Abstract
Rheumatologic diseases are often associated with ophthalmic findings. Familiarity with and recognition of these ophthalmic presentations are important in the management of both the systemic and ocular manifestations of the disease. Identification of ocular signs and symptoms of rheumatologic disease will allow a more comprehensive approach to treatment of patients with rheumatic conditions.
Introduction
The eye is an important indicator of rheumatologic disease. A wide variety of ophthalmic clinical manifestations exist including inflammation of the cornea, conjunctiva, lacrimal gland, episclera, sclera, uvea (including anterior, intermediate, and posterior uveitis), and ophthalmic blood vessels. The most common presenting signs of inflammation of these structures include pain, photophobia, decreased vision, burning, itching, watering (epiphora), and redness. Various ocular signs and symptoms are characteristic of different rheumatologic diseases (See Table 1). The ophthalmic manifestations as well as diagnosis and management of each condition will be discussed in this review. Additionally, ophthalmic screening for a variety of medications used to treat rheumatic disease will be outlined.
Table 1.
| Ophthalmologic Diagnosis | Signs and Symptoms | Rheumatologic Association |
|---|---|---|
| Dry Eye (keratoconjunctivitis sicca) | Ocular burning, itching, watering, foreign body sensation, corneal opacification/ulceration | Rheumatoid arthritis Systemic lupus erythematosus Scleroderma Primary Sjogren’s syndrome Graft-vs-Host disease |
|
| ||
| Episcleritis | Self-limited, mild pain/discomfort, diffuse or segmental redness, may be nodular | Idiopathic (50%) Rheumatoid arthritis Vasculitis Inflammatory bowel disease Relapsing polychondritis |
| Scleritis | Deep boring pain; injection of deep scleral vessels, may be nodular (violaceous nodules), may be necrotizing and may lead to corneal opacification | Psoriatic arthritis Behcet’s disease Wegener’s granulomatosis |
|
| ||
| Keratitis (corneal disease) | Corneal opacification; peripheral corneal thinning, decreased vision, pain, injection, photophobia | Sjogren’s syndrome Rheumatoid arthritis Vasculitis, i.e. Wegener’s granulomatosis, Microscopic polyangitis, Churg-Strauss syndrome Relapsing Polychondritis SLE Behcet’s syndrome IBD Psoriatic arthritis Sarcoidosis |
|
| ||
| Retinal Vasculitis | Loss of vision | SLE Polyarteritis nodosa Churg-Strauss Behcet’s disease Wegener’s granulomatosis Sarcoidosis Relapsing polychondritis Sjogren’s A antigen Rheumatoid arthritis HLA-B27 associated uveitis Crohn’s disease Postvaccination Dermatomyositis Takayasu’s disease Buerger’s disease Polymyositis |
|
| ||
| Optic Neuropathy | Vision loss, central or peripheral | Giant cell artertitis SLE Antiphospholipid antibodies syndrome |
Uveitis
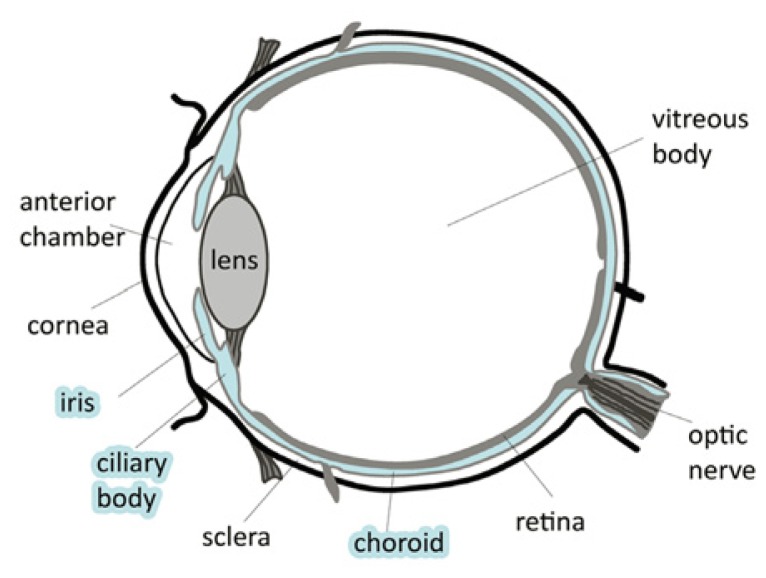
The uvea is the middle, pigmented layer of the eye that consists of vascular structures including the iris, ciliary body, and choroid (See Figure 1). Inflammation of the uvea is referred to as uveitis, the etiology of which is classified into infectious or autoimmune causes.
Figure 1.
Diagram of the eye.44 The uvea is colored blue.
Anterior uveitis is one of the most common reasons for ophthalmology consultation.1 Anterior uveitis (also called iritis or iridocyclitis) may be unilateral or bilateral and usually presents with pain, photophobia, and blurred vision, with or without red eye. Symptoms may range from a quiet white eye to a very painful red eye. The mean age of onset is 37.2 years with a male-to-female ratio of 1:1.4. Slit lamp biomicroscopy demonstrates white blood cells and flare (or protein exudation) in the anterior chamber, cellular deposits on the posterior surface of the cornea (keratic precipitates or KP), and occasionally adhesions (synechiae) between the iris and lens. Hypopyon (or layering of white blood cells in the anterior chamber) is seen with diseases associated with HLA-B27 and Behçet’s syndrome. Anterior uveitis can be a manifestation of many rheumatologic diseases such as juvenile rheumatoid arthritis/juvenile idiopathic arthritis (JRA/JIA), ankylosing spondylitis, HLA-B27 positive non-specific arthropathy, sarcoidosis, reactive arthritis (Reiter’s syndrome), systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), psoriatic arthropathy, rheumatoid arthritis (RA), Wegener’s granulomatosis, and relapsing polychondritis (listed in decreasing incidence). The prevalence of these rheumatologic disease entities varies depending on geographic location.2, 3, 4, 5, 6 In the United States, there is no associated rheumatologic disorder or other cause (infectious or lens-induced) identified in 37.8% of patients who present with anterior uveitis.7 Idiopathic anterior uveitis also appears to be the most common entity in China and was identified in approximately 27% of new anterior uveitis patients in one study.6 Those with HLA-B27 positive disease are likely to have earlier onset with more severe manifestations and these patients are more likely to have ankylosing spondylitis, reactive arthritis, or other spondyloarthropathies.
Various enteric or similar infections (i.e. Klebsiella or Yersinia sp.) have been associated with anterior uveitis, reactive arthritis, and anklyosing spondylitis. It is hypothesized that an unknown microbial peptide which shares structural similarities to antigens normally present in the eyes and joints, has affinity for the HLA-B27 binding cleft and can therefore activate immune responses of cytotoxic T cells upon antigen presentation; thereby inciting inflammation.8 Additional mechanisms through which infectious etiologies are thought to lead to autoimmunity include molecular mimicry, polyclonal T cell activation and failure of bystander suppression.8 (See Figure 1).
The mainstay of therapy is topical cycloplegics (e.g. atropine or homatropine) to lessen sequellae such as posterior synechiae (attachment of the iris to the lens which may result in decreased vision and pupillary block glaucoma). Dilation of the pupil also relieves pain and helps stabilize the blood-aqueous barrier. Topical steroids decrease intraocular inflammation. Occasionally, subconjunctival or systemic steroids are needed. Immunomodulatory medications may benefit patients with recalcitrant or sight-threatening uveitis who require prolonged treatment with corticosteroids or are intolerant to corticosteroid treatment, such as pediatric patients with juvenile idiopathic arthritis (JIA). The categories that are particularly important for uveitis are oligoarthritis (10–30% develop anterior uveitis), rheumatoid factor (RF)-negative polyarthritis (10% develop anterior uveitis), psoriatic arthritis (10% develop anterior uveitis), and enthesitis-related arthritis.9, 10 Ophthalmic screening guidelines have been suggested. Uveitis is rare in children with systemic arthritis and RF-positive polyarthritis.
Short courses of oral corticosteroids may be used in children but long-term use should be avoided due to significant ocular and systemic side effects, including glaucoma, cataract formation, growth retardation, osteoporosis and bone fractures, Cushingoid appearance, diabetes mellitus, peptic ulcers, myopathy, hypertension, altered mental status, pseudotumor cerebri, and increased risk of infection. Methotrexate is one systemic immunosuppressant which may be useful for treatment of both arthritis and uveitis.11, 12 Azathioprine, mycophenolate mofetil, and leflunomide are less commonly used. Cyclosporine is used alone or in combination with steroids and methotrexate for uveitis. Tumor necrosis factor (TNF) inhibitors, including infliximab, adalimumab, and etanercept have been used for refractory uveitis.13, 14 Infliximab has better results than etanercept for treatment of uveitis,15,16 as etanercept has been reported to exacerbate uveitis.17 Daclizumab (humanized murine anti-interleukin-2 receptor) has also been shown to effectively control non-infectious intraocular inflammation in pediatric patients and adults who had failed other immunomodulatory therapy in a retrospective case series.18 Although corticosteroids are used for initial therapy, immunomodulatory agents are also recommended for treatment of Behçet’s disease, Vogt-Koyanagi-Harada (VKH) syndrome, sarcoidosis and necrotizing sclerouveitis to improve long-term prognosis and lessen visual morbidity, as discussed above. In patients with Behçet’s disease interferon-alpha-2a and infliximab have been able to induce remission in disease-modifying anti-rheumatic drug (DMARD)-resistant uveoretinitis. An alternative approach has been to induce tolerance by oral administration of the 336–351 peptide of the human heat shock protein 60 (HSP 60) linked to recombinant cholera B-toxin B-subunit. HSP 60 is thought to have a pathogenic role in Behçet’s disease-associated uveitis. The preliminary results have shown that tolerization is safe and effective in preventing relapses of uveitis.19
Anterior uveitis, as discussed above, is the most common reason for ophthalmologic consultation and is the most common type of uveitis in patients referred to a tertiary eye care center (51.6%), followed by posterior uveitis (19.4%), then panuveitis (16.0%), and lastly, intermediate uveitis (13.0%)7 (See Table 2).
Table 2.
Uveitis and Rheumatologic Associations (listed in decreasing incidence)7
| Anterior Uveitis | Idiopathic Juvenile rheumatoid arthritis/juvenile idiopathic arthritis (JRA/JIA) Ankylosing spondylitis HLA-B27 positive non-specific arthropathy Sarcoidosis Reactive arthritis (Reiter’s syndrome) Systemic lupus erythematosus (SLE) Inflammatory bowel disease (IBD) Psoriatic arthropathy Rheumatoid arthritis (RA) Wegener’s granulomatosis Relapsing polychondritis Kawasaki syndrome |
| Posterior Uveitis | Idiopathic SLE Sarcoidosis Behçet’s disease Temporal arteritis Polyarteritis nodosa Wegener’s granulomatosis |
| Panuveitis | Idiopathic Sarcoidosis Behçet’s disease (more common in Turkey and China) SLE Vogt-Koyanagi-Harada syndrome (more common in China) HLA-B27 associated Relapsing polychondritis Polyarteritis nodosa Dermatomyositis Progressive systemic sclerosis |
| Intermediate Uveitis | Idiopathic Sarcoidosis Multiple sclerosis |
Posterior uveitis is characterized by intraocular inflammation involving the retina and/or choroid. Patients commonly present with blurring, vision loss, and floaters. Inflammatory cells may be scattered diffusely throughout the vitreous cavity, overlying foci of active inflammation, or on the posterior vitreous face. Etiologies include SLE, sarcoidosis, Behçet’s disease, temporal arteritis, polyarteritis nodosa, and Wegener’s granulomatosis. The most common entity is idiopathic. Retinal vasculitis involving small or large vessels, usually phlebitis (which has an appearance of sheathing or cuffing of retinal vessels due to surrounding exudates) with retinal hemorrhages, can occur most commonly as part of sarcoidosis, Behcet’s disease, and SLE. Treatment is usually initiated with high dose corticosteroids tapered to a level at which inflammation is appropriately controlled. Cyclosorin A, tacrolimus and rapamycin have also been shown to be effective through inhibition of cytokines such as interleukin-2 (IL-2).21, 22, 23 Mycophenolate mofetil and methotrexate can also be used. Azathioprine, cyclophosphamide, and chlorambucil are rarely used due to severe adverse effects. Monoclonal antibodies to CD25 (anti-TAC),24 CD20 (rituximab) are more specific therapies that have been used with some success.25 Interferon alpha-2a and 2b have been used successfully in refractory cases of posterior uveitis.26, 19
Episcleritis and Scleritis
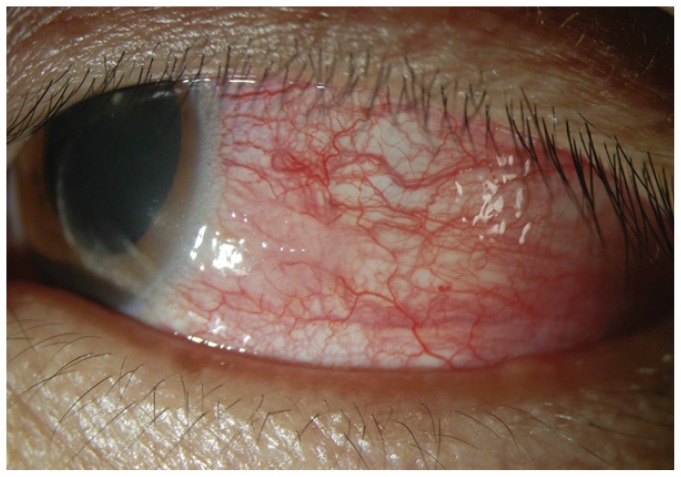
Episcleritis is generally a benign, self-limited inflammation of the episcleral tissues associated with segmental (70%) or diffuse (30%) episcleral redness that is bright red or salmon pink (See Figure 2)27. There may be unilateral or bilateral involvement and a simple or nodular appearance. Unlike deeper scleral vessels, episcleral vessels blanch with 2.5% topical phenylephrine. It is usually found in patients 20–50 years of age and is associated with mild ocular discomfort or tenderness. The work-up for an underlying rheumatologic association (i.e. Sjogren’s syndrome, rheumatoid arthritis or gout) is usually unnecessary except after multiple episodes. Artificial tears and NSAIDs are the mainstay of treatment. Rarely, a short course of topical corticosteroids is warranted.28, 29
Figure 2.
Episcleritis.45 Diffuse inflammation of the episclera is present.
Scleritis often occurs in patients in the fourth to sixth decades of life and is rare in children. Compared to episcleritis there is a much more severe inflammation of the deeper scleral vessels which often presents with severe, deep boring pain which may be referred to the head or face. There may also be a violaceous hue of the inflamed sclera and criss-crossing of deep vessels with associated scleral and episcleral edema noted with slit-lamp biomicroscopy. Scleritis is caused by immune-mediated vasculitis leading to the destruction of the sclera and is often associated with an underlying autoimmune condition such as rheumatoid arthritis, vasculitis, inflammatory bowel disease, relapsing polychondritis, psoriatic arthritis, Behçet’s disease, and Wegener’s granulomatosis. An underlying autoimmune association may be found in up to 50% of patients.30, 31,32 Annual work-up is indicated in those individuals whose initial evaluation is negative.28, 33 There is no evidence of autoimmunity at this point; however, scleral biopsies show immune complex deposition and neutrophil invasion of vessels, generalized MHC class II expression, and T cell and macrophage infiltration.34 Scleral thinning is thought to be secondary to matrix metalloproteinase (MMP) secretion by plasma cells, cytotoxic T cells, and macrophages.
Necrotizing scleritis with inflammation typically presents with severe pain and a localized patch of scleral inflammation. Over time the sclera will thin and produce a blue-gray appearance due to the visibility of the underlying choroid. Necrotizing scleritis without inflammation is a distinct form of anterior scleritis with minimal pain known as scleromalacia perforans. In this condition, the sclera is thin and the underlying dark uveal tissue becomes apparent. Spontaneous perforation is rare but the eye may rupture with minimal trauma.
Nearly 60% of patients with scleritis will need oral corticosteroids or immunosuppressive drugs to control the disease. Treatment of non-necrotizing diffuse scleritis is initiated with ibuprofen 600 mg three times daily. Occasionally, topical corticosteroids may also reduce ocular inflammation in mild cases. Severe nodular disease and necrotizing disease in rheumatoid arthritis-associated scleritis almost always requires initiation of oral corticosteroids followed by tumor necrosis factor (TNF) inhibitors such as infliximab (Remicade). Subconjunctival corticosteroids may also be effective in reducing scleral inflammation but are generally contraindicated due to scleral necrosis and exacerbation of epithelial defects. Treatment failure may be defined as progression to a more severe form or lack of response in two to three weeks, and warrants initiation of alternative treatment. Corticosteroids given orally or in high dose pulsed treatments may be effective. Systemic immunosuppressive therapy with methotrexate, cyclosporine or cyclophosphamide is recommended for treatment failure. Those with rheumatoid arthritis are generally placed on methotrexate whereas those with active vasculitic disease (i.e. Wegener’s granulomatosis) are prescribed cytotoxic agents (i.e. cyclophosphamide).35, 28
Dry Eye Syndrome (Keratoconjunctivitis sicca)
Keratoconjunctivitis sicca (KCS) is a multifactorial disease of the tears and ocular surface that results in ocular discomfort (ranging from mild to disabling), dryness, foreign body sensation, photophobia, decreased vision, and pain. The most common cause is inflammatory lacrimal damage that is seen in autoimmune disorders such as Sjogren’s syndrome and non-Sjogren syndrome dry eye. Inflammation causes both tissue destruction and potentially reversible neurosecretory block. Circulating antibodies to the M3 receptor can also cause a neurosecretory block. Chronic lymphocytic infiltration of the lacrimal and salivary glands, the accessory lacrimal glands, and the conjunctival and epithelial layers is found in biopsy specimens of Sjogren’s syndrome. Antibodies to SS-A, SS-B, fodrin, alpha-amylase, and carbonic anhydrase have been detected.36
The mainstay of treatment for KCS is topical lubricants (i.e. eye drops, gels, or ointments). Warm compresses and eyelid scrubs are used to help bolster the lipid layer of tears. Humidifiers and moisture chambers at night may also be used. If these measures have failed, punctual occlusion and lateral tarsorrhaphy may be considered. In addition, topical cyclosporine A 0.05% (Restasis) is used in treatment of inflammatory dry eye. Finally, autologous serum may be prepared for topical use and oral sialogogues, pilocarpine and cevimeline, may yield improvement in sicca symptoms. Anti-tumor necrosis factor agents have not shown clinical efficacy, and larger controlled trials are needed to establish the efficacy of rituximab.37
Corneal Disease (Keratitis)
Peripheral ulcerative keratitis (PUK) is an inflammatory disease which affects the cornea leading to keratolysis and stromal thinning of the peripheral cornea (usually within 2 mm of the corneoscleral limbus). There is commonly an area of vascular hyperemia in the limbus adjacent to the region of corneal thinning from episcleritis or scleritis. PUK is typically associated with an underlying rheumatologic condition, most commonly rheumatoid arthritis and Wegener’s granulomatosis. A similar clinical picture is present in cases of Mooren’s ulcer which is also characterized by peripheral corneal thinning. Unlike PUK, Mooren’s ulcer is thought to be secondary to an autoimmune response to calgranulin C produced by neutrophils in the presence of infection with the nematode Onchocerca volvulus. Calgranulin C binds to the cell surface of the parasite to stimulate a host response. Corneal keratocytes also produce calgranulin C and this may be the target of the host response as well, leading keratolysis.38 Treatment consists of systemic immunosuppression with adjunctive topical therapy. Ritubximab has been used successfully to treat PUK associated with Wegener’s granulomatosis39 and rheumatoid arthritis40 and infliximab has been recently shown to produce excellent suppression of PUK in Crohn’s disease.41 Topical antibiotics, aggressive tear replacement, matrix metalloproteinase (MMP) inhibition with doxycycline, inhibition of collagenase activity with topical medroxyprogesterone acetate, and conjunctival resection to reduce inflammatory mediators delivered to the cornea through the conjunctival vessels. In the case of corneal perforation, temporizing measures such as a bandage contact lens or cyanoacrylate glue may be used. Larger defects may require corneal patch grafts or therapeutic corneal transplantation; however, the rate of graft failure is very high due to persistence of inflammatory mediators.38
Corneal disease can also result from secondary causes, such as dry eye syndrome leading to corneal ulceration and perforation. An alternative mechanism leading to ocular surface disease results from necrotizing scleritis. This may lead to conjunctival scarring and tear deficiency, entropion, trichiasis, and poor eyelid closure producing exposure keratopathy and secondary infections, ulceration, and possible corneal perforation.
Conclusion
Rheumatologic disease may manifest as a variety of ophthalmologic conditions with a wide range of signs and symptoms (See Table 1). Such signs and symptoms include ocular burning, itching, redness, pain, photophobia, blurred or decreased vision and warrant a referral for ophthalmologic evaluation. Additionally, many medications used to treat rheumatologic disease, including plaquenil (hydroxychloroquine), chloroquine, and systemic corticosteroids, may adversely affect the eye and require routine screening (See Table 3). A low threshold for ophthalmologic consultation should exist when evaluating and treating a diversity of rheumatologic disease.
Table 3.
Ophthalmic Screening for Medications Used in Rheumatologic Disease
| Medication | Screening Recommendations | |
|---|---|---|
|
Hydroxychloroquine (Plaquenil) or Chloroquine Ocular Changes: Corneal verticillata, Bull’s eye maculopathy, toxic retinopathy, paracentral scotoma, peripheral pigmentation, toxic neuropathy |
Low risk
|
|
|
|
||
| Age 20–29 years | at least once during period | |
| Age 30–39 years | at least twice during period | |
| Age 40–64 years | every 2–4 years | |
| Age >65 years | every 1–2 years | |
| *Annual examinations after 5 years of use | ||
|
| ||
|
Systemic and Topical Steroids Ocular Changes: Cataracts, Glaucoma, Infection, Central Serous Chorioretinopathy |
||
Biography
Carisa K. Petris, MD, PhD, is an Ophthalmology Resident, and Arghavan Almony, MD, is an Assistant Professor of Ophthalmology. Both are in the Department of Ophthalmology at the University of Missouri.
Contact: AlmonyA@health.missouri.edu


Footnotes
Disclosure
None reported.
References
- 1.Hamideh F, Prete PE. Ophthalmologic manifestations of rheumatic diseases. Semin Arthritis Rheum. 2001;30(4):217–41. doi: 10.1053/sarh.2001.16639. [DOI] [PubMed] [Google Scholar]
- 2.Bodaghi B, Cassoux N, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001;80(4):263–70. doi: 10.1097/00005792-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Islam SM, Tabbara KF. Causes of uveitis at The Eye Center in Saudi Arabia: a retrospective review. Ophthalmic Epidemiol. 2002;9(4):239–49. doi: 10.1076/opep.9.4.239.1507. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Gupta V, et al. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol. 2004;52(2):121–5. [PubMed] [Google Scholar]
- 5.Sengun A, Karadag R, et al. Causes of uveitis in a referral hospital in Ankara, Turkey. Ocul Immunol Inflamm. 2005;13(1):45–50. doi: 10.1080/09273940590909121. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Zhang Z, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30(11):943–8. doi: 10.1080/02713680500263606. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez A, Calonge M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch Ophthalmol. 1996;114(5):593–9. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 8.Forrester JV. Autoimmunity and autoimmune disease of the eye. Dev Ophthalmol. 1999;30:167–86. doi: 10.1159/000060742. [DOI] [PubMed] [Google Scholar]
- 9.Oren B, Sehgal A, et al. The prevalence of uveitis in juvenile rheumatoid arthritis. J Aapos. 2001;5(1):2–4. doi: 10.1067/mpa.2001.111017. [DOI] [PubMed] [Google Scholar]
- 10.Kotaniemi K, Savolainen A, et al. Recent advances in uveitis of juvenile idiopathic arthritis. Surv Ophthalmol. 2003;48(5):489–502. doi: 10.1016/s0039-6257(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 11.Giannini EH, Brewer EJ, et al. Methotrexate in resistant juvenile rheumatoid arthritis. Results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric Rheumatology Collaborative Study Group and The Cooperative Children’s Study Group. N Engl J Med. 1992;326(16):1043–9. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 12.Woo P, Southwood TR, et al. Randomized, placebo-controlled, crossover trial of low-dose oral methotrexate in children with extended oligoarticular or systemic arthritis. Arthritis Rheum. 2000;43(8):1849–57. doi: 10.1002/1529-0131(200008)43:8<1849::AID-ANR22>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Agle LM, Vazquez-Cobian LB, et al. Clinical trials in pediatric uveitis. Curr Rheumatol Rep. 2003;5(6):477–81. doi: 10.1007/s11926-003-0060-2. [DOI] [PubMed] [Google Scholar]
- 14.Qian Y, Acharya NR. Juvenile idiopathic arthritis-associated uveitis. Curr Opin Ophthalmol. 2010;21(6):468–72. doi: 10.1097/ICU.0b013e32833eab83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards JC, Tay-Kearney ML, et al. Infliximab for juvenile idiopathic arthritis-associated uveitis. Clin Experiment Ophthalmol. 2005;33(5):461–8. doi: 10.1111/j.1442-9071.2005.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Saurenmann RK, Levin AV, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology (Oxford) 2006;45(8):982–9. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 17.Taban M, Dupps WJ, et al. Etanercept (enbrel)-associated inflammatory eye disease: case report and review of the literature. Ocul Immunol Inflamm. 2006;14(3):145–50. doi: 10.1080/09273940600659393. [DOI] [PubMed] [Google Scholar]
- 18.Bhat P, Castaneda-Cervantes RA, et al. Intravenous daclizumab for recalcitrant ocular inflammatory disease. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):687–92. doi: 10.1007/s00417-009-1043-4. [DOI] [PubMed] [Google Scholar]
- 19.Pipitone N, Olivieri I, et al. New approaches in the treatment of Adamantiades-Behcet’s disease. Curr Opin Rheumatol. 2006;18(1):3–9. doi: 10.1097/01.bor.0000197995.27579.9b. [DOI] [PubMed] [Google Scholar]
- 20.Kilmartin DJ, Forrester JV, et al. Tacrolimus (FK506) in failed cyclosporin A therapy in endogenous posterior uveitis. Ocul Immunol Inflamm. 1998;6(2):101–9. doi: 10.1076/ocii.6.2.101.4051. [DOI] [PubMed] [Google Scholar]
- 21.Jabs DA, Rosenbaum JT, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 22.Lightman S, Kok H. Developments in the treatment of uveitis. Expert Opin Investig Drugs. 2002;11(1):59–67. doi: 10.1517/13543784.11.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Nussenblatt RB, Fortin E, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci U S A. 1999;96(13):7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim L, Suhler EB, et al. Biologic therapies for inflammatory eye disease. Clin Experiment Ophthalmol. 2006;34(4):365–74. doi: 10.1111/j.1442-9071.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 25.Kotter I, Zierhut M, et al. Human recombinant interferon-alpha2a (rhIFN alpha2a) for the treatment of Behcet’s disease with sight-threatening retinal vasculitis. Adv Exp Med Biol. 2003;528:521–3. doi: 10.1007/0-306-48382-3_104. [DOI] [PubMed] [Google Scholar]
- 26.Jabs DA, Mudun A, et al. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol. 2000;130(4):469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 27.Williams CP, Browning AC, et al. A randomised, double-blind trial of topical ketorolac vs artificial tears for the treatment of episcleritis. Eye (Lond) 2005;19(7):739–42. doi: 10.1038/sj.eye.6701632. [DOI] [PubMed] [Google Scholar]
- 28.Akpek EK, Thorne JE, et al. Evaluation of patients with scleritis for systemic disease. Ophthalmology. 2004;111(3):501–6. doi: 10.1016/j.ophtha.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Okhravi N, Odufuwa B, et al. Scleritis. Surv Ophthalmol. 2005;50(4):351–63. doi: 10.1016/j.survophthal.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Raiji VR, Palestine AG, et al. Scleritis and systemic disease association in a community-based referral practice. Am J Ophthalmol. 2009;148(6):946–50. doi: 10.1016/j.ajo.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Watson PG, Young RD. Scleral structure, organisation and disease. A review. Exp Eye Res. 2004;78(3):609–23. doi: 10.1016/s0014-4835(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 32.Fong LP, Sainz de la Maza M, et al. Immunopathology of scleritis. Ophthalmology. 1991;98(4):472–9. doi: 10.1016/s0161-6420(91)32280-2. [DOI] [PubMed] [Google Scholar]
- 33.Hakin KN, Ham J, et al. Use of cyclosporin in the management of steroid dependent non-necrotising scleritis. Br J Ophthalmol. 1991;75(6):340–1. doi: 10.1136/bjo.75.6.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox RI, Tornwall J, et al. Evolving concepts of diagnosis, pathogenesis, and therapy of Sjogren’s syndrome. Curr Opin Rheumatol. 1998;10(5):446–56. doi: 10.1097/00002281-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Casals M, Tzioufas AG, et al. Treatment of primary Sjogren syndrome: a systematic review. Jama. 2010;304(4):452–60. doi: 10.1001/jama.2010.1014. [DOI] [PubMed] [Google Scholar]
- 36.El-Asrar AM, Herbort CP, et al. A clinical approach to the diagnosis of retinal vasculitis. Int Ophthalmol. 2010;30(2):149–73. doi: 10.1007/s10792-009-9301-3. [DOI] [PubMed] [Google Scholar]
- 37.Marmor MF, Carr RE, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109(7):1377–82. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 38.Marmor MF, Kellner U, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Stepien KE, Han DP, et al. Spectral-domain optical coherence tomography and adaptive optics may detect hydroxychloroquine retinal toxicity before symptomatic vision loss. Trans Am Ophthalmol Soc. 2009;107:28–33. [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen JA, Barkhof F, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 41.Yeh EA, Weinstock-Guttman B. Fingolimod: an oral disease-modifying therapy for relapsing multiple sclerosis. Adv Ther. 2011;28(4):270–8. doi: 10.1007/s12325-011-0004-6. [DOI] [PubMed] [Google Scholar]
- 42.Renfro L, Snow JS. Ocular effects of topical and systemic steroids. Dermatol Clin. 1992;10(3):505–12. [PubMed] [Google Scholar]
- 43.Carvalho-Recchia CA, Yannuzzi LA, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–7. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 44.Petris CK. Diagram of the Eye. Original Illustration 2011 [Google Scholar]
- 45.Episcleritis. Image database.