutionized treatment. Tumor necrosis factor-α (TNF) inhibitors reduce symptoms, improve function and quality of life and may attenuate spinal inflammation.17,18 A list of current treatment options is outlined in Table 2. Prior to initiating a disease-modifying antirheumatic drug (DMARD), patients should have renal and liver function testing, a complete blood count, hepatitis B and C testing, and a chest radiograph. Similar baseline testing should be done before initiating biologic agents, including an evaluation for active or latent Mycobacterium tuberculosis. Assessing for prior or current infections, other Mycobacteria or fungal infections (e.g. Cryptococcus, Histoplasmosis, etc.) is also prudent., is prudent. Biologic agents are contraindicated in patients with NYHA Class III and IV congestive heart failure (CHF), and should be used cautiously in patients with lesser degrees of CHF. Immunosuppressed patients should be carefully monitored for infections. Prescribing providers should be familiar with all risks associated with these medications.
Table 2.
Common Treatments for SpA
| Medication | AS | ReA | PsA | IBD-related |
|---|---|---|---|---|
| NSAIDs | + | + | + | + |
| Sulfasalazine | + (a) | + (a) | + (a) | + (a) |
| Methotrexate | + (a) | + (a) | + (a) | + (a) |
| Leflunomide | − | − | + (a) | − |
| Cyclosporine | − | − | + (a) | − |
| Azathioprine | − | − | + (a) | + (a) |
| Mesalamine | − | − | − | + (a) (b) |
| Adalimumab | + | − | + | + |
| Etanercept | + | + (b) | + | + |
| Infliximab | + | − | + | + |
| Golimumab | + | − | + | − |
| Certolizumab | + (b) | − | − | − |
| Abatacept | − | − | + (e) | − |
| Corticosteroids | + (c) | + (c) | + (d) | + |
a = limited effect on axial disease but possible benefit for peripheral disease
b = anecdotal; efficacy seen in case studies
c = intraarticular
d = intraarticular; oral may worsen/cause pustular psoriasis; never inject through psoriatic skin lesion
e = 2011 study showed efficacy
Reactive Arthritis
Reactive arthritis (ReA) was defined in 1969 as an arthritis that develops soon after or during an extra-articular infection in which the infection does not enter the joint.19 The annual incidence is estimated at 30–40 cases per 100,000 and the prevalence is 1–7 %.20, 21 Typically, ReA occurs one to six weeks following an enteric or urogenital infection. The risk of ReA after one of these infections is between 1–4% but is increased to as high as 25% in patients who are HLA-B27 positive. Typical pathogens associated include Salmonella, Shigella, Yersina, Campylobacter, Chlamydia trachomatis, and Mycoplasma genitalium, among others.
Clinical Presentation and Diagnosis
Patients usually present with lower extremity large joint oligoarthritis. Enthesitis, dactylitis, rash, conjunctivitis, iritis, and nail changes resembling psoriasis, can also occur. Keratoderma blenorrhagicum, a papulosquamous rash affecting the palms and soles, is a classic ReA rash. Plaque-like and hyperkeratotic shallow ulcers on the glans and shaft of the penis, known as circinate balanitis, can be seen. Approximately 30% will develop sacroiliitis, often unilateral.4 Urethritis and/or cervicitis from a triggering pathogen may be seen in patients with sexually-acquired ReA.
No specific criteria exist for the diagnosis of ReA. Specific labs and appropriate cultures are aimed at identifying the triggering pathogen. Inflammatory markers are often elevated and a urinalysis may show pyuria. An arthrocentesis should be done to exclude septic arthritis. In chronic cases, radiographic evaluation for spondylitis and sacroiliitis should occur.
Etiopathogenesis/Genetics
Up to 80% of patients with ReA are HLA-B27 positive (See Table 3). Research has shown HLA-B27 positive cells kill Salmonella less efficiently than control cells, and lipopolysaccharide stimulation results in increased nuclear factor κB activation and TNF-α secretion in HLA-B27 positive cells supporting a direct role for HLA-B27 in this disease.22, 23 Other data suggests molecular mimicry as an etiopathogenic process.24
Table 3.
Association of Spondyloarthropathy with HLA-B27
| Disease | Approximate Prevalence of HLA-B27, % |
|---|---|
| Ankylosing spondylitis | 90 |
| Reactive Arthritis | 60–80 |
| Psoriatic Arthritis | 40–50 |
| IBD-Associated | 25–80 |
Treatment
Treatment is specific to the underlying pathogen when one is identified. Data are mixed regarding the use of antibiotics without a well-defined infection with a tendency against long term antibiotics in patients with chronic arthritis. NSAIDs are the initial drug of choice followed by intraarticular steroids and SSZ for those with persistent large joint inflammation. Two-thirds of patients recover completely in six months, while 50% experience further episodes. Approximately 15% will develop chronic erosive disease or sacroiliitis requiring additional pharmacological treatment.25 A list of current treatment options is available in Table 2.
Arthritis Associated With Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) classically includes Crohn’s disease (CD) and ulcerative colitis (UC). About 25–35% of patients have extra-intestinal manifestations (EIMs).26, 27, 28 Ophthalmologic findings, mucocutaneous manifestations, and hepatobiliary disease may be seen.26, 27, 28 Musculoskeletal EIMs, found in 4–50% of IBD patients, include peripheral and axial arthritis, tendonitis, enthesitis, dactylitis, myositis, myalgia, osteopenia, and occasionally granulomatous bone lesions. 6, 27, 28, 29 The extent of colonic involvement and of disease activity influences the risk for EIMs, including arthritis.27 Arthritis occurs equally in men and women.
Clinical Presentation and Diagnosis
IBD arthritis is classified into three types.16, 26, 27, 28, 29, 30 Type I is peripheral pauci-arthritis of less than five joints with an abrupt, painful onset of swollen, large weight bearing joints, although other joints may be involved. Often self-limited, it leaves no deformity, but persists in about 10–20% of patients. The arthritis precedes IBD or occurs with IBD activity, especially UC. Type II is a peripheral arthritis of more than five joints and includes small joints of the hands. It is usually asymmetrical, independent of IBD activity, and can persist after colostomy. Lasting months to years, it can precede or follow the onset of IBD, and may be associated with uveitis. Overall peripheral arthritis is more common with extensive UC or colonic CD, and is seen in 5–20% of IBD patients.16, 26, 27, 28, 29, 30
Type III axial arthritis (spondylitis and sacroiliitis) is found in 3–25% of patients and may precede, accompany, or follow IBD onset.15, 16, 26, 27, 28, 29, 30, 31 No association between IBD severity and spinal involvement has been found. 28 Peripheral arthritis may accompany axial disease. Often unilateral, SI disease can be an isolated finding. Asymptomatic SI disease is reported in 11–52%, with plain radiographic evidence in 14–20%.26, 29 If imaged with MRI, CT, or bone scan, SI disease is noted in nearly 50%.15, 16, 26, 29, 30, 31 Overall, 30% of patients describe inflammatory back pain.26 Radiographic features are similar to AS. Type III can also include CC involvement.
Etiopathogenesis and Genetics
HLA-B27 is present in 25–80% with spinal disease.15,16,27,28,30,32,33,34 HLA-B27 associations are noted in Table 3. HLA-B51, a marker for Behcet’s disease, may be seen in about 15% of patients. 1 Other HLA associations reported include HLA-DR B1*0103, HLA-B35, HLA-B44 and MICA (MHC I chain related gene A) polymorphisms.16, 28, 31, 33 Polymorphisms of the CARD 15 gene which produces a protein to handle bacteria may be important.15, 27, 30, 32, 33
The gut-joint connection starts with intestinal mucosal dysfunction.15, 16, 30, 36 Bacteria and dietary antigens may cross mucosal barriers and engage innate cells (epithelial cells, dendritic cells, macrophages, monocytes and others). Innate cells release cytokines (such as TNF-α, IL-12, IL-18, IL-23) and present antigen to T cells.32, 35 In CD, activated T cells (Th-1, Th-17) produce IFN-γ, TNF-α, IL-6, IL-17, IL-22, and others.30, 35, 37 These may further stimulate macrophage release of TNFα, IL-1 and IL-637 CD8 and T regulatory cells are also activated.30, 32, 35, 36 IFN-γ (from Th-1 cells) expression may be less prominent, suggesting Th-17 cells as more pivotal in SpA.30, 37 T cell profiles are less clear in UC.30 While the IL-23/IL-17 axis is important in IBD inflammation, its role in joint disease is evolving and other pathogenic processes may be equally important.16, 30,35, 6,37
Current gut-joint hypotheses include gut macrophages trafficking to the synovium, producing inflammatory cytokines (IL-1 and TNF-α), stimulating release of vasoactive amines and destructive metalloproteinases (MMP).30, 35 The macrophages also present antigen to T cells. Another theory suggests a recirculation, activation and aberrant localization of gut-primed T cells (including CD8 cells) to synovium either related to bacterial antigen circulation to the joint, or to homing of gut T cells to synovium.30, 35 It is suggested HLA-B27 may be involved in CD8 cell priming.30, 35
Treatment
Initially, NSAIDs may be used to treat arthritis. Caution is warranted as NSAIDs can increase gut permeability, and occasionally flare colitis.26, 27, 28 Limited use of steroid injections may be helpful. Oral steroids, uncommonly used for arthritis, tend not to help axial disease. Therapeutic ultrasound and physical therapy can improve axial symptoms. SSZ and MTX might provide some benefit for peripheral arthritis.28, 29 Mesalamine used for IBD may decrease peripheral arthritis symptoms in some.32 Biologic agents have expanded treatment possibilities for IBD arthritis.26, 27, 34, 35, 29, 48 Current medication options are noted in Table 2.
Surgical removal of diseased UC colon may improve peripheral but not axial arthritis. However, removal of diseased CD colon tends not to affect peripheral arthritis.29
Psoriatic Arthritis
Psoriasis affects about 3% of the population and 6–42% of patients develop clinical or radiographic arthritis.14, 37, 38, 39, 40, 49 Psoriatic arthritis (PsA) gained independent classification in 1888.14 Men and women are affected equally, in contrast to a female predominance in rheumatoid arthritis (RA).49 PsA often manifests between the ages of 30–45 years, but there is a wide age range. An increased risk for arthritis is noted in first degree relatives of psoriatic patients.
Clinical Presentation and Diagnosis
PsA includes peripheral arthritis, enthesitis, tenosynovitis, dactylitis and axial spondylo-sacroiliac disease. Disease severity ranges from mild to erosive and destructive.39,41,49 Peripheral arthritis is often asymmetric in a ray-like (all joints of a digit) distribution and includes distal interphalangeal (DIP) joints, in contrast to symmetric RA.49 Psoriasis may precede, develop simultaneously, or follow the arthritis onset. In most, skin disease precedes arthritis. Extra-articular features, found in about 13%, include mucous membrane lesions, urethritis, and aortic root dilation49 as well as, nonspecific colitis and uveitis seen in about 15–30% and 15–18% respectively.14,49 Nail changes (onycholysis, pitting, ridging) can occur in nearly 80–90 % of patients.49
Clinical patterns described by Moll and Wright14, 40, 42,43 in the 1970s include:
Oligoarticular asymmetric arthritis with fewer than five tender and swollen joints;
Polyarticular arthritis;
Spondylitis predominant;
Predominant distal interphalangeal disease often with nail changes;
Arthritis mutilans with “telescoping” shortened digits.
These subsets often overlap and while most peripheral arthritis presents as oligoarticular, 50–60% progress to polyarticular.14, 48 And although 50–78% of patients develop axial disease, many do not report symptoms.14, 43 All levels of the spine can be involved, and SI arthritis is frequently asymmetric. Dactylitis may be associated with erosions and flexor tenosynovitis is not uncommon. Psoriatic nail changes, often with DIP disease, may represent enthesitis near the nail.43
The CASPAR (Classification of Psoriatic Arthritis Study Group) criteria have significant specificity and sensitivity.14, 40, 42 Meeting criteria requires inflammatory disease of peripheral joints, spine or entheseal sites in addition to three or more points from the following:
-
Psoriasis (recorded by dermatologist or rheumatologist)
current psoriasis
history of psoriasis
first or second degree family member with psoriasis
Typical psoriatic dystrophic nail changes
A negative test for rheumatoid factor (RF)
Dactylitis (recorded by rheumatologist)
a. current dactylitis
b. history of dactylitis
Radiographic juxtaarticular bony proliferation appearing as ill-defined ossification by joint margins (excluding osteophytes)
*current psoriasis score a 2, other items score a 1
X-ray findings include bony proliferation around joints, and bone erosions such as pencil in cup deformity of the digits.39, 49 Joint ankylosis can occur49. Spondylitis, syndesmophytes and sacroiliitis are also seen.14, 39, 49 MRI has increased the sensitivity in identifying joint disease and can identify swelling, erosions, as well as entheseal, and bone edema.14, 39, 43 49 Bone edema may represent a pre-erosive lesion.14 Ultrasound may also help identify entheseal disease.
Etiopathogenesis and Genetics
An association with genetic markers including HLA-B17, 13, 27, 38, 39, HLA-DRB107, HLA-cw6, MICA polymorphisms has been reported with PsA.14, 40, 44, 45, 49 A positive HLA-B27 is not uncommon in patients with spinal disease, uveitis, or colitis.14, 49 A significant number of patients with peripheral arthritis have a positive anti-cyclic citrullinated peptide antibody (anti-CCP).29, 46
Activation of innate cells is a triggering event in skin. These cells, including dendritic cells and keratinocytes, release cytokines (TNF-α, IL-12, and IL-23) and activate T cell populations to release cytokines such as IFN-γ, IL-17, IL-21, IL-22, which further stimulate innate cells.14, 35 The IL-23/IL-17 axis appears important in skin disease, and its role in joint disease is emerging.14, 38, 39, 45 Various reports show elevated levels or expression of IL-17, IL-23, IL 23 subunits, or IL-22 in patients’ skin, sera, synovial fluid or synovial tissue.35, 37, 39, 45 Compared to controls, higher IL-17 serum levels have been reported in AS, undifferentiated SpA and ReA.35, 37, 39, 45
Elevated levels of TNF-α, IL-1, IL-6, IL-8 and MMP have been found in patient synovial fluid, and increased TNF-α in patient skin and sera.39, 40 TNF-α, plays a role in psoriasis and PsA. It stimulates further release of IL-1 and IL-6, release of MMP, osteoclastic maturation and activation, activation of T cells and angiogenesis.39, 45 Other cytokine elevations have also been described in PsA.40
Treatment
NSAIDs help mild disease and spinal pain but do not prevent progression.38, 39 Local steroid injections for persistent, focal joint involvement or tendonitis may be helpful. MTX and SSZ, used for peripheral disease, may not prevent radiographic progression. In addition, no benefit for spinal symptoms is seen.8, 39, 43 Cyclosporine with methotrexate can improve peripheral joint disease, decrease inflammatory markers and decrease psoriasis severity.38, 39 Leflunomide shows benefit for peripheral arthritis.38, 39, 51, 52 The biologic agents are a mainstay of treatment. Anti-TNF therapy may attenuate peripheral disease progression. Medication options are reviewed in Table 2. 17, 39, 47, 48, 49, 50, 52
Biography
Gregory Wilson, DO, is a Fellow, and Darcy Folzenlogen, MD, FACP, is an Associate Clinical Professor. Both are at the University of Missouri School of Medicine, Division of Immunology and Rheumatology.
Contact: Wilsongj@health.missouri.edu


Footnotes
Disclosure
None reported.
References
References exceeded space. Email author for list of references.