Abstract
Background
The nonfluent/agrammatic variant of primary progressive aphasia (agPPA) is a heterogeneous diagnosis, wherein some individuals have apraxia of speech (AOS). When agPPA includes AOS, a tauopathy is likely the underlying pathology. Recently, [18F]AV-1451 was developed for the in vivo assessment of tau. In this study, we compare patterns of tau tracer uptake in agPPA patients, with and without apraxia of speech.
Methods
Nine agPPA patients (four without AOS) underwent tau-PET imaging with [18F]AV-1451. Uptake of [18F]AV-1451 was assessed as cortical to cerebellar crus ratios (SUVr) in cortical regions of interest measured using the MCALT atlas and compared voxel-wise in SPM12. Each patient was age- and sex-matched to three controls.
Results
agPPA without AOS showed uptake in the left frontal and temporal lobes, while agPPA with AOS showed uptake in the bilateral supplementary motor areas, frontal lobes, precuneus, and precentral gyrus, relative to controls. The left precentral gyrus had uptake in agPPA with AOS, relative to those without AOS.
Conclusions
This cross-sectional study suggests [18F]AV-1451 uptake in the precentral gyrus is implicated in AOS in agPPA.
Keywords: primary progressive aphasia, apraxia of speech, [18F]AV-1451, tau PET
Introduction
Under current consensus criteria (1), patients with the nonfluent/agrammatic variant of primary progressive aphasia (agPPA) (2, 3), may have either or both agrammatism and apraxia of speech (AOS). This inherent clinical heterogeneity is mirrored by pathological heterogeneity, more so than in other variants of PPA(4). Prior imaging and pathologic evidence suggest that patients who are solely agrammatic may, in fact, be a distinct entity from those who present with concomitant AOS. Research has demonstrated that the agrammatic variant of PPA without AOS was associated with TDP-43 pathology (5); however, there were associations with 4-repeat (4R) tau pathology [e.g. progressive supranuclear palsy (PSP) or a corticobasal (CBS) syndrome] when AOS was also present (6)(7). These findings demonstrate the importance of clear characterization of this patient population.
In vivo imaging of tau pathology, using [18F]AV-1451, may facilitate testing of hypotheses about the pathology underlying a given clinical presentation. A recent autoradiographic study demonstrated low-level [18F]AV-1451 uptake in the left temporal and left frontal lobes in a patient with agPPA without apraxia of speech (8). In another case series, a patient who presented with agPPA, but later met criteria for PSP, demonstrated tracer uptake in the frontal and temporal lobes and cerebellar dentate, with more prominent signal in the basal ganglia and midbrain; it is not mentioned whether the patient also had AOS (9). We have recently demonstrated [18F]AV-1451 uptake in the prefrontal cortex in a group of agPPA subjects (10), although it is unclear how patterns of uptake relate to the presence and absence of concomitant AOS.
Toward that end, the goal of the current study was to investigate differences in [18F]AV-1451 uptake patterns in agPPA patients, with and without AOS. We hypothesized that the patterns of [18F]AV-1451 uptake would differ between the two groups, supporting an association between area of tau tracer uptake and clinical presentation, possibly reflecting more robust binding of [18F]AV-1451 in agPPA with AOS, a likely 4R tauopathy, compared to agPPA without AOS, a possible TDP-43 proteinopathy.
Methods
Participants
The study was approved by the Mayo Clinic Institutional Review Board and written consent was given by all participants. Between February 2015 and September 2017, tau-PET imaging was collected from nine agPPA patients (four without AOS). All patients completed a 3.0 Tesla volumetric head MRI scan and an [18F]AV-1451 tau-PET scan and underwent neurological, speech, and language, evaluations. All patients were 1:3 age and sex-matched to 27 cognitively unimpaired individuals from the Mayo Clinic Study of Aging cohort. All controls were amyloid negative (global Pittsburgh Compound B PET ratio < 1.42) (11) and underwent [18F]AV-1451 tau-PET scans using the identical acquisition parameters to the patient cohort.
Clinical Data
All agPPA patients had a thorough neurological examination and did not meet criteria for another neurodegenerative disease. All patients completed a test of general cognition [Montreal Cognitive Assessment Battery (MOCA), with a score of 26 or above considered normal]. Non-verbal oral apraxia (NVOA) was assessed [a score ≤ 29 suggested the presence of NVOA (12)]. Several speech and language measures were administered, as previously reported (13–15). The Western Aphasia Battery aphasia quotient (WAB-AQ) served as a composite measure of global language ability (a score ≥ 93.8 was considered normal). Grammar was assessed by review of conversational speech and verbal and written picture descriptions. The Northwestern Anagram test (NAT), a non-speech sentence production task, was also administered (16). As previously described (13–15), judgments about motor speech abilities were based on spoken language tasks of the WAB and supplementary speech tasks (including speech alternating and sequential motion rates). AOS severity was rated on 0–4 scale (1 = mild; 4 = severe). Due to the sample size, descriptive rather than inferential statistics were utilized.
Neuroimaging Acquisition and Analysis
Neuroimaging acquisition parameters were identical to those previously reported (17). Briefly, all analyses were performed using two-compartment partial volume corrected tau-PET standard uptake value ratio (SUVr) images with the cerebellar crus grey matter reference region, using the MCALT atlas (https://www.nitrc.org/projects/mcalt/) in SPM12. The cohorts of agPPA patients without AOS (n = 4) and those with AOS (n = 5) were compared to their respective matched sub-group of controls. Age and sex were not included in the models, as comparisons were made between age and sex matched groups. The agPPA patients without AOS and those with AOS were directly compared to each other. Voxel-level comparisons were performed using SPM12, with results assessed at p < .001 (uncorrected) for the direct group comparison and p < .05 [false discovery rate (FDR) corrected] for the patient to control comparison, both with an extent-threshold of 50 voxels.
Results
Clinical Findings
Demographic and clinical data are summarized in Table 1. The agPPA patients without AOS (two female) had a median age of 64.5, median education of 13.5 years, median disease duration of 2.25 years, and median aphasia severity of 2.25 (consistent with median WAB AQ of 80.45 and NAT of 4). The agPPA patients with AOS (one female) had a median age of 68, median education of 16 years, median disease duration of 4 years, and median aphasia severity of 3 (consistent with median WAB AQ of 70.05 and NAT of 5). Median AOS severity was 1 (mild). MOCA scores were lower (worse) in the agPPA patients with AOS; three patients in each group were below the recommended cutoff on 26. All nine patients with agPPA, regardless of AOS, had NVOA.
Table 1.
Demographic, neurologic, speech, and language information for all patients.
| Sex | Age at Evaluation |
Years Since Onset |
Education (years) |
MOCA (/30) |
WAB- AQ (/100) |
Agrammatism in writing? |
Agrammatism in speaking? |
NAT (/10) |
NVOA (/32) |
Aphasia Severity (/4) |
Apraxia Severity (/4) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| agPPA without AOS | ||||||||||||
| 1 | F | 55 | 3 | 12 | 23 | 75.4 | Yes | Yes | 4 | 29 | 3 | 0 |
| 2 | F | 65 | 4.5 | 13 | 18 | 66.5 | Yes | Yes | DNT | 25 | 3 | 0 |
| 3 | M | 64 | 1.5 | 14 | 27 | 90 | Yes | Yes | 5 | 29 | 1 | 0 |
| 4 | M | 72 | 1.5 | 16 | 23 | 85.5 | Yes | No | 2 | 21 | 1.5 | 0 |
| agPPA with AOS | ||||||||||||
| 5* | M | 51 | 4.5 | 20 | DNT | DNT | Yes | Yes | 0 | 0 | 4 | 0.5 |
| 6 | M | 61 | 3 | 16 | 28 | 89 | Yes | No | 10 | 17 | 1.5 | 1 |
| 7 | M | 73 | 6 | 12 | 5 | 53.1 | Yes | Yes | DNT | 16 | 3 | 3 |
| 8 | F | 68 | 4 | 12 | 13 | 58.2 | Yes | Yes | DNT | 13 | 3 | 3 |
| 9 | M | 87 | 2 | 16 | 21 | 81.9 | Yes | Yes | 5 | 24 | 1.5 | 1 |
Note: *=Left handed; F=Female; M=Male; MOCA=Montreal Cognitive Assessment; WAB-AQ=Western Aphasia Battery Aphasia Quotient; NAT=Northwestern Anagram Test; NVOA=Non-Verbal Oral Apraxia; where appropriate, maximum score noted in column header; DNT=did not test.
[18F]AV-1451 findings
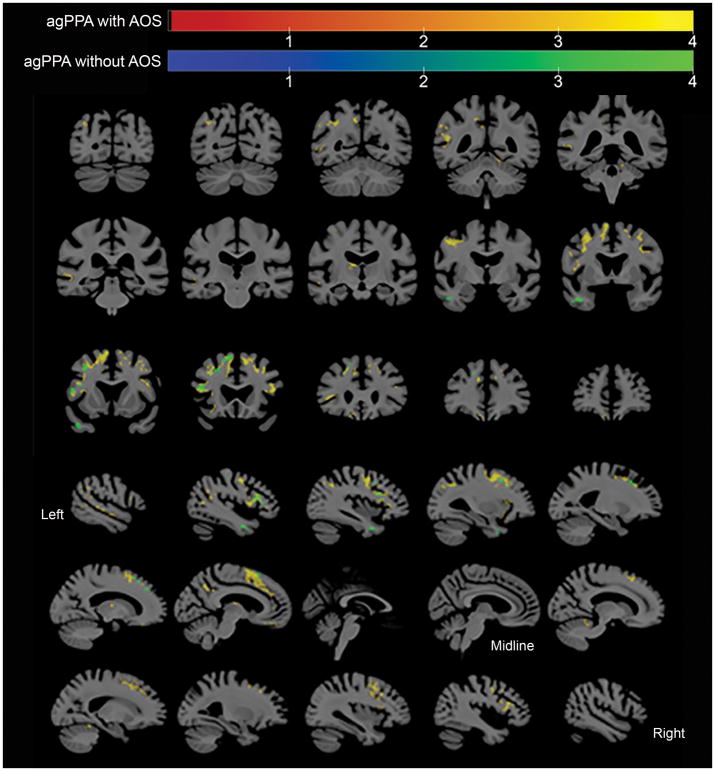
At the voxel-level, the agPPA without AOS cohort showed increased tau-PET uptake the left inferior temporal region, left inferior, middle and superior frontal gyri, and left supplementary motor area (SMA), relative to controls (Figure 1). The agPPA with AOS cohort showed bilateral but left greater than right increased tau-PET uptake in the SMA, inferior, middle and superior frontal gyri, precentral gyrus, and left precuneus, relative to controls (Figure 1). [18F]AV-1451 uptake was never greater in controls than in agPPA patients (with or without AOS).
Figure 1.
Regions where partial volume corrected AV-1451 tau PET SUVr was significantly larger (false discovery rate corrected, p < .05, extent > 50 voxels) in agPPA patients, with and without AOS, than in controls. Results visualized using MRIcroGL.
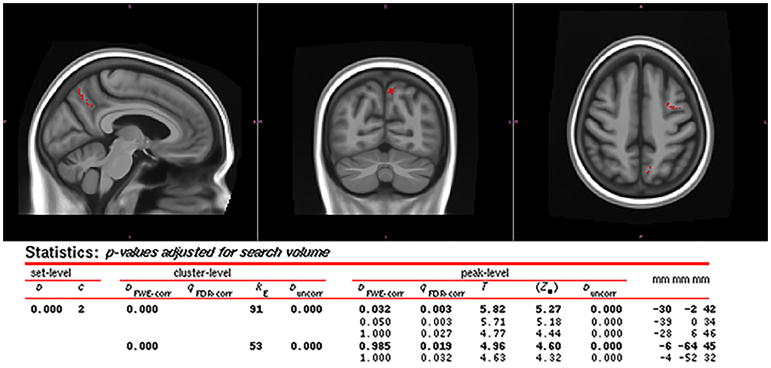
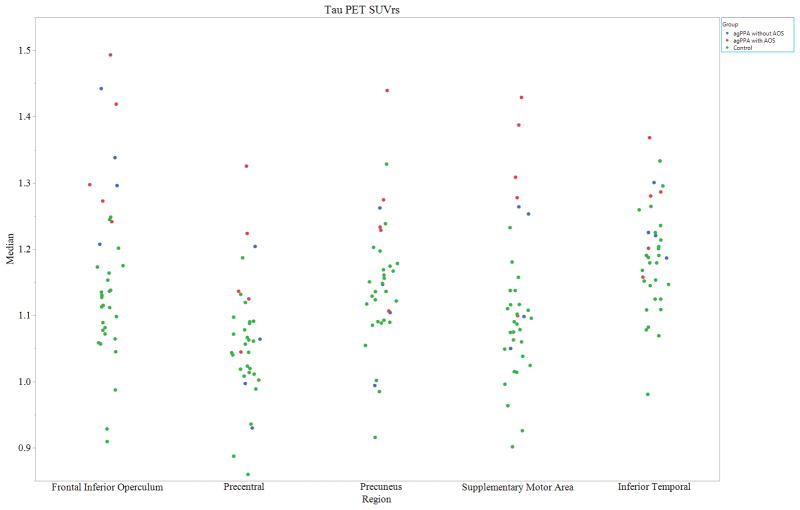
At the voxel-level, the agPPA with AOS cohort showed increased tau-PET uptake in the left precentral gyrus and left precuneus, relative to the agPPA without AOS cohort (Figure 2). [18F]AV-1451 uptake was never greater in agPPA patients without AOS than in agPPA patients with AOS. While region of interest (ROI) level analyses were not formally conducted, standard uptake value ratios (SUVR) were calculated for each participant for selected ROIs. This allowed for a qualitative assessment of whether individuals reflect the pattern seen in the group-level voxel analysis (Figure 3).
Figure 2.
Regions where partial volume corrected AV-1451 tau PET SUVr was significantly larger (uncorrected p < .001, extent > 50 voxels) in agPPA patients with AOS than in agPPA patients without AOS. SPM results included; coordinates correspond to the MCALT template (Schwarz et al., 2017) (https://www.nitrc.org/projects/mcalt/). Results visualized using fslview.
Figure 3.
Partial volume corrected (PVC) tau-PET standard uptake value ratios (SUVrs), normalized by the median uptake in the cerebellar crus gray matter to create SUVr images for left-sided regions of interest (y-axis = median). Controls are shown in green, agPPA without AOS are shown in blue, and agPPA with AOS are shown in Red.
Discussion
This study demonstrated that [18F]AV-1451 uptake was seen in patients with agPPA in regions known to be involved with speech and language, with differing patterns of tracer uptake relative to the presence of apraxia of speech. [18F]AV-1451 uptake was observed in the left precentral gyrus and left precuneus in agPPA when AOS was also present, compared to agPPA alone. This is similar to previous research, where [18F]AV-1451 uptake in the precentral gyrus has been demonstrated in a patient with a dominant apraxia of speech and aphasia, who ultimately had corticobasal degeneration pathology at autopsy (18). Additionally, the precentral gyrus was implicated in stroked-induced pure AOS, with additional areas of involvement accounting for concomitant aphasia (19); importantly, the speech and language network may respond differently to a degenerative process rather than a focal event, such as stroke. [18F]AV-1451 uptake in the precuneus was previously associated with cognitive impairment associated with Alzheimer’s disease, Parkinson’s disease, and Lewy Body dementia (20). While we did not compute correlations, there were overall lower MOCA scores in the group with higher [18F]AV-1451 uptake in the precuneus (i.e. agPPA with AOS). It is possible tracer uptake in this region is associated with longer disease duration in the patients with AOS, and more widespread disease, but it does not appear associated with AOS severity. Importantly, when examining the individual data (Figure 3), it is noticeable that only one patient (Patient 8) has noticeably higher tracer uptake in the precuneus. It is possible this patient is driving this result; nonetheless, there are no clinical or imaging concerns that warrant excluding this participant.
When comparing the patient groups to controls, the results suggest [18F]AV-1451 uptake in the bilateral SMAs and left precentral gyrus is associated with AOS in the context of more severe aphasia (i.e. agPPA with AOS). Interestingly, this pattern of [18F]AV-1451 uptake is consistent with findings in patients with a predominant apraxia of speech and co-occurring aphasia (17). Whether the AOS or aphasia dominates, there is a consistent relationship between [18F]AV-1451 uptake and the clinical appearance; both presentations have been associated with an underlying 4R tauopathy (e.g. CBD or PSP).
There are limitations to the current study. While the sample size was small, it is relatively large in the study of [18F]AV-1451 uptake in agPPA. While the comparison of tau tracer uptake in agPPA with AOS to those without AOS did not survive stringent correction for multiple comparisons (i.e. FDR), a more stringent threshold (.001) was used to assess statistical significance. These findings lay the foundation for future hypothesis driven studies in agPPA. This study demonstrates the importance of clear characterization of participants for the study of this patient population. Confirmation of these cross-sectional findings longitudinally is necessary to support or reject their clinical significance. We hypothesize that patients with agPPA at initial presentation who develop AOS will demonstrate [18F]AV-1451 uptake in the precentral gyrus and have an underlying 4R tauopathy. Of course, past research suggests caution in interpreting results of [18F]AV-1451 uptake, in that there is weak binding with 4R tauopathies and TDP-43 (8). The [18F]AV-1451signal in agPPA without AOS is possibly off-target binding; the same is true, perhaps to a lesser extent, for the agPPA with AOS patients. A portion of these results could be attributable to artifact; nonetheless, their relationship to clinical correlates is impressive. Ultimately, autopsy confirmation will be needed to determine the root pathology in these patients.
In conclusion, there is a relationship between location of [18F]AV-1451 uptake and clinical presentation in agPPA. While a direct association between [18F]AV-1451 uptake in agPPA and pathology is unclear, [18F]AV-1451 uptake in the precentral gyrus is suggestive of the concomitant presence of AOS, suggestive of a 4R tauopathy.
Acknowledgments
Funding: The study was funded by NIH grants R21NS94684, R01DC12519, R01AG11378, and U01AG06786.
The study was funded by NIH grants R21 NS94684 (PI: Josephs), R01 DC014942 (PI: Josephs), R01 DC12519 (PI: Whitwell), R01 AG11378 (PI: Jack), and U01 AG06786 (PI: Petersen). We thank the patients and their families for their time and participation. We acknowledge AVID Radiopharmaceuticals for provision of AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Footnotes
Disclosure of conflict of interest
None.
References
- 1.Gorno-Tempini M, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11(6):592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49(4):425–32. [PubMed] [Google Scholar]
- 4.Harris JM, Gall C, Thompson JC, Richardson AMT, Neary D, du Plessis D, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832–9. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- 5.Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74(1):42–9. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 6.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nature reviews Neurology. 2010;6(2):88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–92. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe V, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4(1):58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, et al. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol. 2017;81(1):117–28. doi: 10.1002/ana.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Josephs KA, Martin PR, Botha H, Schwarz CG, Duffy JR, Clark HM, et al. [18F]AV-1451 tau-PET and primary progressive aphasia. Annals of Neurology. doi: 10.1002/ana.25183. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimer’s & Dementia. 2017;13(3):205–16. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botha HA, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KJ. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014;82(19):1729–35. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(5):1522–36. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81(4):337–45. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, et al. The evolution of primary progressive apraxia of speech. Brain. 2014;137(10):2783–95. doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. American journal of Alzheimer’s disease and other dementias. 2009;24(5):408–16. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utianski RL, Whitwell JL, Schwarz CG, Senjem ML, Tosakulwong N, Duffy JR, et al. Tau-PET imaging with [18F]AV-1451 in Primary Progressive Apraxia of Speech. Cortex. 2018;99:358–74. doi: 10.1016/j.cortex.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs KA, Whitwell J, Tacik P, Duffy J, Senjem M, Tosakulwong N, et al. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta neuropathologica. 2016:931–3. doi: 10.1007/s00401-016-1618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itabashi R, Nishio Y, Kataoka Y, Yazawa Y, Furui E, Matsuda M, et al. Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke. 2016;47(1):31–6. doi: 10.1161/STROKEAHA.115.010402. [DOI] [PubMed] [Google Scholar]
- 20.Gomperts SN, Locascio JJ, Makaretz S, Schultz A, Caso C, Vasdev N, et al. Tau Positron Emission Tomographic Imaging in the Lewy Body Diseases. JAMA Neurology. 2016;73(11):1334–41. doi: 10.1001/jamaneurol.2016.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]