Abstract
Background
A recent analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial-Lipid-Lowering Trial (ALLHAT-LLT) yielded inconclusive results regarding the use of pravastatin for primary prevention of coronary heart disease (CHD) in older adults. Restricted mean survival time (RMST), which summarizes treatment effect in terms of the event-free time in a fixed time period, may be more useful than hazard ratios for communication of treatment effect with older patients.
Design
Secondary analysis of the ALLHAT-LLT trial.
Setting
Ambulatory setting.
Participants
2,867 adults ≥65 years (mean age, 71 years; 49% female) free of cardiovascular disease.
Intervention
Pravastatin 40mg daily (n=1,467) vs usual care (n=1,400).
Measurements
We estimated the difference in RMSTs (95% confidence interval [CI]) for total and CHD-free survival between pravastatin and usual care groups over the 6-year trial period. We also used parametric survival models to estimate the RMST differences projected over 10 years.
Results
Over 6 years, patients treated with pravastatin lived, on average, 33.7 fewer days than usual-care patients (RMST: 2008.1 vs 2041.8 days; RMST difference, −33.7 days; 95% CI, −67.0 to −0.5; p=0.047). Pravastatin-treated patients lived, on average, 18.7 more days free of CHD over 6 years than usual-care patients, but this difference was not statistically significant (RMST: 2088.1 vs 2069.4 days; RMST difference, 18.7 days; 95% CI, −10.4 to 47.8; p=0.209). The 10-year projection showed that pravastatin-treated patients would live 108.1 fewer days (95% CI, −204.5 to −14.1; p=0.028) than usual-care patients, although treated patients would gain 77.9 days (95% CI, 3.8 to 159.6; p=0.046) of CHD-free survival.
Conclusion
RMST provides an intuitive and explicit way to express the effect of pravastatin therapy on CHD-free and overall survival in older adults free of cardiovascular disease. This measure allows a more personalized interpretation of the benefits and risks of a medical intervention for decision-making.
Keywords: Statins, prevention, cardiovascular disease, survival analysis
BACKGROUND
Interpreting treatment effect reported from a clinical trial is fundamental to shared decision-making about a medical treatment in older adults. Yet statistical measures that are routinely used in a clinical trial, such as hazard ratios (HRs) and p-values, provide little information about clinical importance of treatment effect. Since p-values depend on the magnitude of treatment effect as well as the study size and event rates, small p-values may not indicate a clinically important treatment effect; likewise, non-significant p-values should perhaps not be interpreted as evidence for no treatment effect.1–4 Despite popular use, the HR, which represents the ratio of two hazard rates and is commonly estimated using Cox proportional hazards model, is not very intuitive and is subject to misinterpretation.5,6 Without knowing the hazard in the control group, it is difficult to assess how a relative reduction in hazard due to the treatment translates into a clinically measurable benefit or harm. Interpretation becomes more difficult when the ratio of two hazards is not constant over the study period (i.e., HR changes over time).
An alternative way to summarize treatment effect is to compare the median or mean survival time (time from baseline to the event) between the two treatment groups. The median survival time corresponds to the time during the follow-up at which 50% of the population has developed the event. While intuitive, the median survival time is insensitive to long-term survivors and may not be observed from data due to insufficient follow-up. The mean survival time difference can be interpreted as a gain or loss in the average survival time due to the treatment. However, the mean survival time cannot be estimated in most clinical trials due to censoring (i.e., survival time is unknown for patients who do not experience the event by the end of study follow-up). Instead, the restricted mean survival time (RMST) can be obtained by calculating the mean survival time up to the end of follow-up or a clinically meaningful time point within the study duration.7 Graphically, RMST corresponds to the area under the survival curve up to the time point. The difference in RMST is a useful measure of treatment effect that can be interpreted as a gain or loss in the event-free survival time in the specified period due to the treatment.5–12
In this paper, we demonstrate how RMST analysis can enhance clinical interpretation of a clinical trial of pravastatin vs usual care for the primary prevention of coronary heart disease (CHD) in older populations. This topic is particularly relevant as the benefit of statins for primary prevention in older adults is controversial.13–16 In a recent post-hoc analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial—Lipid-Lowering Trial (ALLHAT-LLT) primary prevention cohort,13 there was no statistically significant reduction in CHD in those randomized to pravastatin vs usual care (HR: 0.81; 95% confidence interval [CI], 0.63 to 1.05; p=0.12), with a non-significant increase in mortality (HR: 1.18; 95% CI, 0.97 to 1.42; p=0.09) over 6 years. Although the authors concluded that “no benefit was found… for primary prevention … and a non-significant direction toward increased all-cause mortality was observed…”,13 the wide CI reflects a lack of precision and large uncertainty regarding the true benefit or harm of pravastatin. It does not exclude a possibility of benefit (up to 37% CHD reduction) or harm (up to 42% mortality increase) of pravastatin due to wide CIs of HRs, which depend on the number of events. The clinical importance of such relative risk reduction or increase can be ambiguous to clinicians and patients. Therefore, we reanalyzed the ALLHAT-LLT data using RMST to enhance clinical interpretation of the effect of pravastatin therapy vs usual care for primary prevention in older adults.
METHODS
Data Source
The ALLHAT-LLT was a randomized, open-label, controlled trial of pravastatin vs usual care in adults with hypertension and additional CHD risk factors that was conducted between 1994 and 2002 in the United States. Details of study design and procedures are available elsewhere.17 The ALLHAT-LLT primary prevention post-hoc analysis included 2,867 adults ≥65 years (mean age, 71 years; 49% female; 90% taking antihypertensive medications; 51% with type 2 diabetes; mean low-density lipoprotein cholesterol, 148 mg/dl) and free of cardiovascular disease at baseline who were randomized to pravastatin 40mg daily (n=1,467) or usual care (n=1,400).13 The primary outcome was all-cause mortality and the secondary outcome was CHD events (fatal and nonfatal CHD). The mean follow-up duration (standard deviation) was 4.55 (1.60) years in the pravastatin group and 4.66 (1.58) years in the usual care group.13 Since we reconstructed individual patient-level data from the published survival curves18 to demonstrate RMST analysis, Institutional Review Board approval was not necessary.
RMST Analysis
We calculated RMSTs from baseline to 6 years (end of study follow-up) in each treatment group for all-cause mortality and for CHD events, representing the average survival time and the average CHD-free survival time over 6 years, respectively. For each outcome, treatment effect was summarized by calculating RMST difference between the pravastatin group and the usual care group at 6 years using the established estimation procedure.6–8 We used “surv2sampleComp” package in R software to estimate RMST difference (R code and reconstructed datasets are provided in the Supplementary Appendix S1). The RMST difference is interpreted as the number of event-free days gained or lost in the next 6 years due to pravastatin therapy relative to usual care. To estimate the long-term effect of pravastatin therapy, we fitted parametric Weibull survival models to estimate the RMST difference at 10 years. All analyses were performed in R software version 3.4.
RESULTS
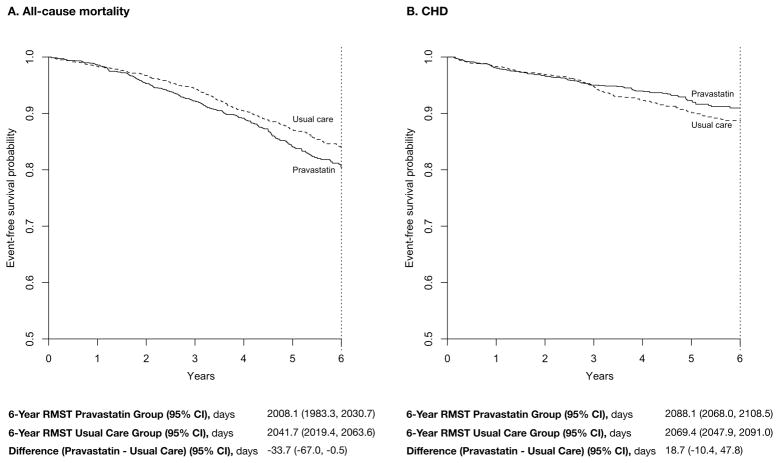
Over 6 years, individuals who were randomized to pravastatin lived, on average, 2008.1 days; this was statistically significantly shorter by 33.7 days (95% CI, −67.0 to −0.5; p=0.047) than usual-care patients who lived, on average, 2041.8 days (Table and Figure Panel A). The 95% CI suggests that the 6-year RMST difference could be as large as 67.0 days, which means that pravastatin-treated patients may live 67 days shorter than usual-care patients over 6 years. The 6-year CHD-free mean survival time was longer by 18.7 days (95% CI, −10.4 to 47.8) in those assigned to pravastatin than in usual-care patients (2088.1 vs 2069.4 days), but this was not statistically significant (p=0.209) (Table and Figure Panel B). Based on the 95% CI, pravastatin-treated patients may live free of CHD events 10.4-days shorter to 47.8-days longer than usual-care patients.
Table.
Restricted Mean Survival Time to Interpret the Effect of Pravastatin vs Usual Care in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial—Lipid-Lowering Trial Primary Prevention Cohort
| Outcome | Pravastatin (95% CI) |
Usual Care (95% CI) |
Difference (95% CI) |
Interpretation |
|---|---|---|---|---|
| Overall survival | N=1466 | N=1400 | ||
| 6-y RMST, d | 2008.1 (1984.6, 2030.9) |
2041.8 (2019.3, 2063.5) |
−33.7 (−67.0, −0.5) |
Pravastatin-treated patients lived, on average, 33.7 fewer days (67.0 to 0.5 fewer days) than usual-care patients over 6 years (p=0.047). |
| 10-y RMST,a d | 3061.8 (2991.0, 3132.8) |
3169.9 (3095.7, 3234.5) |
−108.1 (−204.5, −14.1) |
Pravastatin-treated patients lived, on average, 108.1 fewer days (204.5 to 14.1 fewer days) than usual-care patients over 10 years (p=0.028). |
|
| ||||
| CHD-free survival | N=1543 | N=1387 | ||
| 6-y RMST, d | 2088.1 (2064.8, 2107.3) |
2069.4 (2048.5, 2090.3) |
18.7 (−10.4, 47.8) |
Pravastatin-treated patients lived, on average, 18.7 more days (10.4 fewer days to 47.8 more days) free of CHD than usual-care patients over 6 years (p=0.209). |
| 10-y RMST,a d | 3378.6 (3323.9, 3431.3) |
3300.7 (3239.3, 3359.2) |
77.9 (3.8, 159.6) |
Pravastatin-treated patients lived, on average, 77.9 more days (3.8 to 159.6 more days) free of CHD than usual-care patients over 10 years (p=0.046). |
Abbreviations: CHD, coronary heart disease; d, days; RMST, restricted mean survival time; y, years.
The 10-year restricted mean survival time was estimated from parametric Weibull survival models that were fit based on the reconstructed data.
Figure. Restricted Mean Survival Time for Overall Survival (A) and Coronary Heart Disease-Free Survival (B) Over 6 Years.
The restricted mean survival time (RMST) represents the mean event-free survival time from the trial baseline to the trial end (6 years), which is heuristically equivalent to the area under the Kaplan-Meier curve from the beginning of the curve to vertical dotted line. The difference in RMST between the two groups indicates the number of days gained or lost in terms of overall survival (A) and coronary heart disease-free survival (B) due to 6 years of pravastatin therapy compared with usual care.
The 10-year RMST difference estimated from parametric models showed that pravastatin-treated individuals live 108.1 days shorter (95% CI, −204.5 to −14.1) than individuals treated with usual care, although the treated individuals gain 77.9 days (95% CI, 3.8 to 159.6) of CHD-free survival (Table).
DISCUSSION
The recent post-hoc analysis of ALLHAT-LLT left several unanswered questions about the efficacy and safety of pravastatin therapy in older adults without cardiovascular disease.13 Although the authors of the paper interpreted a HR of 0.81 (95% CI, 0.63 to 1.05; p=0.12) as evidence for no benefit of pravastatin therapy for primary prevention, the 95% CI included previously reported HRs in middle-aged19 and older adults.20 Conversely, the non-significant HR of 1.18 with a wide CI (95% CI, 0.97 to 1.42; p=0.09) for mortality suggests a lack of precision that does not exclude the possibility of harm from pravastatin therapy. As a result, clinicians and patients may not be satisfied with this interpretation.
RMST analysis is a useful approach that can improve clinical interpretability of HRs by placing the results on a time scale that can be intuitively understood.5–12 Our analysis of the ALLHAT-LLT primary prevention cohort using RMST showed that individuals treated with pravastatin could live free of CHD, on average, for an additional 18.7 days in 6 years, yet their overall survival was 33.7 days shorter than those who received usual care. Even if the cardiovascular benefit of pravastatin was projected to 10 years, the average gain in CHD-free survival was 77.9 days as opposed to an average loss of 108.1 days in overall survival. These results provide strong evidence that pravastatin therapy for primary prevention in older adults is unlikely to provide a clinically important survival benefit over 6 or 10 years. Our results also suggest that individuals with a remaining life expectancy of 10 years may gain CHD-free survival from pravastatin therapy, despite a reduction in overall survival; the clinical meaning of the magnitude of treatment effect and trade-off between CHD-free and overall survival will depend on one’s personal values. As such, summarizing treatment effect in terms of the number of event-free days within a pre-specified time frame offers a more explicit way to realize the risks and benefits of a treatment.
In addition to interpretability, RMST analysis has methodological advantages over HRs. In a Cox proportional hazards model,21 which is widely used to estimate HRs, a key assumption is that the ratio of the hazard rate in the treatment group to that in the control group is constant throughout the study follow-up (proportional hazards assumption). Violation of this assumption is common. In an analysis of 54 cancer clinical trials, evidence for non-proportionality was observed in 24%.22 In the ALLHAT-LLT post-hoc analysis, the cumulative incidence curves for CHD crossed between the two groups (Figure 2 in the published paper13), indicating non-proportionality. When this assumption is breached, a single HR is no longer an informative summary of treatment effect.23 In contrast to HRs, calculating a RMST difference does not require any model assumption. When the hazards are not proportional, RMST analysis has more statistical power, resulting in more precise estimates.6–8,11 One caveat of RMST analysis is that the time frame to compute RMST should be chosen a priori at the design stage of a clinical trial such that it represents a clinically meaningful time frame to evaluate the treatment benefit. In a post-hoc analysis, the end of trial follow-up can be used, as we have done in the ALLHAT-LLT analysis. A long-term projection beyond the trial duration is possible by fitting a parametric survival model, assuming that the parametric model provides a good approximation.
As clinicians are encouraged to engage in shared decision-making conversations with patients regarding the use of statins for primary prevention,24 clear communication of the benefit and risk is critical. HRs tend to exaggerate treatment effect and may misinform treatment decisions, which can be mitigated by presenting absolute rates.22,25 Some may be concerned that RMST difference tends to understate treatment effect when the majority of patients do not experience the event. The best way to present the evidence for clinical decision-making remains to be determined. To individualize a drug therapy in older adults, it is recommended to compare the time-to-benefit from a treatment with the patient’s life expectancy.26 However, time-to-benefit is not readily estimated from clinical trials. Further, the time-to-benefit, even if estimated, is often too uncertain to guide clinical decisions.27 RMST analysis that quantifies treatment effect within a pre-specified, clinically relevant time frame (e.g., 5 years for patients with intermediate prognosis and 10 years for patients with good prognosis) can be a useful alternative to the time-to-benefit estimation.
Our results need to be interpreted within the context of limitations of the original ALLHAT-LLT study. Analysis of the primary prevention cohort was not specified a priori. Open-label design might have resulted in differential changes in lifestyle factors and high rates of crossover (22–29%) between the two arms,13 which may explain the modest CHD reduction with pravastatin during the study period. We did not examine subgroups that might have a greater RMST gain, and this should be investigated in future research.
In conclusion, RMST provides an intuitive and explicit way to express the effect of pravastatin on CHD-free and overall survival for primary prevention in older adults. Compared with HRs, RMST allows a more personalized interpretation of the benefits and risks of treatment. Since RMST is intuitively interpreted and conveniently estimated using standard statistical software, we recommend that it be routinely reported in conjunction with HRs and absolute rates in clinical studies evaluating a medical intervention. Future research is warranted to investigate how RMST can improve communication between clinicians and patients and influence treatment choices.
Supplementary Material
Supplementary Appendix S1. RMST analysis in R software
Acknowledgments
Author Contributions:
Study concept and design: Wei, Kim.
Acquisition, analysis, and interpretation of data: Orkaby, Rich, Sun, Lux, Wei, Kim.
Drafting of the manuscript: Orkaby, Kim.
Critical revision of the manuscript for important intellectual content: Orkaby, Rich, Sun, Lux, Wei, Kim.
Statistical analysis: Kim, Sun.
Administrative, technical, or material support: Kim.
Study supervision: Wei, Kim.
Conflict of Interest Disclosures: None
Funding/Support:
Dr. Kim is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, the American Federation for Aging Research, the John A. Hartford Foundation, and Atlantic Philanthropies. He is also supported by the Boston Claude D. Pepper Older Americans Independence Center/Pilot and Exploratory Studies Core (P30AG031679) and Boston Roybal Center Pilot Award (P30AG048785).
Dr. Orkaby is supported in part by a career development award from the Boston Claude D. Pepper Older Americans Independence Center, National Institute on Aging (P30AG013679).
Role of the Funder/Sponsor: The funding sources had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication.
Funding Sources: Dr. Orkaby is supported in part by the Boston Claude D. Pepper Older Americans Independence Center, NIA grant P30-AG013679. Dr. Kim is supported by a Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, the American Federation for Aging Research, the John A. Hartford Foundation, and Atlantic Philanthropies. Funding sources have no role in study design; in the collection, analysis, and interpretation of data; in writing the report; and in the decision to submit the article for publication.
Footnotes
Conflict of Interest Disclosures: The authors report no relevant conflicts of interest.
Impact Statement: We certify that this work is novel because we demonstrate the usefulness of restricted mean survival time (as opposed to conventional hazard ratios and hypothesis testing using p-values) in interpreting the clinical benefit of pravastatin in older populations free of heart disease.
References
- 1.Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasserstein RL, Lazar NA. The ASA’s Statement on p-Values: Context, Process, and Purpose. Am Stat. 2016;70(2):129–133. [Google Scholar]
- 3.Hackshaw A, Kirkwood A. Interpreting and reporting clinical trials with results of borderline significance. BMJ. 2011;343:d3340. doi: 10.1136/bmj.d3340. [DOI] [PubMed] [Google Scholar]
- 4.Ioannidis JPA. The Proposal to Lower P Value Thresholds to .005. Jama. 2018;319(14):1429–1430. doi: 10.1001/jama.2018.1536. [DOI] [PubMed] [Google Scholar]
- 5.Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uno H, Wittes J, Fu H, et al. Alternatives to Hazard Ratios for Comparing the Efficacy or Safety of Therapies in Noninferiority Studies. Ann Intern Med. 2015;163(2):127–134. doi: 10.7326/M14-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L, Claggett B, Tian L, et al. On the restricted mean survival time curve in survival analysis. Biometrics. 2016;72(1):215–221. doi: 10.1111/biom.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Royston P, Parmar MK. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30(19):2409–2421. doi: 10.1002/sim.4274. [DOI] [PubMed] [Google Scholar]
- 9.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A’Hern RP. Restricted Mean Survival Time: An Obligatory End Point for Time-to-Event Analysis in Cancer Trials? J Clin Oncol. 2016;34(28):3474–3476. doi: 10.1200/JCO.2016.67.8045. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Uno H, Wei LJ. Restricted Mean Survival Time as a Measure to Interpret Clinical Trial Results. JAMA Cardiol. 2017;2(11):1179–1180. doi: 10.1001/jamacardio.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uno H, Claggett B, Tian L, et al. Adding A New Analytical Procedure With Clinical Interpretation in the Tool Box of Survival Analysis. Ann Oncol. 2018 doi: 10.1093/annonc/mdy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han BH, Sutin D, Williamson JD, et al. Effect of Statin Treatment vs Usual Care on Primary Cardiovascular Prevention Among Older Adults: The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):955–965. doi: 10.1001/jamainternmed.2017.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurwitz JH, Go AS, Fortmann SP. Statins for Primary Prevention in Older Adults: Uncertainty and the Need for More Evidence. Jama. 2016;316(19):1971–1972. doi: 10.1001/jama.2016.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orkaby AR, Gaziano JM, Djousse L, Driver JA. Statins for Primary Prevention of Cardiovascular Events and Mortality in Older Men. J Am Geriatr Soc. 2017;65(11):2362–2368. doi: 10.1111/jgs.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich MW, Pieper CF. Statins for Primary Prevention in Older Adults: An Unresolved Conundrum. J Am Geriatr Soc. 2017;65(11):2352–2353. doi: 10.1111/jgs.15063. [DOI] [PubMed] [Google Scholar]
- 17.ALLHAT. [Accessed April 15, 2018];The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Available at: https://ccct.sph.uth.tmc.edu/allhat/
- 18.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–2024. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary Prevention With Statin Therapy in the Elderly: New Meta-Analyses From the Contemporary JUPITER and HOPE-3 Randomized Trials. Circulation. 2017;135(20):1979–1981. doi: 10.1161/CIRCULATIONAHA.117.028271. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 22.Trinquart L, Jacot J, Conner SC, Porcher R. Comparison of Treatment Effects Measured by the Hazard Ratio and by the Ratio of Restricted Mean Survival Times in Oncology Randomized Controlled Trials. J Clin Oncol. 2016;34(15):1813–1819. doi: 10.1200/JCO.2015.64.2488. [DOI] [PubMed] [Google Scholar]
- 23.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161(4):270–280. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Kim CM. Individualizing Prevention for Older Adults. J Am Geriatr Soc. 2017 doi: 10.1111/jgs.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. doi: 10.1136/bmj.e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1. RMST analysis in R software