Abstract
The nucleus accumbens (NAc) is a critical brain reward region that mediates the rewarding effects of drugs of abuse, including those of morphine and other opiates. Drugs of abuse induce widespread alterations in gene transcription and dendritic spine morphology in medium spiny neurons (MSNs) of the NAc that ultimately influence NAc excitability and hence reward-related behavioral responses. Growing evidence indicates that within the NAc small GTPases are common intracellular targets of drugs of abuse where these molecules regulate drug-mediated transcriptional and spine morphogenic effects. The RhoA small GTPase is among the most well-characterized members of the Ras superfamily of small GTPases, and recent work highlights an important role for hippocampal RhoA in morphine-facilitated reward behavior. Despite this, it is unclear how RhoA pathway signaling in the NAc is affected by withdrawal from morphine. To investigate this question, using subcellular fractionation and subsequent protein profiling we examined the expression of key components of the RhoA pathway in NAc nuclear, cytoplasmic, and synaptosomal compartments during multiple withdrawal periods from repeated morphine administration. Further, using in vivo viral-mediated gene transfer, we determined the consequences of revealed RhoA pathway alterations on NAc MSN dendritic spine morphology. Our findings reveal an important role for RhoA signaling cascades in mediating the effects of long-term morphine withdrawal on NAc MSN dendritic spine elimination.
Keywords: Opiates, opiate withdrawal, PDZ-RhoGEF, Rock1, dendritic spine
Graphical abstract
The expression profile of the RhoA network was examined in nuclear, cytosolic, and synaptosomal fractions from nucleus accumbens tissue following multiple withdrawal periods from repeated morphine administration. We found that short-term morphine withdrawal increases the expression of a key component of the RhoA network in nuclear fractions, and this increase is directed toward specific actin cytoskeleton states. Long-term morphine withdrawal, on the other hand, increases expression of a component of the RhoA network in synaptosomal fractions. These findings have implications for understanding the mechanisms that contribute to drug-seeking behavior during opiate withdrawal.
Introduction
The actin cytoskeleton is critical for the stability and dynamic regulation of cellular morphology. In neurons, actin is a key regulator of the formation, stability, and experience-dependent remodeling of dendritic spines, the regions at which most excitatory connections in the central nervous system occur (Bosch & Hayashi 2012, Penzes & Cahill 2012). Additionally, recent studies indicate that actin has critical non-structural roles in cells, including the regulation of gene transcription (Rajakyla & Vartiainen 2014, Olson & Nordheim 2010, Wu et al. 2006). Similar to other cell types, our recent studies indicate that actin regulatory pathways control the nuclear distribution of specific transcription factor networks in neurons (Cahill et al. 2017, Cahill et al. 2016). Guanine nucleotide exchange factors (GEFs) are potent regulators of actin function in neurons; however, in most instances GEFs interact with actin indirectly through a cascade of intermediary proteins. Notably, via their tandem Dbl-homology/plekstrin-homology (DH/PH) domains, GEFs induce a guanosine diphosphate (GDP) to guanosine-5′-triphosphate (GTP) exchange on small GTPases thereby resulting in small GTPase activation. Small GTPases, in turn, regulate the activity of a broad class of molecules referred to as small GTPase effectors. These effectors influence the activity of actin binding proteins responsible for regulating actin structure and stability (Tolias et al. 2011, Penzes & Cahill 2012).
The nucleus accumbens (NAc) is a primary brain reward region located in the ventral striatum. The NAc receives glutamtergic input from the medial prefrontal cortex, basolateral amygdala, and ventral hippocampus, as well as dopaminergic input from the ventral tegmental area (Russo & Nestler 2013). The immediate rewarding properties of drugs of abuse stem in large part from their ability to alter the excitability of medium spiny neurons (MSNs) of the NAc, largely through augmented reciprocal dopaminergic and glutamatergic drive (D’Souza 2015). During both acute and long-term withdrawal from repeated drug exposures, dynamic alterations in the density and morphology of dendritic spines on NAc MSNs are believed to increase the propensity toward relapse. Such pro-reward neuroadaptations in NAc MSNs enable drugs of abuse to promote a state of heightened reward circuit function and concomitant increased drug seeking behavior even long after drug administration has ceased (Russo & Nestler 2013).
Recent studies have demonstrated that the reinforcing properties of drugs of abuse stem in part from their ability to alter the expression and activity of GEF-small GTPase networks in NAc MSNs during both acute and protracted withdrawal periods (Nagai et al. 2016, Cahill et al. 2016, Dietz et al. 2012, Wang et al. 2013, Covington et al. 2011). While most studies to date have focused on the Rac1, Rap1, and Ras small GTPases, recent work suggests that drugs of abuse also engage the RhoA small GTPase network. In particular (Fakira et al. 2016) found that 1 day withdrawal from 4 days of escalating doses of morphine triggers an increase in the expression of RhoA in hippocampal synapses, and this RhoA induction, in turn, is essential for the formation of morphine-mediated conditioned place preference. Nevertheless, the effects of morphine withdrawal on the RhoA network in the NAc remain unclear. Given the demonstrated importance of small GTPase signaling in NAc MSNs in mediating cellular and behavioral responses during withdrawal from stimulant drugs of abuse (Cahill et al. 2016), knowledge of RhoA pathway regulation in the NAc in response to morphine withdrawal remains an important question. To address this, we investigated the expression profile of individual components of the RhoA pathway in the NAc following both acute and protracted withdrawal from repeated morphine administration. At each withdrawal period, subcellular fractionation was used to gain insight into not only which RhoA network proteins are regulated by morphine withdrawal, but also where in the cell this regulation occurs. Finally, the consequences of morphine withdrawal-based regulation of the RhoA pathway on NAc neuronal morphology was investigated. Overall, our findings reveal that RhoA pathways are engaged during both early and protracted withdrawal form morphine, and our findings suggest that RhoA signaling contributes to morphine withdrawal-mediated dendritic spine remodeling in the NAc.
Materials and methods
Mice
Male C57BL/6J mice were purchased from Jackson labs and were young adults at the time of tissue harvesting (10–14 weeks of age; ~25 grams). Mice were housed in groups of 5 per cage on a 12 hour light/dark cycle in a temperature controlled environment with ad libitum access to food and water. Bedding enrichment was provided for all cages. For mouse surgeries, animals were anesthetized with a ketamine/xylazine cocktail. All animal procedures were approved by the Institutional Animal Care and Use Committee at Mount Sinai (LA12-00051).
Morphine administration and tissue harvesting
Mice were injected with morphine dissolved in 0.9% sterile saline at 20 mg/kg body weight. Mice received a single intraperitoneal injection per day for 7 consecutive days (injections given in late morning); IP injections are the conventional way for delivering experimenter-administered morphine. All injections were done in the home cage of the animals; cages were randomly assigned to receive either saline or morphine injections by an experimenter. Injections of morphine and saline were done within approximately 10 minutes of each other to assure mice were handled and injected at roughly the same time each day. Mice were then withdrawn from the repeated morphine injections for 24 hours, 1 week, or 2 weeks at which time NAc tissue was dissected from 1 mm thick coronal brain sections using a 14 gauge tissue punch.
Western blotting and antibodies
Total protein levels of P1, P2, and S2 fractions were determined using a Bio-Rad DC protein assay and equal amounts of protein were placed into a buffer containing SDS and DTT as a reducing agent. Samples were heated to 95°C for 5 minutes and loaded onto 4–15% Tris-HCL gels (BioRad) for SDS-PAGE. Proteins were transferred onto Immobilon-P PVDF membranes (Milipore) for 1 hour at 100 V in buffer containing 15% methanol. Membrane blocking steps were performed using 5% bovine serum albumin (BSA) in Tris buffered saline containing 0.1% Tween-20. Primary and secondary antibody incubations were carried out in the same buffer as used for blocking. Peroxidase-conjugated secondary antibodies (Vector Labs) were used to label primary antibodies. Proteins were illuminated using chemiluminescence and protein levels quantified using densitometry (Image J). For all protein quantification, levels of the protein of interest were normalized to the corresponding levels of total actin, which itself was unchanged in any cellular fractions by the experimental conditions used. Antibodies purchased from Cell Signaling include SRF (Cat# 5147S; RRID: AB_10694554) and Rock1 (Cat# 4035S; RRID: AB_2238679). Actin antibody was purchased from MP Biomedicals (Cat# 08691002; RRID: AB_2335304). Antibodies purchased from Santa Cruz Biotechnology include PDZ-RhoGEF (Cat# sc-67024; RRID: AB_2059886) and MRTF-A (MAL) (Cat# sc-21558; RRID: AB_2142498). RhoA antibody was purchased from Cytoskeleton (Cat# ARH03-A; RRID: AB_10708069). Antibody validations are available from each company.
Cell fractionation
The isolation of P1, S2, and P2 fractions from NAc tissue was performed using our established procedures (Cahill et al. 2016, Cahill et al. 2017). Briefly, NAc tissue was homogenized using a Teflon homogenizer (40 strokes at 400 rpm) in ice cold HEPES-buffered sucrose solution (320 mM sucrose and 4 mM HEPES plus protease and phosphatase inhibitors). Homogenates were centrifuged at 1000 x g for 10 minutes which produced a S1 supernatant and an unwashed P1 fraction pellet. The P1 pellet was further purified by resuspension in HEPES-buffered sucrose solution and again centrifuged at 1000 x g. The resulting P1 pellet is the washed P1 fraction which was subsequently stored at −80°C until resolved via SDS PAGE. The S1 fraction was additionally centrifuged multiple times to remove any residual P1 fraction material. The purified S1 fraction was then centrifuged at 10,000 x g for 10 minutes which produced a S2 fraction supernatant and an unwashed P2 fraction. The S2 fraction was removed and stored at −80°C. The unwashed P2 fraction was resuspended in HEPES-buffered sucrose solution and again centrifuged at 10,000 x g for 10 minutes. The resulting washed P2 fraction pellet was then isolated and stored at −80°C.
Isolation of G-actin and F-actin subfractions
G-actin and F-actin subfractions were derived from NAc P1 fractions as detailed previously using a G-actin/F-actin in vivo assay kit (Cytoskelton, Cat# BK037) (Cahill et al. 2016, Cahill et al. 2017). Briefly, NAc P1 pellets were resuspended in warmed (37°C) lysis buffer and F-actin stabilization buffer containing protease inhibitors and ATP and incubated at 37°C for 15 minutes. Samples were then centrifuged at 350 x g for 5 minutes at room temperature. Insoluble debris was discarded and the supernatant was again centrifuged at 350 x g. This step was repeated two additional times. Samples were then centrifuged at 100,000 x g for 60 minutes (at 37°C). The resulting supernatant contained the G-actin subfraction which was removed and rapidly frozen at −80°C. The resulting F-actin subfraction pellet was resuspeneded in F-actin depolymerization buffer and stored on ice for 1 hour with periodic mixing. Sample was then frozen at −80°C until resolved via SDS-PAGE.
Viral-mediated gene transfer
All viruses used were herpes simplex viruses (HSVs) that overexpress green fluorescent protein (GFP). HSV-GFP and HSVs that overexpress GFP plus RhoA-GEF have been described and validated in our previous work (Cahill et al. 2016, Cahill et al. 2017). Mice were infused with 0.5 μl of virus per hemisphere into the NAc using Hamilton 33-gauge syringe needles at a flow rate of 0.1 μl per minute, followed by a 5 minute period of waiting in which viral diffusion is allowed to occur uninterrupted. The following stereotaxic coordinated were used to target the NAc (relative to Bregma): anterior/posterior 1.6 mm; medial/lateral 1.5 mm; dorsal/ventral −4.4 mm at a 10° syringe angle.
Dendritic spine imaging and analysis
Mice were analyzed for dendritic spines 5 days post viral infusion. Mice were transcardially perfused with phosphate buffered saline (PBS) for 30 seconds with subsequent perfusion with 4% paraformaldehyde (PFA) for 7 minutes. Brains were removed and postfixed in 4% PFA overnight. Brains were coronally sectioned using a Vibratome and 100 μm thick NAc sections obtained. Sections were washed in PBS and incubated in blocking solution containing 3% normal donkey serum and 0.3% Triton-X. Native green fluorescent protein (GFP) expression from viral constructs was boosted by overnight incubation in a GFP primary antibody (Aves Labs, Cat# GFP-1020; RRID: AB_10000240). GFP primary antibody was labeled with an AlexaFluor488 secondary antibody (Jackson Immunoresearch). Sections were coverslipped using VectaShield (Vector Labs). Dendrite segments were imaged using a LSM 780 confocal microscope (Carl Zeiss) via a 100x objective lens and 2.0 digital zoom. Dendrites were only imaged if they could be traced back to their parent soma and were at least 50 μm from the soma. Imaging and quantification was done blind to experimental conditions by coding of viral condition. For each cell a 40–50 μm segment of dendrite was imaged and spines semi-automatically classified as thin, stubby, or mushroom using Neuronstudio (http://research.mssm.edu/cnic/tools-ns.html). Several criteria are used to classify spines into discrete morphologies, including spine length to head diameter ratios, spine head to neck ratios, and diameter of the spine head. The critical numerical values for each criterion listed above used to classify spines as thin, stubby, or mushroom are detailed in our previous work (Cahill et al. 2017).
Statistics
Statistical analyses were performed using Graphpad Prism 5. The number of animals examined, statistical tests used, and significance levels for individual experiments are reported in the figure legends. The critical criteria for significance was set at p<0.05. All t-tests were two-tailed. No specific methods were used to assess normal distribution. For experiments, animals were randomly assigned to groups by an experimenter using a simple randomization method. This study was not pre-registered. Number of animals used for experiments was based on previous experience with similar experimental designs, and thus no sample size calculation was perfomed. Sample size differences from the beginning to the end of an experiments occurred in two instances: Grubbs’ test was used for potential outlier detection; outlier was detected in a single instance and indicated in the corresponding figure legend, and for one Western blot the protein band of interest for 4 of the leftmost lanes was completely blocked by a smear of high background that was not able to be eliminated (to make up for the loss of sample size for this protein, samples from additional animals were analyzed). For all biochemistry experiments the n for statistical analyses was the number of brains for each condition (detailed in each figure legend).
Results
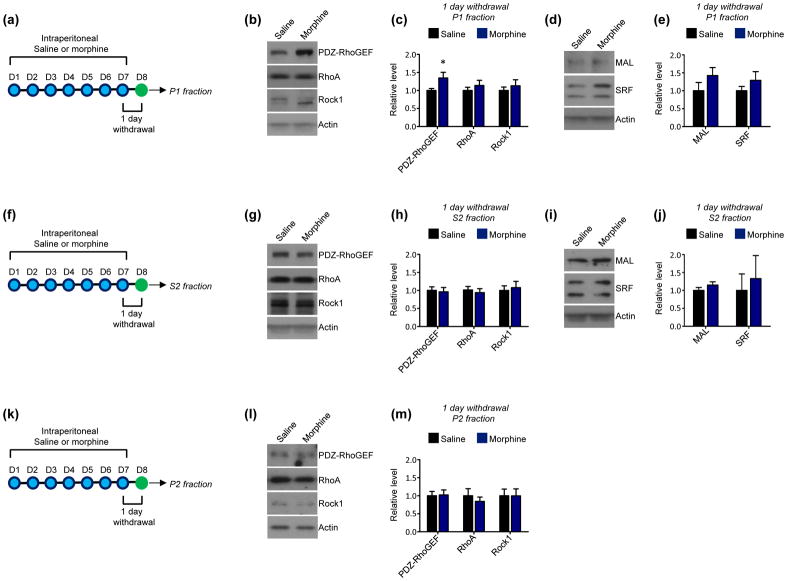
Within neurons the RhoA network is enriched in nuclei, cytoplasmic regions, and synapses (Cahill et al. 2016). Alterations in cytoplasmic RhoA network expression drive morphological phenotypes such as altered dendritic length and complexity, while synaptic RhoA pathways are potent regulators of dendritic spine formation and stability (Li et al. 2010, Warren et al. 2012, Zhu et al. 2012). In addition, RhoA pathway activity in the nucleus has a well-characterized role in facilitating the transcription of specific gene targets of the serum response factor (SRF) transcription factor and its co-activator, MAL (Gineitis & Treisman 2001, Cen et al. 2003, Liu et al. 2003). To determine the effects of acute morphine withdrawal on RhoA network expression in the NAc, mice underwent 24 hours of withdrawal from 7 days of morphine administration. NAc homogenate was subcellularly fractionated into P1 (crude nuclear), P2 (crude synaptoneurosomal), and S2 (crude cytosolic) fractions using established procedures (Cahill et al. 2017, Cahill et al. 2016). We then examined the expression of RhoA as well as that of a NAc-enriched RhoA GEF, PDZ-RhoGEF (Cahill et al. 2016), and a primary RhoA effector, Rock1, in each fraction. In addition, we investigated MAL and SRF levels in P1 and S2 fractions as RhoA pathway activity has been previously shown to influence cytoplasmic to nuclear shuttling of these molecules (Liu et al. 2003). We found that 24 hours morphine withdrawal increased the expression of PDZ-RhoGEF in NAc P1 fractions, with no change in P1 levels of RhoA or Rock1 (Fig. 1a–c). Further, no changes in the P1 expression of MAL or SRF were detected (Fig. 1d–e). No alterations in the expression of members of the RhoA pathway or alterations in MAL and SRF were detected in S2 fractions (Fig. 1f–j). Finally, the P2 expression of the RhoA pathway was not altered by 24 hours morphine withdrawal (Fig. 1k–m).
Fig 1. Effect of short-term (24 hour) withdrawal from repeated morphine exposure on the RhoA network in the NAc.
(a) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 24 hours following the final injection P1 fractions were derived from NAc lysates.
(b and c) Western blots and quantification indicate that short-term morphine withdrawal increased PDZ-RhoGEF levels in NAc P1 fractions relative to saline-treated mice (n=26 saline, 25 morphine; t=2.130, *p<0.05). NAc P1 levels of RhoA and Rock1 were not affected (n=21 saline mice, 21 morphine mice; RhoA t=0.8201, Rock1 t=0.6812, p>0.05).
(d and e) Western blots and quantification indicate that short-term morphine withdrawal did not alter the expression of MAL (t=1.313, p>0.05) or SRF (t=1.095, p>0.05) in NAc P1 fractions. n=11 saline mice and 10 morphine mice.
(f) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 24 hours following the final injection S2 fractions were derived from NAc lysates.
(g and h) Western blots and quantification indicate that short-term morphine withdrawal did not affect the levels of PDZ-RhoGEF (t=0.2562, p>0.05), RhoA (t=0.5070, p>0.05), or Rock1 (t=0.3627, p>0.05) in NAc S2 fractions. n=21 saline mice and 20 morphine mice.
(i and j) Western blots and quantification indicate that short-term morphine withdrawal did not alter the expression of MAL (t=1.200, p>0.05) or SRF (t=0.4179, p>0.05) in NAc S2 fractions. n=5 saline and morphine mice per condition.
(k) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 24 hours following the final injection P2 fractions were derived from NAc lysates.
(l and m) Western blots and quantification indicate that short-term morphine withdrawal did not affect the levels of PDZ-RhoGEF (n= 20 saline mice and 21 morphine mice; t=0.1250, p>0.05), RhoA (n=20 saline mice and 21 morphine mice; t=0.6960, p>0.05), or Rock1 (n=12 saline mice and 13 morphine mice; t=0.01777, p>0.05) in NAc P2 fractions.
All summary data are the mean + SEM.
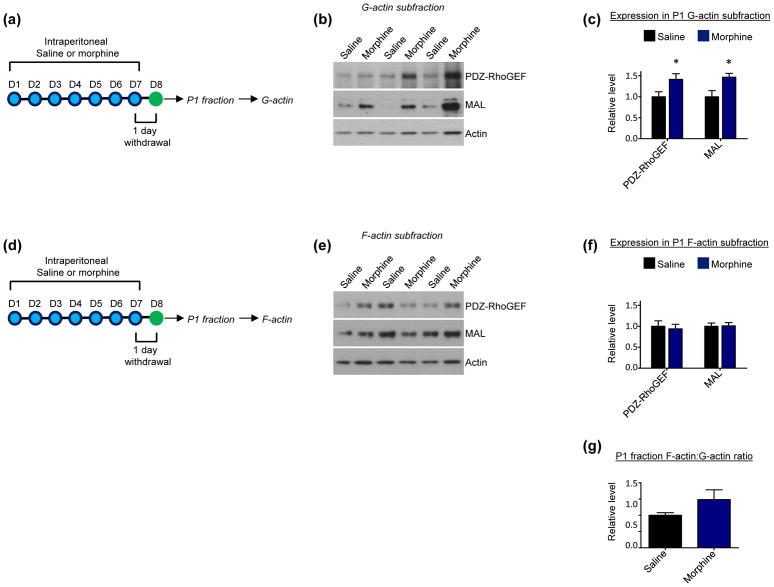
Within nuclei the actin cytoskeleton directly dictates the ability of MAL to interact with SRF (Stern et al. 2009, Kokai et al. 2014, Vartiainen et al. 2007). As MAL’s interaction with SRF enhances SRF’s ability to initiate gene transcription in large part by facilitating RNA polymerase II recruitment (Esnault et al. 2014), actin has pronounced effects in the regulation of SRF-mediated gene transcription. In particular, actin’s polymerization state is the critical determinant, as MAL’s interaction with nuclear filamentous actin (F-actin) likely places MAL in close proximity to SRF bound to its target sites in gene promoter regions, which would lead to a predicted increase in SRF-mediated gene transcription. By contrast, MAL’s interaction with nuclear monomeric actin (G-actin) sequesters MAL away from SRF and likely away from gene promoter regions (Baarlink et al. 2013, Vartiainen et al. 2007, Kokai et al. 2014, Cahill et al. 2017). Within NAc P1 fractions, we previously showed that PDZ-RhoGEF and MAL are present in both G-actin and F-actin subfractions, and our recent findings indicate that 24 hours of withdrawal from repeated cocaine exposure specifically increases the enrichment of MAL in P1 F-actin pools (Cahill et al. 2016, Cahill et al. 2017). To determine if morphine has effects reminiscent of cocaine, NAc P1 fractions were prepared from mice after 24 hours of withdrawal from repeated morphine exposure and further processed to derive G-actin and F-actin subfractions. Surprisingly we found that, opposite to cocaine, morphine increased MAL and PDZ-RhoGEF expression in NAc P1 G-actin subfractions (Fig. 2a–c), with no changes found in F-actin subfractions (Fig. 2d–f). No differences in the ratio of total F-actin to that of total G-actin were found in the P1 fraction following 24 hour morphine withdrawal (Fig. 2g).
Fig 2. Effect short-term (24 hour) withdrawal from repeated morphine exposure on protein enrichment in P1 G-actin and F-actin subfractions of NAc.
(a) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 24 hours following the final injection G-actin subfractions were derived from NAc P1 fractions.
(b and c) Western blots and quantification indicate that short-term morphne withdrawal increases levels of PDZ-RhoGEF (n=12 saline mice, 14 morphine mice; t=2.266, *p<0.05; 1 Grubbs’ test outlier in saline group) and MAL (n=13 saline mice, 14 morphine mice, t=2.736, *p<0.05)
(d) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 24 hours following the final injection F-actin subfractions were derived from NAc P1 fractions.
(e and f) Western blots and quantification indicate that short-term morphine withdrawal does not affect levels of PDZ-RhoGEF (t=0.3661, p>0.05) or MAL (t=0.1090, p>0.05) in NAc P1 F-actin subfractions. n=13 saline mice, 14 morphine mice.
(g) Quantification indicates that short term morphine withdrawal does not alter the ratio of total F-actin to total G-actin in the P1 fraction (t=1.504, p>0.05). n=13 saline mice, 14 morphine mice.
All summary data are the mean + SEM.
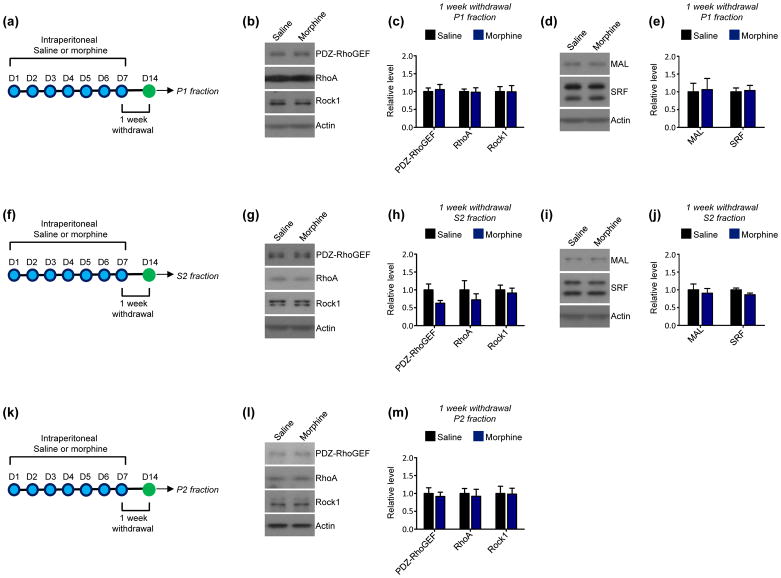
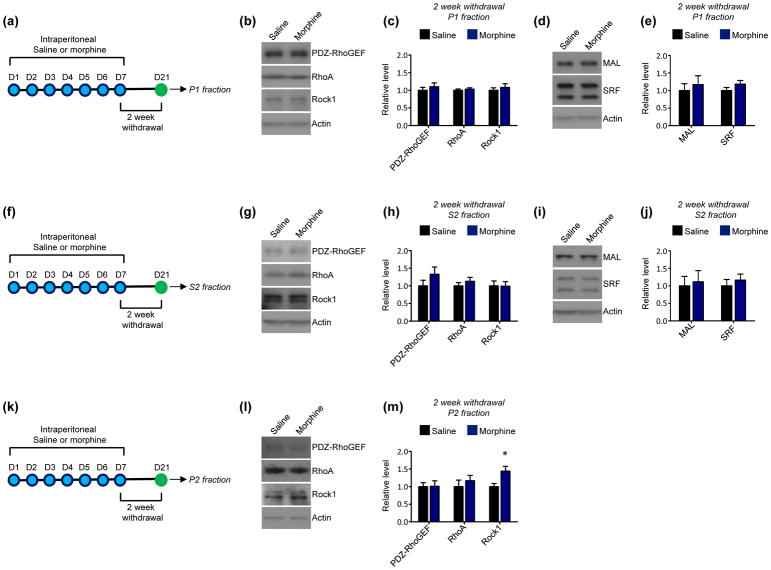
To determine if the induction of PDZ-RhoGEF expression at this early morphine withdrawal time point is maintained during more protracted withdrawal periods, NAc expression of PDZ-RhoGEF and other components of the RhoA network were examined 1 week following the cessation of repeated morphine administration. We detected no alterations in the expression of any components of the RhoA network or in the expression of MAL or SRF in NAc P1 (Fig. 3a–e) or S2 fractions (Fig. 3f–j). Further, no changes in PDZ-RhoGEF, RhoA, or Rock1 were detected in NAc P2 fractions at this time point (Fig. 3k–m). Overall, this suggests that the induction of P1 PDZ-RhoGEF by repeated morphine administration is specific to early withdrawal periods. Similar to 1-week withdrawal, 2-week withdrawal from repeated morphine exposure produced no significant alterations in the expression of the RhoA network or in levels of MAL and SRF in NAc P1 (Fig. 4a–e) or S2 fractions (Fig. 4f–j). However, this withdrawal period was marked by a significant increase in the expression of Rock1 in NAc P2 fractions, with no changes in other portions of the RhoA pathway detected in these fractions (Fig. 4k–m).
Fig 3. Effect of 1 week of withdrawal from repeated morphine exposure on the RhoA network in the NAc.
(a) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 1 week following the final injection P1 fractions were derived from NAc lysates.
(b and c) Western blots and quantification indicate that 1 week of morphine withdrawal does not affect PDZ-RhoGEF (t=0.3266, p>0.05), RhoA (t=0.1282, p>0.05), or Rock1 (t=0.02593, p>0.05) levels in NAc P1 fractions relative to saline-treated mice. n=12 saline mice, 11 morphine mice.
(d and e) Western blots and quantification indicate that 1 week of morphine withdrawal did not alter the expression of MAL (t=0.1606, p>0.05) or SRF (t=0.2064, p>0.05) in NAc P1 fractions. n=12 saline mice, 11 morphine mice
(f) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 1 week following the final injection S2 fractions were derived from NAc lysates.
(g and h) Western blots and quantification indicate that 1 week of morphine withdrawal did not affect the levels of PDZ-RhoGEF (t=1.991, p>0.05), RhoA (t=0.8842, p>0.05), or Rock1 (t=0.4595, p>0.05) in NAc S2 fractions. n=12 saline mice, 11 morphine mice.
(i and j) Western blots and quantification indicate that 1 week of morphine withdrawal did not alter the expression of MAL (t=0.4299, p>0.05) or SRF (t=1.918, p>0.05) in NAc S2 fractions. n=12 saline mice, 11 morphine mice.
(k) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 1 week following the final injection P2 fractions were derived from NAc lysates.
(l and m) Western blots and quantification indicate that 1 week of morphine withdrawal did not affect the levels of PDZ-RhoGEF (t=0.4244, p>0.05), RhoA (t=0.3352, p>0.05), or Rock1 (t=0.07281, p>0.05) in NAc P2 fractions. n=12 saline mice, 11 morphine mice.
All summary data are the mean + SEM.
Fig 4. Longer-term (2 week) withdrawal from repeated morphine exposure on the RhoA network in the NAc.
(a) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 2 weeks following the final injection P1 fractions were derived from NAc lysates.
(b and c) Western blots and quantification indicate that longer-term morphine withdrawal does not affect PDZ-RhoGEF (t=0.7444, p>0.05), RhoA (t=0.6194, p>0.05), or Rock1 (t=0.6625, p>0.05) levels in NAc P1 fractions relative to saline-treated mice. n=10 mice per condition.
(d and e) Western blots and quantification indicate that longer-term morphine withdrawal did not alter the expression of MAL (t=0.5354, p>0.05) or SRF (t=1.369, p>0.05) in NAc P1 fractions. n=10 saline mice, 10 morphine mice.
(f) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 2 weeks following the final injection S2 fractions were derived from NAc lysates.
(g and h). Western blots and quantification indicate that longer-term morphine withdrawal did not affect the levels of PDZ-RhoGEF (t=1.264, p>0.05), RhoA (t=0.9068, p>0.05), or Rock1 (t=0.04323, p>0.05) in NAc S2 fractions. n=10 saline mice, 10 morphine mice.
(i and j) Western blots and quantification indicate that longer-term morphine withdrawal did not alter the expression of MAL (t=0.2759, p>0.05) or SRF (t=0.6634, p>0.05) in NAc S2 fractions. n=10 saline mice, 10 morphine mice
(k) Experimental design. Mice were intraperitoneally injected with saline or morphine 1x per day for 7 consecutive days. 2 weeks following the final injection P2 fractions were derived from NAc lysates.
(l and m) Western blots and quantification indicate that longer-term morphine withdrawal increased levels of Rock1 in NAc P2 fractions (n=13 saline mice, 14 morphine mice, t=2.673, *p<0.05). Levels of PDZ-RhoGEF (n=10 saline mice, 10 morphine mice, t=0.04549, p>0.05) and RhoA (n=10 saline mice, 10 morphine mice, t=0.7014, p>0.05) on the other hand were not affected under these conditions.
All summary data are the mean + SEM.
The increased expression of Rock1 in NAc P2 fractions following long-term morphine withdrawal is indicative of the engagement of the RhoA network in NAc synapses. While studies in hippocampal pyramidal neurons indicate that the RhoA pathway exerts profound effects on the formation and stability of dendritic spines (Mizuki et al. 2016, Tashiro et al. 2000, Nakayama et al. 2000), comparatively little is known regarding the effects of RhoA network expression on spine morphogenesis in NAc MSNs. To this end, we virally overexpressed the enzymatic DH/PH domain of a RhoA GEF in the NAc in vivo (Fig. 5a). Importantly, we recently showed that this viral construct produces a significant, yet modest, increase in NAc MSN RhoA network engagement (Cahill et al. 2016), allowing for the assessment of physiologically-relevant effects on spines. Viral constructs also co-expressed green fluorescent protein (GFP) enabling the visualization of viral targeting and assessments of neuronal morphology (Cahill et al. 2017, Cahill et al. 2016). Dendritic spines were semi-automatically classified as possessing a thin, stubby, or mushroom phenotype based on their length and head diameter (Fig. 5b). Stubby spines typically have a head size intermediate to that of thin and mushroom spines, and the lack of a discernable neck is the defining feature of this spine subtype. Thin and mushroom spines have a distinct neck region and are distinguishable from each other chiefly on the basis of spine head area, such that mushroom spines have a larger head area than thin spines (Nimchinsky et al. 2002). Across spine subtypes we found a main effect for RhoA GEF overexpression on spine density. Specifically, we found that augmentation of the RhoA pathway resulted in a significant decrease in the density of thin dendritic spines on NAc MSNs. No significant effects on the density of stubby or mushroom spines were detected (Fig. 5c and d).
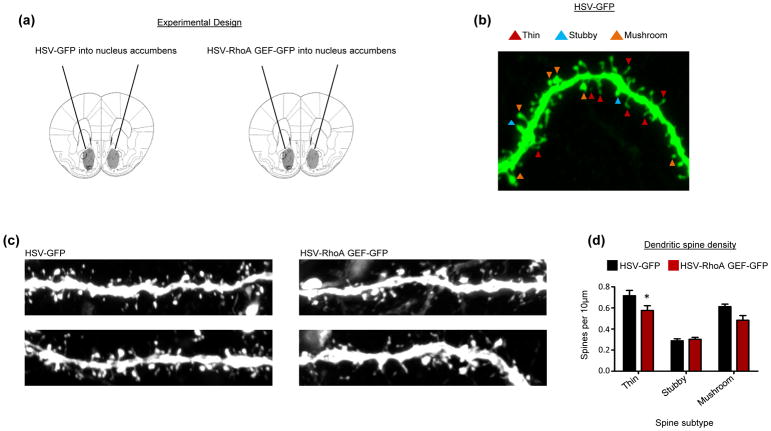
Fig 5. Effect of the RhoA network on dendritic spines in NAc MSNs.
(a) Experimental design. HSV-GFP and HSV-RhoA-GEF-GFP were infused into the NAc. 5 days post viral infusion, NAc MSNs were imaged.
(b) Representative image shows dendritic segment from a NAc MSN infected with HSV-GFP. Different spine subtypes, including thin, stubby, and mushroom spines, are indicated.
(c) Representative images of NAc MSN dendritic segments from mice infused with HSV-GFP or HSV-RhoA-GEF-GFP into the NAc.
(d) Quantification indicates that NAc MSNs expressing HSV-RhoA-GEF-GFP show a significant reduction in the density of thin spines relative to HSV-GFP controls. The density of stubby and mushroom spines was not affected by this manipulation (two-way ANOVA, main effect of RhoA-GEF-GFP overexpression F=6.633, *p<0.05; Bonferroni posthoc thin spines, *p<0.05, Bonferroni posthoc stubby and mushroom spines, p>0.05). n=34 GFP cells and 15 RhoA GEF cells.
All summary data are the mean + SEM.
Discussion
The main findings of this work indicate that early and long-term withdrawal from repeated morphine exposure engages the RhoA network in the NAc, that the subcellular regions displaying increased RhoA network signaling differ during early and protracted morphine withdrawal, and that heightened RhoA network activity is sufficient to induce synaptic structural alterations in NAc MSNs reminiscent of the known effects of morphine.
We found that after 24 hours of morphine withdrawal, there are increased levels of PDZ-RhoGEF within NAc P1 fractions, and that this increased expression is due to a selective enrichment in the P1 G-actin, and not F-actin, subfraction. The accumulation of PDZ-RhoGEF in the G-actin subfraction is surprising given our previous findings that, although PDZ-RhoGEF is expressed in pools of both actin subtypes, it is preferentially enriched in NAc P1 F-actin subfractions under basal conditions (Cahill et al. 2017). Indeed PDZ-RhoGEF contains a direct F-actin binding domain N-terminus to its enzymatic DH/PH domain that promotes F-actin bundling and thereby increased F-actin stability (Banerjee et al. 2009). Also, through the activation of canonical RhoA signaling, PDZ-RhoGEF likely promotes F-actin stability by attenuating F-actin depolymerization (Siehler 2009). The reason for the selective accumulation of PDZ-RhoGEF in the G-actin subfraction after short-term morphine withdrawal is not entirely clear. One possibility is that following such withdrawal there are insufficient nuclear F-actin anchoring sites for PDZ-RhoGEF, which then leads to its default accumulation in G-actin rather than F-actin pools. While PDZ-RhoGEF can promote F-actin stability, it might not be independently capable of driving de novo F-actin formation from G-actin monomers. As such, the accumulation of PDZ-RhoGEF at nuclear F-actin sites might necessitate enhanced F-actin formation over basal levels via distinct actin polymerization pathways that are not engaged during short-term morphine withdrawal. As signaling networks that drive F-actin formation also facilitate the depletion of MAL from G-actin pools (Cahill et al. 2016), the accumulation of MAL in NAc P1 G-actin, but not F-actin, subfractions after short-term morphine withdrawal is further suggestive of a lack of sufficient de novo F-actin docking sites for these molecules.
As our recent findings indicate that histones are enriched in NAc P1 F-actin subfractions, with little to no presence in G-actin subfractions (Cahill et al. 2017), the accumulation of MAL in G-actin subfractions during short-term morphine withdrawal likely places MAL away from its potential DNA binding sites (Supplementary Fig. 1). This is in direct contrast to our recent work demonstrating that short-term withdrawal from repeated cocaine administration increases the ratio of MAL in NAc P1 F-actin vs. G-actin subfractions (Cahill et al. 2016), which likely contributes to cocaine-mediated enhancements in SRF-mediated gene transcription. Nevertheless, morphine-mediated enrichment of MAL in NAc P1 G-actin subfractions could effectively prime MAL to foster SRF-mediated gene transcription very rapidly in the event that G-actin binding sites for MAL are reduced by the enhanced activity of nuclear F-actin polymerization pathways. Additional factors capable of driving actin polymerization in NAc nuclei during short-term morphine withdrawal remain unclear. While in our study mice were administered morphine in their home cage, it is possible that if morphine was administered in a novel environment, subsequent exposure to the novel environment could drive nuclear actin polymerization in the NAc during the acute withdrawal period. If so, this could foster the transition of PDZ-RhoGEF and MAL toward F-actin pools where PDZ-RhoGEF could increase the stability of newly formed F-actin and thereby prolong MAL’s ability to foster SRF-mediated gene transcription. Comparative studies that investigate the effects of morphine administration in novel vs. home cage environments on the RhoA pathway merit future attention.
In contrast to short-term withdrawal, our results show that 2 weeks post-morphine administration the RhoA network is excessively engaged in NAc synaptic regions. We show further that increased expression of the RhoA network in this brain region facilitates the selective elimination of thin spines on NAc MSNs. Recent work indicates that long-term morphine withdrawal causes a selective elimination of thin spines in NAc MSNs (Graziane et al. 2016). Together with our findings, this work suggests that the RhoA network is a potential contributor to the elimination of this spine subset during protracted stages of morphine withdrawal. Similar to other drugs of abuse, seeking behavior for opiates following abstinence is greater during long term withdrawal (e.g., 2 weeks) as compared to 1 day withdrawal (Kuntz et al. 2008). This suggests that the elimination of thin spines in NAc MSNs during multi-week morphine withdrawal potentially contributes to the incubation of opiate craving responses with a concomitant increased propensity toward drug relapse.
NAc MSNs can be categorized into two distinct groups defined by their predominant expression of either dopaminergic D1 or D2 receptors. Multiple studies indicate that NAc D1-expressing MSNs facilitate drug-mediated reward behavior, while the activity of D2-expressing MSNs confers anti-reward responses (Lobo & Nestler 2011, Calipari et al. 2016, Lobo et al. 2010). Studies indicate that longer-term (10–14 days) morphine withdrawal increases the frequency of miniature excitatory postsynaptic currents (mEPSCs) in D1-expressing NAc MSNs while simultaneously decreasing mEPSCs in D2-expressing NAc MSNs (Hearing et al. 2016). While synaptic strength correlates with spine head size and hence thin spines are typically the sites of weaker synapses as compared to mushroom spines (Tada & Sheng 2006), the majority of thin spines on NAc MSNs nevertheless are responsive to glutamate, indicating that they are important regulators of neuronal excitability (Khibnik et al. 2016). As such, the loss of thin spines on D2-expressing NAc MSNs could account for the reported decrease in mEPSCs on this neuronal subtype by morphine. Future studies aimed at determining if the RhoA pathway is selectively induced in synapses of D2-expressing NAc MSNs during longer-term morphine withdrawal will enable a more precise understanding of the role for aberrant RhoA network regulation in contributing to the synaptic structural hallmarks of repeated morphine exposure and withdrawal. Indeed, the specific loss of synaptic connectivity on D2 NAc MSNs would be expected to increase drug-mediated reward responsivity by downshifting activity in an anti-reward system and hence tipping the balance toward a D1-mediated pro-reward circuit.
A recent study found that 1 day withdrawal from repeated morphine administration decreased thin spine density in hippocampal neurons, and this loss of thin spines was associated with increased expression of total RhoA in hippocampal synapses. These effects were present when morphine was administered in a novel environment (conditioning chamber) and not elicited following home cage administered morphine (Fakira et al. 2016). This suggests that hippocampal synaptic RhoA induction encodes associations between environmental cues and drug exposure rather than frank morphine reward. Given that our findings in the NAc were triggered by home cage morphine administration, the RhoA alterations identified in our study might control the fundamental rewarding and motivating properties of morphine.
Several pharmacological compounds that selectively target components of the RhoA pathway have been developed (Guan et al. 2013). Notably, pharmacological inhibition of the RhoA pathway selectively in the hippocampus reduces morphine-induced place preference, suggestive of a reduction in drug-seeking behavior (Fakira et al. 2016). We found that RhoA pathway activity reduces spine density in NAc MSNs, thereby recapitulating the effects of multi-week morphine withdrawal. As multi-week morphine withdrawal is associated with intensified drug-seeking responsivity as compared to more acute withdrawal (Kuntz et al. 2008), it is conceivable that RhoA pathway inhibition in the NAc will dampen morphine seeking behavior similar to the reported effects of RhoA inhibition in the hippocampus. If so, this could implicate general forebrain RhoA hyper-engagement as a contributing factor to morphine seeking behavior and hence the propensity toward drug relapse. RhoA inhibition, in turn, could become viable treatment strategy in controlling opiate relapse.
Finally, as this work examined effects on the RhoA pathway spanning from 1 day to 2 weeks following the final dose of repeated morphine administration, our findings pertain to morphine withdrawal rather than strict morphine administration per se. Indeed, whereas striking effects on NAc MSN synaptic structure are evident spanning from 1 day to several weeks post-morphine administration, chronic morphine fails to elicit more immediate (within 1 hour) synaptic structural effects on NAc MSNs (Diana et al. 2006). This suggest that the 1 day time point post-repeated morphine administration is a bonafide withdrawal period with effects that differ in severity and kind from the physiological effects of morphine itself. Nevertheless, the requirement of withdrawal period on the identified effects in this work will require future studies that examine the more immediate effects of morphine administration on the expression and function of the RhoA network in NAc MSNs.
Supplementary Material
Acknowledgments
This work was supported by NIH grants P01DA008227 (EJN) and R01DA014133 (EJN).
Abbreviations used
- MSN
medium spiny neuron
- GEF
guanine nucleotide exchange factor
- NAc
nucleus accumbens
- GFP
green fluorescent protein
- SRF
serum response factor
Footnotes
conflict of interest disclosure
The authors declare no competing financial interests or other sources of conflict of interest.
Open Science Badges
This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
References
- Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–867. doi: 10.1126/science.1235038. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Fischer CC, Wedegaertner PB. The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry. 2009;48:8032–8043. doi: 10.1021/bi9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Bagot RC, Gancarz AM, et al. Bidirectional Synaptic Structural Plasticity after Chronic Cocaine Administration Occurs through Rap1 Small GTPase Signaling. Neuron. 2016;89:566–582. doi: 10.1016/j.neuron.2016.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Walker DM, Gancarz AM, Wang ZJ, Lardner CK, Bagot RC, Neve RL, Dietz DM, Nestler EJ. The dendritic spine morphogenic effects of repeated cocaine use occur through the regulation of serum response factor signaling. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A. 2016;113:2726–2731. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol. 2003;23:6597–6608. doi: 10.1128/MCB.23.18.6597-6608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, Sun H, et al. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71:656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Spiga S, Acquas E. Persistent and reversible morphine withdrawal-induced morphological changes in the nucleus accumbens. Ann N Y Acad Sci. 2006;1074:446–457. doi: 10.1196/annals.1369.045. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault C, Stewart A, Gualdrini F, East P, Horswell S, Matthews N, Treisman R. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014;28:943–958. doi: 10.1101/gad.239327.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakira AK, Massaly N, Cohensedgh O, Berman A, Moron JA. Morphine-Associated Contextual Cues Induce Structural Plasticity in Hippocampal CA1 Pyramidal Neurons. Neuropsychopharmacology. 2016;41:2668–2678. doi: 10.1038/npp.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gineitis D, Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem. 2001;276:24531–24539. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Sun S, Wright WJ, et al. Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci. 2016;19:915–925. doi: 10.1038/nn.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Xu X, Chen M, Hu H, Ge H, Wen S, Zhou S, Pi R. Advances in the studies of roles of Rho/Rho-kinase in diseases and the development of its inhibitors. Eur J Med Chem. 2013;70:613–622. doi: 10.1016/j.ejmech.2013.10.048. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, Schmidt C, Larson EB, Thomas MJ. Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci U S A. 2016;113:757–762. doi: 10.1073/pnas.1519248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, Russo SJ. Stress and Cocaine Trigger Divergent and Cell Type-Specific Regulation of Synaptic Transmission at Single Spines in Nucleus Accumbens. Biol Psychiatry. 2016;79:898–905. doi: 10.1016/j.biopsych.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai E, Beck H, Weissbach J, et al. Analysis of nuclear actin by overexpression of wild-type and actin mutant proteins. Histochem Cell Biol. 2014;141:123–135. doi: 10.1007/s00418-013-1151-4. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacol Biochem Behav. 2008;90:344–348. doi: 10.1016/j.pbb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhao CT, Wang Y, Yuan XB. The transcription factor Cux1 regulates dendritic morphology of cortical pyramidal neurons. PLoS One. 2010;5:e10596. doi: 10.1371/journal.pone.0010596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HW, Halayko AJ, Fernandes DJ, et al. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki Y, Takaki M, Sakamoto S, Okamoto S, Kishimoto M, Okahisa Y, Itoh M, Yamada N. Human Rho Guanine Nucleotide Exchange Factor 11 (ARHGEF11) Regulates Dendritic Morphogenesis. Int J Mol Sci. 2016:18. doi: 10.3390/ijms18010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Nakamuta S, Kuroda K, et al. Phosphoproteomics of the Dopamine Pathway Enables Discovery of Rap1 Activation as a Reward Signal In Vivo. Neuron. 2016;89:550–565. doi: 10.1016/j.neuron.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME. Deconstructing signal transduction pathways that regulate the actin cytoskeleton in dendritic spines. Cytoskeleton (Hoboken) 2012;69:426–441. doi: 10.1002/cm.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakyla EK, Vartiainen MK. Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases. 2014;5:e27539. doi: 10.4161/sgtp.27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol. 2009;158:41–49. doi: 10.1111/j.1476-5381.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Debre E, Stritt C, Berger J, Posern G, Knoll B. A nuclear actin function regulates neuronal motility by serum response factor-dependent gene transcription. J Neurosci. 2009;29:4512–4518. doi: 10.1523/JNEUROSCI.0333-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex. 2000;10:927–938. doi: 10.1093/cercor/10.10.927. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- Wang X, Cahill ME, Werner CT, et al. Kalirin-7 mediates cocaine-induced AMPA receptor and spine plasticity, enabling incentive sensitization. J Neurosci. 2013;33:11012–11022. doi: 10.1523/JNEUROSCI.1097-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, Greer CA, Taylor JR, Koleske AJ. Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J Neurosci. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan JL. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat Cell Biol. 2006;8:756–763. doi: 10.1038/ncb1433. [DOI] [PubMed] [Google Scholar]
- Zhu YB, Kang K, Zhang Y, Qi C, Li G, Yin DM, Wang Y. PLD1 Negatively Regulates Dendritic Branching. J Neurosci. 2012;32:7960–7969. doi: 10.1523/JNEUROSCI.5378-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.