Abstract
In order to maintain the health and wellbeing of all mammals, numerous aspects of physiology are controlled by neuroendocrine mechanisms. These mechanisms ultimately enable communication between neurons and glands throughout the body and are centrally mediated by neuropeptides and/or steroid hormones. A recent session at the International Workshop in Neuroendocrinology highlighted essential roles for some of these neuropeptide and steroid hormone mediators in the neuroendocrine regulation of stress-, reproduction-, and behavior- related processes. The present review, accordingly, highlights topics presented in this session, including the role of the neuropeptides corticotropin releasing factor and gonadotropin releasing hormone in stress and reproductive physiology, respectively. Additionally, it details an important role for gonadal sex steroids in the development of behavioral sex preference. Overall, this review suggests that the neuroendocrine regulation of numerous physiological processes cannot occur without the involvement of neuropetides and/or steroid hormones.
Keywords: stress, reproduction, CRF, GnRH, neuropetides, neuroactive steroids
Introduction
This review showcases a session presented at the International Workshop in Neuroendoocrinology (IWNE) in Concón, Chile in August 2017. Talks in the session emphasized diverse stress, reproduction and behavior related processes that are all subject to neuroendocrine regulation. This review, therefore, details advances in our understanding of how key hypothalamic neuropetides and steroid hormones enable the neuroendocrine control of these processes.
Since their discovery about 4 decades ago, hypothalamic neuropeptides have greatly advanced our understanding of the mechanisms underlying neuroendocrine regulation.1 This is especially true for the neuropeptides corticotropin releasing factor (CRF) and gonadotropin releasing hormone (GnRH), which are key mediators of stress and reproductive physiology, respectively. CRF was first characterized by Wylie Vale and colleagues in 1981 and has since been established as the pivotal regulator of the endocrine network that drives the body wide response to stress- the hypothalamic pituitary adrenal (HPA) axis.2 GnRH, alternatively, was characterized by Andrew Shally’s group in the 1970s and is now accepted as the central regulator of the endocrine network that drives reproductive functions and behaviors- the hypothalamic pituitary gonadal (HPG) axis.1,3 In addition to their involvement in these classical neuroendocrine networks, CRF and GnRH regulate other stress and reproduction related functions. For example CRF has been implicated in autonomic, behavioral and metabolic activities in response to stress; and GnRH plays a role in reproductive cancer biology.4,5
Like neuropeptides, steroid hormones are important mediators of neuroendocrine regulation of various physiological functions. Steroid hormones, including corticosteroids made by the adrenal gland and sex steroids made in the gonads, act on numerous tissues to alter stress, reproduction and behavior related processes, among others.6,7 The discovery of steroid hormone receptors in the brain in the 1960s and 1970s paved the way for studies of steroid hormone effects on brain functions.8 It is now understood that corticosteroids, such as glucocorticoids can act within various brain regions to influence stress reactivity, cognition, arousal, memory, mood, and behavior.6 Similarly, sex steroids, such as testosterone and estradiol, act throughout the brain to alter stress reactivity, sexual differentiation, and sex preference behavior.7,9,10
Ultimately, dynamic, bidirectional interactions exist between the endocrine and nervous systems that are largely mediated by neuropeptides and steroid hormones. In this review, we specifically detail advances in our understanding of how the stress response and reproduction are controlled by the neuropeptides CRF and GnRH, respectively. Additionally, we present developments in our understanding of how sex steroids influence behavioral sex preference. As we review the diverse topics presented at the IWNE, we hope to ultimately emphasize the ongoing importance of these neuropeptide and sex hormone mediators in neuroendocrine regulatory mechanisms.
Control of the stress response by CRF
Stress is defined as any real or perceived threat to an organism’s well being, or homeostasis; and it is induced by factors that fall within two broad catergories: physical and psychological stressors.11 Physical stressors involve any external or internal condition that brings physical harm, danger, pain or discomfort.11 Psychological stressors, alternatively, are factors that result in uncertainty, helplessness, or social conflict.11 In rodents, for example, hemorrhage induces physical stress, whereas restraint predominantly induces psychological stress.12 Notably, different types of stressors activate varying neural networks to drive physiological responses (see Herman and Cullinan 1997 for review).12
The neuropeptide CRF is an important mediator of responses to physical and psychological stressors alike. CRF expressed by hypothalamic neurons is the central regulator of the HPA axis, the endocrine network that ultimately drives hormonal responses to both types of stressors.12 Furthermore, CRF is an important regulator of autonomic and behavioral responses to varying stressors.4 In addition to its synthesis by and secretion from neurons of the hypothalamic paraventricular nucleus (PVN), which activates the HPA axis, CRF is expressed throughout the central nervous system.13 It has notably high expression in the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) where it is important for numerous autonomic, immune and behavioral changes during stress.4
In order to regulate various aspects of the stress response, CRF and the related peptides, urocortins 1, 2 and 3, all bind to one or two of two types of CRF receptors (CRF1 and CRF2) with varying affinity.14 Like CRF, CRF receptors and urocortins are widely expressed throughout the central nervous system and periphery and have been implicated in the regulation of various stress responses.14
Because CRF integrates numerous aspects of the stress response, its tight regulation is essential for an organism’s wellbeing and survival in the face of environmental challenges. Here we provide examples of brain CRF’s regulation of and by stress related processes that emphasize the necessity of CRF for neuroendocrine mechanisms enabling well balanced stress physiology.
CRF and the modulation of cardiovascular responses to stress
CRF and the related peptides, as well as its receptors, are located in various CNS areas known to regulate cardiovascular function, such as the CeA, BNST, and dorsal motor nucleus of the vagus (DMV).4,13,15,16 Thus, CRF neurotransmission is favorably positioned to mediate the neuroendocrine control of autonomic and cardiovascular responses to environmental challenges. Indeed, several studies support involvement of CRF neurotransmission in the control of cardiovascular responses to a variety of physical, psychological and chronic stressors, as will be discussed below.
Acute physical stressors
The first evidence for the involvement of brain CRF receptors in the control of cardiovascular responses to stress was obtained in a study that evaluated the cardiovascular responses during treadmill exercise in rats.17 Intacerebroventricular (i.c.v.) administration of the nonselective CRF receptor antagonist, α-helical-CRF9–41, decreased exercise-induced elevations of blood pressure, heart rate, mesenteric vascular resistance, and iliac blood flow.17 Additionally, authors reported that the reduced cardiovascular responses in animals treated with the CRF antagonist were followed by compromised function as reflected by the animals’ decreased ability to sustain dynamic exercise.17 These results provided evidence of an important role of brain CRF neurotransmission in expression of cardiovascular adjustment to dynamic exercise. Other initial studies in rats further supported a role for CRF in mediating cardiovascular responses to physical stressors. Treatment with the nonselective CRF receptor antagonist α-helical-CRF9–41 attenuated the elevation of plasma epinephrine levels induced by hemorrhage and hypoglycemia.18 Furthermore, i.c.v. administration of α-helical-CRF9–41 decreased hypertension, tachycardia (heart rate exceeding the resting rate), and hyperthermia induced by treatment with interleukin 1 beta, an important cytokine mediator of the inflammatory response.19 It was also reported that α-helical-CRF9–41 decreased the reduction in baroreflex function induced by bilateral hindlimb ischaemia without affecting the pressor and tachycardiac effects.20
Acute psychological stressors
Endogenous CRF and related peptides also mediate cardiovascular and autonomic changes evoked by psychological stressors. Early studies in rats demonstrated that α-helical-CRF9–41 given i.c.v. decreased the pressor response, tachycardia and hyperthermia induced by acute cage-switch stress.19,21 This cage-switch stress is a mild psychological stressor that is evoked by removing the rat from its home cage and placing it into another identical plastic cage containing one centimeter deep water.19 Other studies reported evidence of decreased circulating epinephrine and norepinephrine in rhesus monkeys subjected to psychosocial stress (intruder paradigm) and treated orally with the centrally acting, selective CRF1 receptor antagonist antalarmin.22 Additionally, α-helical-CRF9–41 treatment decreased the tachycardiac response to contextual fear conditioning in rats by modulating parasympathetic activity.23 Effects of nonselective and selective CRF receptor antagonists on tachycardia following auditory fear conditioning, however, were not observed.24 The results of this study using auditory fear conditioning are difficult to interpret because absolute values rather than changes in heart rate during the conditioned stimulus were analyzed. Thus, it is hard to determine how much pre-stimulus bradycardia (heart rate below the resting rate) evoked by CRF affected the results obtained during the conditioned stimulus. Nevertheless, the contradicting findings of such fear conditioning studies suggest that the involvement of CRF neurotransmission in cardiovascular changes during conditioned threats may be stimulus specific.
Whereas CRF in the CNS enhances cardiovascular responses to many psychological stressors, it can also decrease them depending on the nature of the stressor. For instance, i.c.v. administration of the nonselective CRF receptor antagonist D-PheCRF12–41 increased the bradycardiac responses evoked by airpuff startle stress.25 Increased bradycardia evoked by swim stress was also reported in rats systemically treated with the centrally acting, selective CRF receptor 1 antagonist antalarmin.26 These studies suggest that CRF neurotransmission has an inhibitory influence on cardiovascular responses to airpuff startle and swim stressors. However, it is important to note that bradycardia, rather than the typical stress-evoked tachycardia, was reported in these studies.25,26 Thus, an inhibitory influence of CRF neurotransmission in bradycardiac responses is well aligned with evidence that CRF increases cardiovascular function by inhibiting parasympathetic activity, which drives bradycardia.18,23,27 Accordingly, the increase in swim stress-evoked bradycardia by antalarmin was completely antagonized by systemic treatment with a peripherally acting cholinergic antagonist, methylatropine nitrate, which inhibits parasympathetic activity.26
CRF signaling may not play a role in cardiovascular responses to other psychological stressors. One study did not identify an effect of i.c.v administration of the nonselective CRF receptor antagonist astressin on pressor and tachycardiac responses evoked by acute restraint stress.28 Earlier findings also did not observe effects of CRF administered i.c.v. on restraint-evoked tachycardia.29 Why treatment effects are absent in these studies is not clear, especially because recent results identified involvement of CRF receptors in the BNST in restraint-evoked cardiovascular responses.30 Some findings have provided evidence that a treatment time longer than 10 minutes may be necessary for CRF given i.c.v. to affect stress-evoked cardiovascular responses.25 Additionally, it is possible that drug administration i.c.v. does not allow for an adequate concentration of the drug to reach brain sites where endogenous CRF and related peptides act to modulate the cardiovascular responses to stress.
Chronic stressors
CRF neurotransmission in the brain was also reported to be involved in cardiovascular changes evoked by chronic stressors. For instance, repeated i.c.v. administration of the nonselective CRF receptor antagonist astressin prior to each stress session inhibited increases in the baroreflex bradycardiac response following exposure to five sessions of restraint stress.28 Systemic treatment with the selective CRF1 receptor antagonist NBI-30775 also blocked the autonomic imbalance evoked by chronic social stress (resident–intruder stress).31
CNS sites involved in the modulation of stress-evoked cardiovascular responses by CRF
The studies reviewed above clearly indicate a role of CRF neurotransmission in the control of cardiovascular responses evoked by different stressors. However, information regarding the specific sites within the CNS where CRF receptors are activated to modulate stress-evoked cardiovascular responses is much more limited. Some initial studies found different effects of CRF on neuronal activation in CNS structures controlling autonomic activity during aversive threats. For example, i.c.v. administration of CRF decreased a cold restraint stress-induced increase in fos-positive neurons in the DMV; but it increased fos labelling in the nucleus of the solitary tract.32 These results suggest that control of cardiovascular responses to stress by CRF neurotransmission may be brain structure-specific. Additionally, a recent study reported an increase in the number of CRF-positive neurons in the CeA evoked by air-jet stress, suggesting that stress exposure can affect CeA CRF neurotransmission control of stress-evoked cardiovascular responses.33
Studies directly evaluating sites in the CNS that mediate the control of cardiovascular responses to stress by CRF neurotransmission provided evidence for a role of the DMV and the BNST.23,24,30 A role for the DMV was identified in experiments in which ovine CRF was microinjected into the DMV resulting in decreased tachycardia following auditory fear conditioning in mice.24 This effect of CRF was dose-dependent and antagonized by pretreatment with the CRF receptor antagonist astressin.24 The control of cardiovascular responses by CRF neurotransmission within the BNST, on the other hand, seems to be stress-specific. One study documented that microinjection of the nonselective CRF receptor antagonist α-helical-CRF9–41 in the BNST enhanced the tachycardiac response to contextual fear conditioning, possibly by modulating parasympathetic activity.23 Conversely, microinjection of selective CRF1 or CRF2 receptor antagonists into the BNST dose-dependently decreased the pressor, tachycardiac and sympathetically-mediated cutaneous vasoconstriction responses evoked by acute restraint stress.30 Taken together, these results indicate that CRF neurotransmission within the BNST plays an inhibitory role in cardiac responses to conditioned aversive stimuli, whereas it plays an excitatory role in the expression of cardiovascular changes in response to unconditioned stimuli.
CRF and the regulation of the hormonal response to stress
The importance of CRF in directing the hormonal responses to stress has been well- recognized since its characterization by Wylie Vale and colleagues.2 In the presence of both physical and psychological stressors, CRF markedly increases in and is secreted from PVN neurons, which activates the HPA axis.2 Upon its secretion into the hypophyseal portal vasculature, CRF stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary.2 ACTH, in turn, induces downstream secretion of glucocorticoids by the adrenal cortex in order to organize body wide, adaptive responses to stress.2 Acute elevations in glucocorticoids are beneficial, however, unrestrained glucocorticoid levels can lead to stress-related pathologies, such as cardiovascular disease, obesity and major depressive disorder.6 Thus, PVN CRF expression is tightly controlled by a glucocorticoid negative feedback loop and represents an important nexus in the control of glucocorticoid production.34
Substantial evidence suggests that PVN crf is a target of feedback regulation by glucocorticoids. In the rat, removal of most circulating glucocorticoids by adrenalectomy (ADX) increased PVN CRF messenger RNA (mRNA)35 and protein36 whereas peripheral administration of glucocorticoids decreased them in ADX’d animals.37 Additionally, glucocorticoid implants in the PVN decreased CRF mRNA levels in ADX’d rats, suggesting that glucocorticoids act directly upon PVN CRF neurons to decrease crf expression.38
Ultimately, CRF is essential in the neuroendocrine mechanisms that limit activation of the HPA axis. This subsection will therefore detail advances in our understanding of how crf expression is regulated by glucocorticoids to shed light on how it may act to keep stress induced HPA axis activity in check.
An important role for the glucocorticoid receptor
In order to decrease PVN CRF expression and prevent the detrimental health consequences of excess glucocorticoids, stress-induced glucocorticoids act through the intracellular glucocorticoid receptor (GR).39 The GR functions as a nuclear receptor and, accordingly, exists as a cytoplasmic multiprotein complex consisting of one receptor molecule and stress-responsive heat shock proteins, including heat shock protein (hsp)90, and hsp70. Binding of glucocorticoids triggers the dissociation of heat shock proteins, multiple phosphorylation steps, nuclear translocation, dimerization, and ultimately the increased affinity of the receptor for DNA elements where it can influence gene transcription.39 Notably, the GR is not the only receptor that can bind glucocorticoids and influence gene transcription. The mineralocorticoid receptor (MR) is also sensitive to glucocorticoids.40 However, the limited localization of the MR in hypothalamic areas and its higher affinity for corticosterone results in its selective occupation by basal glucocorticoid levels.40 The GR, alternatively, is abundantly expressed in hypothalamic areas, and its lower affinity for corticosterone results in its occupation by stress-induced glucocorticoids.40 Hence, the GR plays a greater role in mediating glucocorticoid negative feedback following a stressor.
Within PVN CRF neurons, the ligand-bound GR can directly alter crf transcription. To do so, GR can either (i) bind the crf promoter and recruit transcriptional co-regulators, (ii) interact with other transcription factors and modify their interaction with DNA, (iii) modify signal transduction pathways, and/or (iv) it can affect the half-life of CRF transcripts.4 All mechanisms are important for controlling crf transcription; however, here we will detail advances in our understanding of a direct DNA binding mechanism in which ligand-bound GR recruits co-regulators to the crf promoter. Initial support for these direct actions of GR comes from the localization of GR immunoreactivity in CRF neurons.41 Furthermore, the crf promoter contains a negative glucocorticoid response element (nGRE) that mediates repression of crf expression by the GR.42
A role for Methyl-CpG-binding protein 2 (MeCP2)
The mechanism whereby DNA-bound GR represses PVN crf is not fully understood; yet, recent in vitro evidence suggests that GR induces methylation in the crf promoter to facilitate transcriptional repression.43 Hypermethylation of the crf promoter may allow methyl-CpG binding domain (MBD) proteins to bind and recruit unique sets of co-repressors, such as histone deacetylases to the promoter.44 A number of MBD proteins have been identified (Methyl-CpG-binding proteins 1 and 2 (MeCP1 and MeCP2) and MBD1–4), and all but MBD3 specifically bind to methylated DNA in vitro and in vivo.44 Most of these MBD proteins could contribute to transcriptional repression of the crf gene; however, MeCP2 is notable because loss-of-function mutations in MeCP2 cause Rett Syndrome, a severe neurodevelopmental disorder characterized by an abnormal stress response.45 Unlike MeCP1, which has a high affinity for densely methylated DNA, MeCP2 can bind singly methylated CpG sites, such as those present in the crf promoter.46 Additionally, MeCP2 has been implicated in crf transcriptional repression in vivo since increased PVN CRF mRNA was shown in mice expressing a mutated MeCP2 protein.46 Concurrently, GR agonist administration increased MeCP2 occupancy of the crf promoter in hypothalamic cells in vitro, where it presumably interacted with co-repressors to decrease transcription.43 Together these studies point to significant involvement of MeCP2 in crf regulation by GR, but this remains to be directly demonstrated. Further investigation will greatly advance our understanding of the mechanisms controlling PVN CRF expression and HPA axis activity.
Conclusion
The neuropeptide CRF is an essential mediator of the neuroendocrine processes that facilitate body wide coping with various types of stressors. In response to stress, CRF not only activates the HPA axis to drive the hormonal stress response, but it also coordinates autonomic, behavioral and metabolic reactions.4 In this section we particularly highlighted a role for CRF in brain regions outside the PVN (i.e. the bNST, CeA and DMV) in driving the cardiovascular responses to physical and psychological stressors. Even though the mechanisms through which CRF exerts its effects on cardiovascular function are not fully understood, the indispensible role CRF plays in cardiovascular responses to stressors is undeniable.
CRF is also an important regulator of the hormonal stress response, as it is a target of feedback regulation by glucococorticoids.34 Glucocorticoids produced by the HPA axis in response to stressors feed back to act on numerous brain regions that express CRF.4 Yet, PVN CRF is a particularly significant target, as it is the central regulator of the HPA axis.4 Without CRF mediating glucocorticoid feedback inhibition, the activity of the HPA axis would largely go unrestrained and result in severe pathological consequences.6 Thus, understanding how PVN CRF may limit HPA activation certainly warrants further investigation.
Ultimately, these diverse examples highlight the significance of CRF in neuroendocrine mechanisms that control numerous aspects of stress physiology.
Control of reproduction by GnRH
The neuropeptide GnRH is the pivotal regulator of the endocrine network that drives reproductive function. This network, the HPG axis, is activated by GnRH synthesis in the medial preoptic area and arcute nucleus of the hypothalamus.47 Following its release from the hypothalamus into the median eminence, GnRH stimulates the synthesis of the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary.47 These gonadotropins are released from the pituitary in response to GnRH pulses during the reproductive cycle and act on the gonads to regulate steroid synthesis and gamete production.47 In females, FSH prompts the growth and maturation of ovarian follicles, and stimulates estrogen secretion; LH induces ovulation, formation of the corpus luteum, and stimulates progesterone secretion.48 In males, on the other hand, FSH and LH stimulate spermatogenesis and androgen secretion respectively.49 Ultimately, GnRH, via its control of the HPG axis, is an important mediator of both direct and indirect neuroendocrine regulation of reproductive function as will be demonstrated in the following examples.
Direct neuroendocrine regulation by GnRH
GnRH exerts its effects on reproductive functions and behaviors by acting through a receptor that belongs to a large family of G Protein-Coupled receptors (GPCRs).49 This GnRH receptor has seven transmembrane domains and responds to extracellular cues by triggering a cascade of intracellular events to alter neuroendocrine processes such as those regulating reproductive function.49 Studies in the last 40 years have shown that in addition to its presence in the pituitary gonadotroph cells, the GnRH receptor is present in numerous extrapituitary tissues.50 Reflecting the diverse expression pattern of the GnRH receptor, GnRH peptide has been shown to exert functions outside of stimulating the production of LH and FSH. For instance, GnRH plays an antitumorigenic role in reproduction associated cancer biology, and regultates cardiac contractile function5,51
A GnRH metabolite complicates what we know about GnRH biology
GnRH neuroendocrinology is complicated by the production of a GnRH metabolite that functions independently of the parent peptide.52 Full-length GnRH is enzymatically metabolized by the zinc metalloendopeptidase EC3.4.24.15 (EP24.15) in brain and peripheral tissues to generate a pentapeptide, GnRH-(1–5). This metabolite is biologically active by binding the novel GPCRs, GPR173 and GPR101 and may contribute to the normal migration of GnRH neurons during development as well as cancer biology.53,54 Thus, GnRH-(1–5) signaling is a topic of great interest because altered GnRH neuronal migration can lead to multiple pathologies including delayed pubertal onset and infertility.55 Additionally, GnRH-(1–5) signaling may increase risk for cancers of the reproductive system.56 In this subsection, therefore, we detail advances in our understanding of GnRH-(1–5) signaling in GnRH neuronal migration during development and cancers.
GnRH-(1–5) signaling and the migration of developing GnRH neurons
During development, GnRH-secreting neurons originate outside the central nervous system in the nasal region and migrate along the vomeronasal tract traversing the cribiform plate to target the basal forebrain.57 As these neurons populate the basal forebrain, they send their nerve terminals to the median eminence where, eventually, GnRH is released into the hypophyseal portal system from hypothalamic neurons in a pulsatile manner.
Recent evidence suggests that GnRH-(1–5) binding to GPR173, in particular, is important for regulating GnRH neuronal migration during development. In an immature GnRH neuronal cell line, GN11, GnRH-(1–5) activation of GPR173 inhibited neuronal migration as measured by the in vitro wound closure assay and Boyden chamber assay.53 The wound closure assay involves creating a scratch in a cell monolayer and examining the rate at which cells migrate to close the scratch and return to their preferred state. The Boyden chamber assay, alternatively, is a filter membrane migration assay in which the number of cells that pass through a microporous membrane to enter a compartment containing chemoattractants is examined. Accordingly, GnRH-(1–5) activation of GPR173 inhibited the migration of GN11 neurons as evidenced by decreased migration into a wound as well as through a microporous membrane. Furthermore, GnRH-(1–5) binding to GPR173 recruited β-arrestin and phosphatase and tensin homolog (PTEN) as adaptor proteins to inhibit the phosphorylation of signal transducer and activator of transcription 3 (STAT3) leading to decreased migration.58 Thus, GnRH-(1–5) may modulate the migration rate of GnRH neurons into the basal forebrain during development assuring that they are positioned properly to make and receive appropriate connections.
GnRH-(1–5) signaling and cancers of the reproductive system
Whereas antiproliferative effects of the full-length GnRH have been reported,5 proliferative effects of GnRH-(1–5) on endometrial cancer cell lines have also been found.56 The effect of GnRH-(1–5) on these cells is mediated by GPR101, which stimulates phosphorylation of the epidermal growth factor receptor (EGFR) to enhance cell proliferation, as measured by increased cellular migration in an in vitro wound closure assay.56 To phosphorylate EGFR, GnRH-(1–5) binds to GPR101 and mobilizes matrix metalloproteinase 9, which cleaves membrane bound epidermal growth factor (EGF).56 Although GnRH-(1–5) signaling through GPR101 has a known role in cancer biology, its normal physiological functions remain under investigated. Exploration of GnRH-(1–5) signaling through GPR101 in the brain especially will be important, since GPR101 is expressed in many brain regions and has been implicated in numerous neuroendocrine-related functions.59
Indirect neuroendocrine regulation by GnRH
GnRH also alters reproductive function indirectly via its regulation of the production of gonadotropins, FSH and LH. This subsection will discuss how FSH may regulate ovarian cell fate, thereby highlighting an indirect role for GnRH in the control of reproductive processes.
Neuroendocrine regulation of granulosa cell fate
Studies on the neuroendocrine mechansims governing reproductive function have long emphasized a role for the ovary, whose gamete and hormone production are tightly controlled by the nervous system. The ovary is partially controlled by the brain via its regulation of the HPG axis and production of the pituitary hormones FSH and LH.60 To complement the actions of pituitary hormones, fibers within the sympathetic nervous system also form connections between the hypothalamus and the ovary.61 Sympathetic nerve fibers are found in most areas of the ovary. However, granulosa cells of the ovarian follicles are an interesting exception, since nerve fibers do not cross the basal lamina between the theca and granulosa cell compartments.62 Thus, pituitary hormones may play an especially predominant regulatory role in the granulosa cells.
The granulosa cell compartment of ovarian follicles hosts the oocyte and changes in size and function dramatically during follicular development and growth.62 This follicular development process, or folliculogenesis, involves the recruitment of a primordial follicle which grows and develops into a specialized preovulatory follicle that contains a fluid-filled antrum.62 Importantly, most ovarian follicles never reach the preovulatory stage. Instead, they undergo follicular atresia, which involves death and degeneration of granulosa cells and the oocyte.62 Because FSH promotes granulosa cell division and subsequent growth of the follicle, this follicular atresia largely occurs during stages with declining FSH concentrations.63 Thus, FSH is a key determinant in the life and death of ovarian follicles, but underlying mechanisms are not fully understood.
The growth stimulating actions of FSH in the avascular compartment of the ovarian follicle may be mediated by a cholinergic system, which has been identified in granulosa cells and implicated in the regulation of their fate (Figure 1).64,65 Evidence suggests that FSH increases activity of this cholinergic system to promote growth in granulosa cells.66 Thus, an indirect neuroendocrine process in which GnRH regulates FSH, and the ovarian cholinergic system, in turn, may be important for controlling granulosa cell fate.
Figure 1.
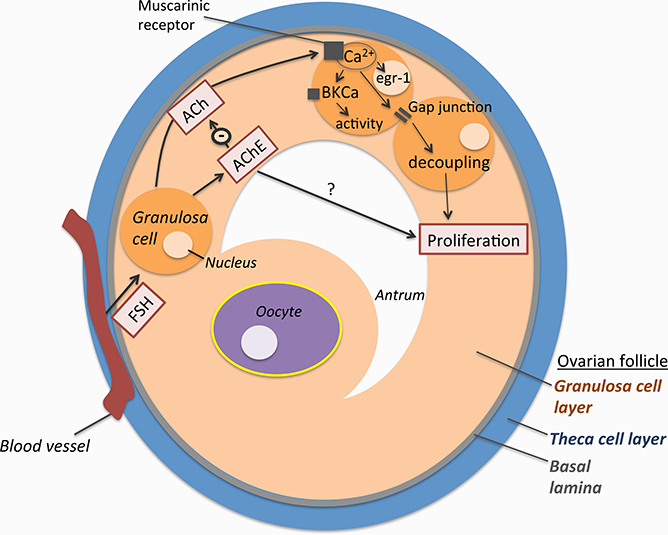
The ovarian follicle contains two main cellular compartments: the granulosa cell layer and the theca cell layer. These compartments are separated by the basal lamina such that blood vessels only contact the theca cells and release FSH there. Upon diffusion, FSH can influence the function of granulosa cells by binding their FSH receptors. FSH acts on granulosa cells to increase the activity of the non-neuronal cholinergic system of the ovarian follicle. Within this system, ACh acts through its muscarinic receptors to elevate intracellular calcium which leads to transcription factor changes, breakdown of gap junction communication, and activation of ion channels. Together, these actions promote growth in granulosa cells. AChE inactivates ACh and thereby limits its actions.
The ovarian cholinergic system and control of granulosa cell fate
Although acetylcholine (ACh) is classically known for its role in the nervous system, recent studies found that granulosa cells also express the ACh synthesizing enzyme (choline acetyltransferase (ChAT)), produce ACh, and express functional muscarinic ACh receptors.65,67,68 The functions of this ovarian, non-cholinergic system were studied in cultured human, in vitro-fertilization (IVF) derived granulosa cells. Experiments found muscarinic receptor-mediated elevations in intracellular Ca2, transcription factor changes, breakdown of gap junction communication, and activation of ion channels.67,69–72 Collectively, these findings suggested that the ovarian cholinergic system mediates trophic, growth-promoting actions in granulosa cells.
Granulosa cells of the human follicle also express the enzyme ACh-esterase (AChE), which inactivates ACh and thus limits its actions.73 AChE inhibition elevated ACh and thereby enhanced survival of cultured IVF-derived granulosa cells.74 Several splice variants of AChE were detected in human granulosa cells, including AChE-R, which has been associated with enzymatic and other, non-enzymatic actions.75 Non-enzymatic roles are due to a unique C-terminal sequence of this AChE splice variant. In granulosa cells administered a synthetic AChE-R peptide (ARP), a striking form of cell death was evident.74 This cell death, deemed regulated necrosis, or necroptosis, involved activation of specific kinases and was distinct from classical apoptosis (see review76).74 Importantly, clear signs of this necroptosis have also been observed in the human and nonhuman primate ovary, supporting its physiological relevance.74
As is the case for humans, AChE inhibition promotes cell growth in rodents and nonhuman primates. In the rat, ovarian ACh and AChE were readily found, and application of the AChE inhibitor Huperzine A, to the ovarian bursa increased follicle growth, ovulation and overall fertility.77 Similarly, Huperzine A treatment of 3D-cultures of monkey ovarian follicles improved growth of small follicles associated with estradiol production (Mayerhofer unpublished data). To inhibit AChE in all of the species examined, Huperzine A acts by binding an allosteric site on AChE to induce a conformational change in its active site and prevent ACh binding and degradation.
A potential role for FSH
Indirect evidence suggests that FSH increases activity of the non-neuronal cholinergic system to promote growth in granulosa cells. For one, the ACh biosynthesis enzyme, choline-acetyltransferase (ChAT), was expressed only during follicular stages that depended on the activity of FSH in mice, rats, and monkeys.65,66 Additionally, cells derived from antral rat follicles that express the FSH receptor, had increased ACh production when treated with FSH.66 Thus, an indirect neuroendocrine process in which GnRH regulates FSH may be important for controlling granulosa cell growth.
Conclusion
The neuropeptide GnRH is an essential mediator of the neuroendocrine mechanisms underlying various reproduction related functions. In addition to its regulatory role in the HPG axis, GnRH has been shown to be important for GnRH neuronal migration during development, as well as for cancers of the reproductive system, via the production of its metabolite GnRH-(1,5). Furthermore, recent studies support indirect GnRH involvement in the control of granulosa cell fate. GnRH stimulates the production of FSH by the anterior pituitary, which likely acts on an ovarian cholingeric system to determine whether ovarian follicles live to reach the preovulatory stage. These examples of GnRH regulation of neuroendocrine processes are admittedly diverse. Nevertheless, they emphasize the overall significance of GnRH for numerous aspects of reproductive physiology.
Steroid hormone regulation of sex preference behavior
One of the most obvious behavioral sex differences among animals is sexual partner preference. Males usually select female partners, while sexually receptive females regularly prefer males.78 However, in several species, including humans, there are male individuals that spontaneously prefer same-sex interactions.79,80 Given these differences, it has been asked whether a neuroendocrinology-mediated process determines sex preference in animals and, even further, if such a mechanism is responsible for human sexual orientation. The answers to these questions are highly controversial. Technical issues preclude the analysis of putative prenatal changes in hypothalamic releasing factors, gonadotropins, and gonadal steroids in animal fetuses that may result in same-sex preference in adulthood. In addition, no endocrine differences have been found between adult male or female homosexuals and their heterosexual counterparts.81 To date, most literature supporting a neuroendocrine hypothesis of sexual preference/orientation refers to the organizational effect of androgens, either directly or via their conversion to estrogens, in modeling the brain.82
In the following section, we provide evidence that sex preference is subject to a neuroendocrine process and that the brains of males that prefer other males differ from those that prefer females. Importantly, we emphasize caution in directly translating these findings to human sexuality.82
Aromatase inhibitors and sex preference
Sexual differentiation of the male brain relies on fetal/neonatal testosterone exposure, which may have direct effects and/or act via its conversion to estradiol, to virilize brain structures regulating sex preference, excitement and behavior.10 The aromatase enzyme, which converts testosterone to estradiol, therefore plays a critical role in promoting sexual differentiation druing critical periods in male rats.83 Studies examining effects of the aromatase enzyme on sex preference behavior have often used the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) to demonstrate that aromatase blockade increases same-sex preference in males.84 However, ATD is not the most selective blocker of aromatase activity, as it is a steroidal compound that can also bind androgen, estrogen and progesterone receptors to potentially influence sex preference.85 Thus, a new third-generation non-steroidal compound, letrozole, fulfills the need for a selective aromatase inhibitor.86
Sex preference can be experimentally altered with letrozole
In rats, the effects of prenatal exposure to letrozole on sexual behavior and partner preference in adulthood have been examined. One study demonstrated that, in male rats, prenatal letrozole decreased male sexual behavior (i.e. recognizing and mounting a receptive female) and increased female typical sexual behavior, such as lordosis, a posture for sexual receptivity.87 Another study similarly showed that prenatal letrozole treatment induced same sex preference in a population (about 30%) of adult male rats.88 The letrozole treated males that preferred other males in this study not only exhibited female sexual behavior (e.g. lordosis when mounted by other males (56%), but they also retained their masculine sexual behavior (i.e. mounts, intromissions and ejacluations) when exposed to sexually receptive females (100%). Collectively, these findings support the possibility that, in males with same sex preference, the brain areas important for sex preference, but not masculine sexual behavior, are demasculinized or even feminized. 89
Brain areas regulating sex preference
Both the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the suprachiasmatic nucleus (SCN) are hypothalamic brain areas that have been associated with sexual preference. Yet, the best examined of the two is the SDN-POA. Destruction of the SDN-POA in male rats and its counterpart in ferrets reverses sexual preferences.90 Additionally, in sheep, differences exist in the size of an ovine SDN (oSDN) that may underlie same sex preference. A subpopulation of domestic rams has smaller oSDNs and shows a sexual preference for other rams, even when they can choose between another ram and a receptive ewe.91 In rams and rodents, the size of the SDN is under the influence of testosterone or estradiol during development, which may explain differences in adult sex preference.92,93
Whether morphological differences underlying sex preference exist in the human hypothalamus that are similar to those described in animals is still under debate. However, support for such morphological differences comes from studies of the interstitial nuclei of the human anterior hypothalamus (INAH1–4) that are considered to be similar to the SDN-POA of the rat.94 One INAH nucleus in particular, INAH3, has a smaller volume in homosexual men compared to that of heterosexuals, which is similar to that of women.95 Additionally, differences exist in the human SCN that are related to sex preference. Both the volume of the SCN and number of vasopressin (AVP) expressing neurons within it is higher in homosexual men than in heterosexual men.96
Aromatase blockade affects brain areas regulating sex preference
In rats, perinatal treatment with ATD, which reverses partner preference in adult males, has also been shown to reduce the volume of the SDN-POA97 and to increase the number of AVP+ neurons in the SCN.98 The effects of letrozole on morphological changes in the SDN and SCN have also been explored. Prenatal treatment with letraozole was found to reduce the volume of and cell number in both the SDN and SCN, suggesting that inhibition of aromatase produced anatomical changes in areas sensitive to estradiol during development. Unexpectedly, such reduction was not linked to a same-sex preference, as males with female or male preference had a similar decrease in the SDN and SCN cell number and volume.99 The discrepancy between these results and those of other experiments may be attributable to several factors including species, the rat strain, the aromatase inhibitor used and its timing of administration. These findings also stress the importance of measuring the size of these and other brain areas in males treated with aromatase inhibitors that retain female preference. Needless to mention, the use of rats with spontaneous same-sex preference may enlighten the possible role of these brain areas in partner preference.100 For the moment, the relation between SDN, SCN, AVP and same-sex preference continues to be an enigma.
Summary and Conclusion
The topics presented in this session of the IWNE highlight the essential involvement of neuropeptides and/or steroid hormones in the neuroendocrine mechanisms controlling stress, reproduction and behavior related functions. The first section of this review, therefore, examined the role of the neuropeptide CRF in stress physiology. Specifically, CRF’s essential roles in mediating cardiovascular responses to stress and in the negative feedback regulation of the HPA axis by glucocorticoids were emphasized. The second section then illustrated the importance of the neuropeptide GnRH for controlling various aspects of reproductive physiology. These included GnRH neuronal migration during development, cancers of the reproductive system, and, albeit indirectly, ovarian follicular growth. Lastly, the third section highlighted the significance of sex steroids during critical developmental windows in determining sex prefence behavior.
Collectively, the sections of this review illustrate the significance of molecular mediators in the dynamic, bidirectional interactions that occur between the endocrine and nervous systems. Without neuropeptides, such as CRF and GnRH, and sex steroids, the fine neuroendocrine control of many physiological functions would not occur. Thus, further study of the involvement of these molecular players in stress, reproduction and behavior related functions is certainly warranted.
Acknowledgements
This work was supported by the folllowing grants: C.C.C was supported by FAPESP grant # 2015/05922–9 and CNPq grant # 456405/2014–3; A.M. was supported by grants from the German Research Foundation (DFG), in part MA1080/19–2; and C.E.R. was supported by National Institutes of Health grant number R01OD011047. D.O.L would like to thank Dr. John Wu, Dr. Marcelo Paez-Pereda, and Dr. Michael Culler for their mentorship and constructive feedback. He would also like to note that he is no longer an employee at Ipsen Bioscience. A.M. would like to thank all colleagues, students and former students, who are or were involved in this line of work over the years, especially Lars Kunz and Hernan Lara. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of Ipsen Bioscience. Additionally, the authors declare that they have no conflicts of interest.
References
- 1.Schally AV, Gual C. Some Recollections of Early Clinical Studies on Hypothalamic Hormones: A Tale of a Successful International Collaboration. Endocrinologist. 2001;11:341–349. [Google Scholar]
- 2.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. [DOI] [PubMed] [Google Scholar]
- 4.Kovács KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33. [DOI] [PubMed] [Google Scholar]
- 5.Gründker C, Günthert AR, Westphalen S, Emons G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur J Endocrinol. 2002;146:1–14. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. [DOI] [PubMed] [Google Scholar]
- 7.Goel N, Bale TL. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology. 2008;149:6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenstein BD. Steroid hormone receptors in the brain. Trends Neurosci. 1978;1:4–6. [Google Scholar]
- 9.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy MM. The Two Faces of Estradiol: Effects on the Developing Brain. Neurosci. 2009;15:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb DH. On the distinction between physical and psychological stressors - A review of the evidence. Motiv Emot. 1979;3:51–61. [Google Scholar]
- 12.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. [DOI] [PubMed] [Google Scholar]
- 13.Palkovits M, Brownstein MJ, Vale W. Distribution of corticotropin-releasing factor in rat brain. Fed Proc. 1985;44:215–219. [PubMed] [Google Scholar]
- 14.Inda C, Armando NG, dos Santos Claro PA, Silberstein S. Endocrinology and the brain: corticotropin-releasing hormone signaling. Endocr Connect. 2017;6:R99–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: Evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 16.Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. [DOI] [PubMed] [Google Scholar]
- 17.Kregel KC, Overton JM, Seals DR, Tipton CM, Fisher LA. Cardiovascular responses to exercise in the rat: role of corticotropin-releasing factor. J Appl Physiol. 1990;68:561–567. [DOI] [PubMed] [Google Scholar]
- 18.Brown MR, Gray TS, Fisher LA. Corticotropin-releasing factor receptor antagonist: effects on the autonomic nervous system and cardiovascular function. Regul Pept. 1986;16:321–329. [DOI] [PubMed] [Google Scholar]
- 19.Nakamori T, Morimoto A, Murakami N. Effect of a central CRF antagonist on cardiovascular and thermoregulatory responses induced by stress or IL-1 beta. Am J Physiol. 1993;265:R834–9. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull AV, Kirkman E, Rothwell NJ, Little RA. The neuropeptide CRF is involved in the modulation of the baroreflex during hindlimb ischaemia in the anaesthetized rat. J Physiol. 1993;468:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J Physiol. 1993;460:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habib KE, Weld KP, Rice KC, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97:6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nijsen MJ, Croiset G, Diamant M, et al. Endogenous corticotropin-releasing hormone inhibits conditioned-fear-induced vagal activation in the rat. Eur J Pharmacol. 2000;389:89–98. [DOI] [PubMed] [Google Scholar]
- 24.Stiedl O, Meyer M, Jahn O, Ogren SO, Spiess J. Corticotropin-releasing factor receptor 1 and central heart rate regulation in mice during expression of conditioned fear. J Pharmacol Exp Ther. 2005;312:905. [DOI] [PubMed] [Google Scholar]
- 25.Youngblood K, Conti L, Printz MP. Central actions of corticotropin releasing factor on sensori-autonomic processing in normotensive and hypertensive rats. Brain Res. 1996;734:175–185. [PubMed] [Google Scholar]
- 26.Wood SK, Verhoeven RE, Savit AZ, Rice KC, Fischbach PS, Woods JH. Facilitation of cardiac vagal activity by CRF-R1 antagonists during swim stress in rats. Neuropsychopharmacology. 2006;31:2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown MR, Fisher LA, Webb V, Vale WW, Rivier JE. Corticotropin-releasing factor: a physiologic regulator of adrenal epinephrine secretion. Brain Res. 1985;328:355–357. [DOI] [PubMed] [Google Scholar]
- 28.Conti LH, Shannon MH, Murry JD, Printz MP. Repeated Restraint Stress-Induced Increase in Baroreceptor Reflex Sensitivity: Role of Corticotropin-Releasing Factor. Neuropeptides. 2001;35:71–81. [DOI] [PubMed] [Google Scholar]
- 29.Herbert J, Howes SR. Interactions between corticotropin-releasing factor and endogenous opiates on the cardioaccelerator, hypothermic, and corticoid responses to restraint in the rat. Peptides. 1993;14:145–152. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira LA, Almeida J, Benini R, Crestani CC. CRF1 and CRF2 receptors in the bed nucleus of the stria terminalis modulate the cardiovascular responses to acute restraint stress in rats. Pharmacol Res. 2015;95–96: 53–62. [DOI] [PubMed] [Google Scholar]
- 31.Wood SK, McFadden KV, Grigoriadis D, Bhatnagar S, Valentino RJ. Depressive and cardiovascular disease comorbidity in a rat model of social stress: A putative role for corticotropin-releasing factor. Psychopharmacology (Berl). 2012;222:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Cardin S, Martínez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. [DOI] [PubMed] [Google Scholar]
- 33.Porter K, Hayward LF. Stress-induced changes in c-Fos and corticotropin releasing hormone immunoreactivity in the amygdala of the spontaneously hypertensive rat. Behav Brain Res. 2011;216:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretz O, Reichardt HM, Schütz G, Bock R. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999;818:488–491. [DOI] [PubMed] [Google Scholar]
- 35.Albeck DS, Hastings NB, McEwen BS. Effects of adrenalectomy and type I or type II glucocorticoid receptor activation on AVP and CRH mRNA in the rat hypothalamus. Brain Res Mol Brain Res. 1994;26:129–134. [DOI] [PubMed] [Google Scholar]
- 36.Sawchenko PE. Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci. 1987;7:1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. [DOI] [PubMed] [Google Scholar]
- 38.Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–223. [DOI] [PubMed] [Google Scholar]
- 39.de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain Corticosteroid Receptor Balance in Health and Disease. Endocr Rev. 1998;19:269–301. [DOI] [PubMed] [Google Scholar]
- 40.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. [DOI] [PubMed] [Google Scholar]
- 41.Uht RM, McKelvy JF, Harrison RW, Bohn MC. Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res. 1988;19:405–411, 468–469. [DOI] [PubMed] [Google Scholar]
- 42.Malkoski SP, Dorin RI. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol Endocrinol. 1999;13:1629–1644. [DOI] [PubMed] [Google Scholar]
- 43.Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone Induces a Putative Repressor Complex and Chromatin Modifications in the CRH Promoter. Mol Endocrinol. 2013;27:1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. [DOI] [PubMed] [Google Scholar]
- 46.McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2006;103:18267–18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marques P, Skorupskaite K, Rozario KS, Anderson RA, George JT. Physiology of Gnrh and Gonadotropin Secretion. Endotext. 2000;1–31. [Google Scholar]
- 48.Howles CM. Role of LH and FSH in ovarian function. Mol Cellular Endocrinol. 2000;161:25–30. [DOI] [PubMed] [Google Scholar]
- 49.Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: tales of mice and men. Metabolism. 2017. [DOI] [PubMed] [Google Scholar]
- 50.Cheung LWT, Wong AST. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275:5479–5495. [DOI] [PubMed] [Google Scholar]
- 51.Dong F, Skinner DC, Wu TJ, Ren J. The Heart: A Novel Gonadotrophin-Releasing Hormone Target. J Neuroendocrinol. 2011;23:456–463. [DOI] [PubMed] [Google Scholar]
- 52.Walters K, Chin YP, Wu TJ. A processed metabolite of luteinizing hormone-releasing hormone has proliferative effects in endometrial cells. Am J Obstet Gynecol. 2007;196:33, e1–5 [DOI] [PubMed] [Google Scholar]
- 53.Larco DO, Cho-Clark M, Mani SK, Wu TJ. The metabolite GnRH-(1–5) inhibits the migration of immortalized GnRH neurons. Endocrinology. 2013;154:783–795. [DOI] [PubMed] [Google Scholar]
- 54.Cho-Clark M, Larco DO, Semsarzadeh NN, Vasta F, Mani SK, Wu TJ. GnRH-(1–5) Transactivates EGFR in Ishikawa Human Endometrial Cells via an Orphan G Protein-Coupled Receptor. Mol Endocrinol. 2014;28:80–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6:311–326. [DOI] [PubMed] [Google Scholar]
- 56.Cho-Clark M, Larco DO, Zahn BR, Mani SK, Wu TJ. GnRH-(1–5) activates matrix metallopeptidase-9 to release epidermal growth factor and promote cellular invasion. Mol Cell Endocrinol. 2015;415:114–125. [DOI] [PubMed] [Google Scholar]
- 57.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. [DOI] [PubMed] [Google Scholar]
- 58.Larco DO, Semsarzadeh NN, Cho-Clark M, Mani SK, Wu TJ. B-arrestin 2 is a mediator of GnRH-(1–5) signaling in immortalized GnRH neurons. Endocrinology. 2013;154:4726–4736. [DOI] [PubMed] [Google Scholar]
- 59.Trivellin G, Bjelobaba I, Daly AF, et al. Characterization of GPR101 transcript structure and expression patterns. J Mol Endocrinol. 2016;57:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christensen A, Bentley GE, Cabrera R, et al. Hormonal regulation of female reproduction. Horm Metab Res. 2012;44:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerendai I, Tóth IE, Boldogkoi Z, Medveczky I, Halász B. Neuronal labeling in the rat brain and spinal cord from the ovary using viral transneuronal tracing technique. Neuroendocrinology. 1998;68:244–256. [DOI] [PubMed] [Google Scholar]
- 62.Williams CJ, Erickson GF. Morphology and Physiology of the Ovary. 2000.
- 63.Son WY, Das M, Shalom-Paz E, Holzer H. Mechanisms of follicle selection and development. Minerva Ginecol. 2011;63:89–102. [PubMed] [Google Scholar]
- 64.Mayerhofer A, Fritz S. Ovarian acetylcholine and muscarinic receptors: Hints of a novel intrinsic ovarian regulatory system. Microsc Res Tech. 2002;59:503–508. [DOI] [PubMed] [Google Scholar]
- 65.Mayerhofer A, Kunz L. A non-neuronal cholinergic system of the ovarian follicle. Ann Anat. 2005;187:521–528. [DOI] [PubMed] [Google Scholar]
- 66.Mayerhofer A, Kunz L, Krieger A, et al. FSH regulates acetycholine production by ovarian granulosa cells. Reprod Biol Endocrinol. 2006;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritz S, Kunz L, Dimitrijevic N, Grünert R, Heiss C, Mayerhofer A. Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metab. 2002;87:1362–1367. [DOI] [PubMed] [Google Scholar]
- 68.English BA, Jones CK. Cholinergic Neurotransmission. In: Primer on the Autonomic Nervous System. 2012:71–74. [Google Scholar]
- 69.Fritz S, Föhr KJ, Boddien S, Berg U, Brucker C, Mayerhofer A. Functional and molecular characterization of a muscarinic receptor type and evidence for expression of choline-acetyltransferase and vesicular acetylcholine transporter in human granulosa-luteal cells. J Clin Endocrinol Metab. 1999;84:1744–1750. [DOI] [PubMed] [Google Scholar]
- 70.Fritz S, Wessler I, Breitling R, et al. Expression of muscarinic receptor types in the primate ovary and evidence for nonneuronal acetylcholine synthesis. J Clin Endocrinol Metab. 2001;86:349–354. [DOI] [PubMed] [Google Scholar]
- 71.Kunz L, Thalhammer A, Berg FD, Berg U, Duffy DM, Stouffer RL, Dissen GA, Ojeda SR, Mayerhofer A. Ca2+-activated, large conductance K+ channel in the ovary: identification, characterization, and functional involvement in steroidogenesis. J Clin Endocrinol Metab. 2002;87:5566–5574. [DOI] [PubMed] [Google Scholar]
- 72.Kunz L, Roggors C, Mayerhofer A. Ovarian acetylcholine and ovarian KCNQ channels: Insights into cellular regulatory systems of steroidogenic granulosa cells. Life Sci. 2007;80:2195–2198. [DOI] [PubMed] [Google Scholar]
- 73.Silver A Species variation in the distribution of cholinesterases in the ovary of the plains viscacha, cat, ferret, rabbit, rat, guinea-pig and roe deer. Histochem J. 1978;10:79–102. [DOI] [PubMed] [Google Scholar]
- 74.Blohberger J, Kunz L, Einwang D, et al. Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis. 2015;6:e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmermann M Neuronal AChE splice variants and their non-hydrolytic functions: Redefining a target of AChE inhibitors? Br J Pharmacol. 2013;170:953–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urra J, Blohberger J, Tiszavari M, Mayerhofer A, Lara HE. In vivo blockade of acetylcholinesterase increases intraovarian acetylcholine and enhances follicular development and fertility in the rat. Sci Rep. 2016;6:30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkins A, Roselli CE. The ram as a model for behavioral neuroendocrinology. Horm Behav. 2007;52:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poiani A Animal Homosexuality: A Biosocial Perspective. New York: Cambridge University Press; 2010. [Google Scholar]
- 80.Roselli CE, Reddy RC, Kaufman KR. The development of male-oriented behavior in rams. Front Neuroendocrinol. 2011;32:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savic I, Berglund H, Lindstrom P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci. 2005;102:7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olvera-Hernández S, Fernández-Guasti A. Perinatal administration of aromatase inhibitors in rodents as animal models of human male homosexuality: Similarities and differences. In: Advances in Neurobiology. 2015;10:381–406. [DOI] [PubMed] [Google Scholar]
- 83.Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: Dogma and beyond. Front Neurosci. 2012;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993;107:480–487. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan ME, McGinnis MY. Effects of ATD on male sexual behavior and androgen receptor binding: A reexamination of the aromatization hypothesis. Horm Behav. 1989;23:10–26. [DOI] [PubMed] [Google Scholar]
- 86.Dutta U, Pant K. Aromatase inhibitors: past, present and future in breast cancer therapy. Med Oncol. 2008;25:113–124. [DOI] [PubMed] [Google Scholar]
- 87.Gerardin DC, Pereira OC. Reproductive changes in male rats treated perinatally with an aromatase inhibitor. Pharmacol Biochem Behav. 2002;71:301–305. [DOI] [PubMed] [Google Scholar]
- 88.Olvera-Hernández S, Chavira R, Fernández-Guasti A. Prenatal letrozole produces a subpopulation of male rats with same-sex preference and arousal as well as female sexual behavior. Physiol Behav. 2015;139:403–411. [DOI] [PubMed] [Google Scholar]
- 89.Vreeburg JT, van der Vaart PD, van der Schoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74:375–382. [DOI] [PubMed] [Google Scholar]
- 90.Paredes RG, Tzschentke T, Nakach N. Lesions of the medial preoptic area/anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res. 1998;813:1–8. [DOI] [PubMed] [Google Scholar]
- 91.Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. [DOI] [PubMed] [Google Scholar]
- 92.Sickel MJ, McCarthy MM. Calbindin-d28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: Developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. [DOI] [PubMed] [Google Scholar]
- 93.Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. [DOI] [PubMed] [Google Scholar]
- 94.Byne W, Tobet S, Mattiace LA, et al. The Interstitial Nuclei of the Human Anterior Hypothalamus: An Investigation of Variation with Sex, Sexual Orientation, and HIV Status. Horm Behav. 2001;40:86–92. [DOI] [PubMed] [Google Scholar]
- 95.LeVay S A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. [DOI] [PubMed] [Google Scholar]
- 96.Swaab DF, Hofman MA. An enlarged suprachiasmatic nucleus in homosexual men. Brain Res. 1990;537:141–148. [DOI] [PubMed] [Google Scholar]
- 97.Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56:535–541. [DOI] [PubMed] [Google Scholar]
- 98.Swaab DF, Slob AK, Houtsmuller EJ, Brand T, Zhou JN. Increased number of vasopressin neurons in the suprachiasmatic nucleus (SCN) of “bisexual” adult male rats following perinatal treatment with the aromatase blocker ATD. Dev Brain Res. 1995;85:273–279. [DOI] [PubMed] [Google Scholar]
- 99.Olvera-Hernández S, Tapia-Rodríguez M, Swaab DF, Fernández-Guasti A. Prenatal administration of letrozole reduces SDN and SCN volume and cell number independent of partner preference in the male rat. Physiol Behav. 2017;171:61–68. [DOI] [PubMed] [Google Scholar]
- 100.García-Cárdenas N, Olvera-Hernández S, Gómez-Quintanar BN, Fernández-Guasti A. Male rats with same sex preference show high experimental anxiety and lack of anxiogenic-like effect of fluoxetine in the plus maze test. Pharmacol Biochem Behav. 2015;135:128–135. [DOI] [PubMed] [Google Scholar]


