Abstract
Background/ Objectives
Determine the association of weight trajectories with health status and mortality in older women.
Design
Cohort study
Setting
Study of Osteoporotic Fractures (US).
Participants
1,323 older, community-dwelling women (mean baseline [1986–88] age of 68 [65–81] and 20-year exam age [2006–2008] of 88 [83–102]), with follow-up through 2016.
Measurements
Body weight measured repeatedly over 20 years (mean 8 weights). Logistic and Cox proportional hazard models were used to evaluate whether 20-year weight trajectory measures were associated with hip fracture, falls, physical performance, and/or mortality. Voluntary and involuntary weight loss could not be distinguished.
Results
In models adjusted for age, clinic, calcium use, Year 20 weight, walking speed, comorbidity score, smoking, self-reported health, and walking for exercise, women with moderate weight loss (>9.0 kg) over 20 years had a 74% increased risk of death (HR=1.74 [95% CI: 1.37, 2.20]) in the 5 years after the Year 20 visit compared to those with no weight loss. They also had a greater than 2-fold increased risk of suffering a hip fracture (HR=2.56 [95% CI: 1.39, 4.70]). They were 3.6 times (OR=3.60 [95% CI: 1.86, 6.95]) more likely to have poor physical function at the Year 20 visit than women with no weight loss. However, there was no increased risk of 2 or more falls in the 1.5 years after the Year 20 visit. Weight variability and abrupt weight decline were not associated with adverse health oucomes (falls, fractures, and mortality), but those in the highest quartile of variability were 2.3 times (OR=2.26 [95% CI: 1.34, 3.80]) more likely to have poor physical function scores.
Conclusions
In women surviving past 80 years of age, moderate weight loss over 20 years was associated with an increased risk of hip fracture, poor physical function, and mortality, but without an apparent increase in falls. Future work should separate voluntary from involuntary weight loss.
Keywords: Mortality, fracture, weight loss, physical function
INTRODUCTION
Studies of the effects of weight loss on health rarely consider age.1 However, weight loss in middle age2–4 likely has different effects on health than weight loss in the elderly (age >65), especially the oldest old (>85).
Although some studies have found that weight loss in the elderly is generally associated with increased morbidity and mortality,5–13 these studies were limited by short duration of follow-up (2–4 years)5–7, 9 and/or reliance on self-reported weight change.9–12
The Study of Osteoporotic Fractures (SOF) measured weights over 20 years among women over age 65 at baseline, providing a unique opportunity to examine the association between long-term weight trajectory and health outcomes.
Using this data, we recently found that rate of weight loss over 20 years, but not abrupt weight decline or weight variability, was associated with development of mild cognitive impairment or dementia in women surviving past 80 years.14 Here, we evaluate whether 20-year weight trajectory is associated with other health outcomes (hip fracture, falls, and death), and physical function. We hypothesized women with greater weight loss, greater weight variability, and/or an abrupt decline in weight would have more limited physical function at Year 20 and be more likely to experience adverse health outcomes in the 1 to 5 years after the Year 20 visit.
METHODS
Study overview and sample
From 1986 to 1988, SOF recruited 9,704 community-dwelling women ages 65 and older (>99% non-Hispanic white) in four U.S. regions: Baltimore County, Maryland; Minneapolis, Minnesota; Portland, Oregon; and the Monongahela Valley near Pittsburgh, Pennsylvania.15 Women unable to walk without assistance and those with bilateral hip replacements were not eligible. African-American women were initially excluded because of a lower prevalence of osteoporotic fractures than Caucasians.15
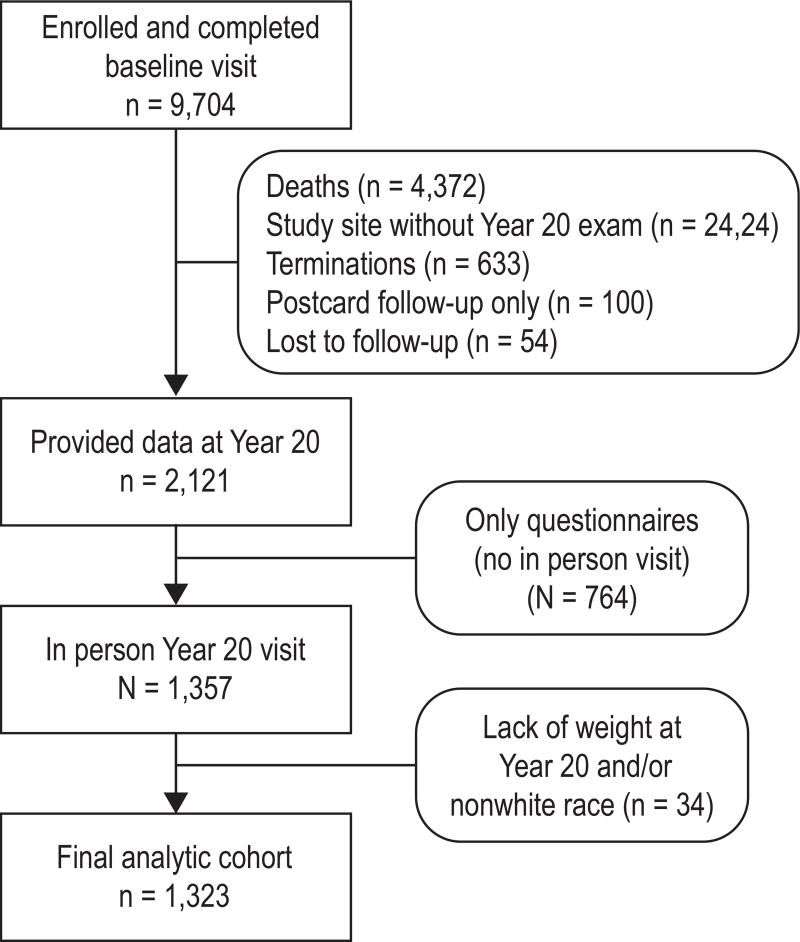
From 2006 to 2008, all surviving participants at three clinical centers (Minneapolis, Portland, and Pittsburgh) were invited to participate in the Year 20 visit. At least minimal questionnaire information was collected on 2,121 of the original cohort; of these, 1,323 (62.4%) also completed a Year 20 examination that included weight measurement (see Figure 1). All women provided written consent. The study was approved by each site’s Institutional Review Board.
Figure 1. Consort diagram.
Weight and body mass index (BMI)
Body weight was measured in light clothing with a standard balance beam (clinic visit) or digital scale (home visit) at 8 visits (every 2–5 years) over the 20-year period. A total of 1,091 (82%) women had all measurements; 97% had at least 6 measurements between baseline and the Year 20 exam. Height was measured with a wall-mounted Harpenden stadiometer (Holtain, Dyved, United Kingdom). The measures were used to compute BMI.15
Other characteristics
At baseline, participants completed questionnaires assessing basic demographics, medical history, estrogen exposure, and educational history. At Year 20, smoking and alcohol consumption, medical history, health behaviors (e.g., walking for exercise), independent activities of daily living, self-reported health, depressive symptoms (assessed with the 15-item Geriatric Depression Scale, GDS16) were determined via questionnaire and interview. A comorbidity index was calculated by summing selected self-reported medical conditions (see Table 1 footnote). Walking speed was also calculated as the average time to complete 2 trials on a 6-meter course. At Year 16, visual acuity was measured with Bailey and Lovie letter charts.17 Areal bone mineral density (BMD) (in g/cm2) was measured in the proximal femur by dual-energy x-ray absorptiometry using QDR 1000 densitometers (Hologic, Bedford, Massachusetts) in 1989 or 1990.15, 18, 19
Table 1.
Overall Characteristics
| Weight change category (median weight change, kg) |
|||||
|---|---|---|---|---|---|
| No weight loss (3.4) |
Minimal weight loss (−6.2) |
Moderate weight loss (−19.4) |
|||
| N=367 | N=773 | N=183 | p-value | ||
| Baseline age, yrs (mean, se) | 67.8 (0.2) | 68.5 (0.1) | 68.6 (0.2) | 0.0002 | |
| Education, =>12 yrs (n, %) | 318 (86.7) | 656 (84.9) | 152 (83.1) | 0.51 | |
| Baseline BMI, kg/m2 (mean, se) | 25.5 (0.2) | 26.4 (0.2) | 30.8 (0.3) | <.0001 | |
| Baseline height, cms (mean, se) | 160.3 (0.3) | 160.1 (0.2) | 161.2 (0.4) | 0.07 | |
| Baseline total hip BMD, g/cm2 (mean, se) | 0.79 (0.01) | 0.78 (0.01) | 0.83 (0.01) | <.0001 | |
| Baseline estrogen use, ever (n, %) | 197 (54.7) | 355 (46.3) | 78 (43.1) | 0.01 | |
| Year 20 calcium use, daily (n, %) | 191 (52.8) | 421 (54.7) | 94 (51.9) | 0.72 | |
| Year 20 weight, kg (mean, se) | 69.9 (0.6) | 60.8 (0.4) | 58.5 (0.8) | <.0001 | |
| Year 20 walking speed, m/sec (mean, se) | 0.73 (0.02) | 0.67 (0.01) | 0.55 (0.02) | <.0001 | |
| Year 20 comorbidity scorea (n, %) | 0.05 | ||||
| 1 | 108 (29.5) | 245 (31.8) | 61 (33.3) | ||
| 2 or more | 53 (14.5) | 140 (18.2) | 42 (23.0) | ||
| Year 16 visual acuity, poor (n, %) | 61 (19.7) | 107 (16.5) | 23 (15.8) | 0.41 | |
| Year 20 ever smoker (n, %) | 115 (31.3) | 264 (34.2) | 59 (32.2) | 0.62 | |
| Year 20 alcohol use (n, %) | 0.01 | ||||
| <3 days/ week | 106 (29.0) | 208 (27.0) | 52 (28.4) | ||
| 3–7 days/ week | 48 (13.1) | 83 (10.8) | 6 (3.3) | ||
| Year 20 self-reported health, good/excellent (n, %) | 301 (82.5) | 610 (79.1) | 131 (71.6) | 0.01 | |
| Year 20 walks for exercise (n, %) | 160 (44.6) | 332 (44.0) | 64 (36.2) | 0.13 | |
| Year 20 depressionb (n, %) | 28 (7.9) | 96 (12.8) | 26 (14.9) | 0.02 | |
Abbreviation: se refers to standard error
Comorbidity index was calculated using the sum (0–7) of seven possible self-reported medical conditions, including ever having a stroke, diabetes, COPD, Parkinson’s disease, heart attack/angioplasty, congestive heart failure, or peripheral vascular disease.
Depressive symptoms were assessed with the 15-item Geriatric Depression Scale (GDS). Those with scores >=6 were considered to have depression.
Outcomes
Falls, fractures, and mortality
After Year 20 [2006–2008], we reached out to participants by telephone every 4 months to ask about falls until 2009 (average follow-up of 1.5 years) and by postcard or telephone every 6 months to ask about fractures until 2016 (average follow-up of 5 years). We completed 95% of these contacts among surviving women. When we learned of deaths from phone calls or postcards, certificates were used to confirm them and, when possible, determine cause. Hip fracture and cause of death were locally adjudicated and confirmed by a physician.
Short Physical Performance Battery (SPPB)
At Year 20, staff administered the SPPB,20–22 which comprises measures of standing balance, usual gait speed, and ability to rise from a chair 5 times without using arms. For each task, participants were assigned scores of 1–4 based on quartiles of performance or 0 if unable to complete.22 A summary score was created by adding scores for each task and categorized as poor (0–3), low intermediate (4–6), high intermediate (7–9), or good (10–12).22 All participants had SPPB data at the Year 20 visit.
Statistical analyses
We compared baseline characteristics using Chi-square for categorical variables and analysis of variance for continuous variables. We examined associations between 20-year weight trajectory and experience of hip fracture, 2 or more falls, or death post Year 20 (until last follow-up). We also examined associations between weight trajectory and categorical SPPB scores.22
Absolute weight change was calculated as the difference in weight from baseline to Year 20 in kilograms. For each outcome, we evaluated weight change per 10 kg of weight loss (primary exposure). We examined a 10 kg decrease based on the population SD (8.99 kg) and clinical relevance. We also evaluated weight change categorically as: 1) no loss (gain or no change [change ≥0]); 2) minimal loss (same as or slightly greater [within 1 SD] than population change [1 SD ≤ change <0]); or 3) moderate loss (amount of loss greater than the SD for population change [change >1 SD]).
Rate of weight loss (kg/year) was calculated using the slope estimate from a linear model fit to each woman’s weight measures over time. We evaluated rate of loss continuously and categorically. We examined a 0.5 kg/year decrease in weight based on population SD (0.44 kg/year) and clinical relevance. We also compared slope of weight change to overall population slope: 1) positive slope ([slope >=0], indicating weight gain); 2) minimally negative slope (rate of loss the same as or slightly less than the SD for population rate [1 SD <= slope <0]); or 3) moderate negative slope (rate of loss greater than the SD for population rate [slope >1 SD]).
We identified abrupt, unrecovered episodes of marked weight loss inconsistent with women’s prior weight trajectories by determining whether trajectories fit quadratic equations, with a relative maximum, and whether loss continued to Year 20 (dichotomous variable of yes/no).
Weight variability was assessed using root mean square percentage error (RMSPE). We evaluated linear and quadratic curves for each woman and calculated RMSPE for the better-fitting model. RMSPE quartiles, as well as the continuous measure, were included in models.
Using age- and clinic-adjusted “base” logistic regression and Cox proportional hazard models, we examined likelihood of experiencing an outcome (2 or more falls, hip fracture, death) for each weight trajectory measure. For hip fracture, we included death as a competing risk using sub-distribution hazards models proposed by Fine and Gray. In these models, women who died prior to experiencing a hip fracture are not censored but remain in the risk set.23 For the ordinal SPPB outcome, we used cumulative logit models to estimate odds ratios. For this method, OR represents the increased odds of having an SPPB score in category “j” or lower versus higher than category “j,” given the specific change in weight measure.
For multivariable models, we considered potentially confounding factors from baseline (education, baseline body composition [height and BMI)], BMD, estrogen use); Year 20 (alcohol use, smoking, self-reported health, weight, depression, walking for exercise, walking speed, comorbidity index, calcium use); and Year 16 (visual impairment). Models were developed separately for each outcome. Each potential covariate was added to the base model and those associated individually with the outcome in base models (p<0.10) were evaluated together in logistic regression or Cox proportional hazard models through manual backwards stepwise selection, where age, clinic, and weight variables were forced. Statistically significant variables remained in final models (p<0.05).24, 25 Each final, fully adjusted model consisted of covariates identified above for each weight measure (see footnotes in Tables 2 and 3). We also conducted sensitivity analyses repeating hip fracture analyses using conventional Cox proportional hazard models where mortality is treated as uninformative censoring. We repeated all analyses excluding cancer deaths, and separately, those with mild cognitive impairment (MCI) or dementia.
Table 2.
Falls, Fractures, Survival: Risk of Mortality, Hip Fractures, and Falls According to Individual Characteristics of Weight Trajectory
| N=1323 Mortality n=839 (63.4) |
N=1195 Hip fracture n=116 (9.7) |
N=1292 Falls, 2 or more n=398 (30.8) |
||||
|---|---|---|---|---|---|---|
| Weight Measurea | Age, clinic adjusted HR (95% CI) |
MV adjustede HR (95% CI) |
Age, clinic adjusted HRh (95% CI) |
MV adjustedf HRh (95% CI) |
Age, clinic adjusted OR (95% CI) |
MV adjustedg OR (95% CI) |
| Weight change baseline to Year 20b | ||||||
| No weight loss | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Minimal weight loss | 1.45 (1.22,1.72) | 1.22 (1.02,1.47) | 1.43 (0.91,2.25) | 1.44 (0.91,2.26) | 1.03 (0.78,1.36) | 0.97 (0.73,1.28) |
| Moderate weight loss | 2.49 (2.00,3.10) | 1.74 (1.37,2.20) | 1.94 (1.08,3.48) | 2.56 (1.39,4.70) | 1.22 (0.83,1.80) | 1.05 (0.70,1.57) |
| Abrupt weight declinec | ||||||
| No | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Yes | 1.21 (1.04,1.41) | 1.12 (0.96,1.31) | 1.42 (0.96,2.11) | 1.40 (0.94,2.09) | 1.22 (0.93,1.59) | 1.18 (0.90,1.55) |
| Weight variability (RMSPE) quartiled | ||||||
| Q1 (least variable) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Q2 | 1.02 (0.84,1.24) | 1.05 (0.86,1.28) | 0.91 (0.56,1.48) | 0.93 (0.57,1.51) | 0.74 (0.52,1.04) | 0.79 (0.55,1.11) |
| Q3 | 1.27 (1.05,1.53) | 1.19 (0.98,1.45) | 0.64 (0.38,1.08) | 0.65 (0.38,1.10) | 0.96 (0.69,1.34) | 0.97 (0.69,1.36) |
| Q4 (most variable) | 1.37 (1.13,1.66) | 1.07 (0.88,1.31) | 0.73 (0.44,1.20) | 0.78 (0.47,1.30) | 1.01 (0.73,1.40) | 0.99 (0.71,1.39) |
Each model includes only the weight measure of interest and the non-weight covariates.
Categories are relative to population change SD (8.99 kg); no weight loss signifies weight change >=0; minimal weight loss indicates weight change was <=1 SD but <0; moderate weight loss indicates weight change was >1 SD.
Presence of an unrecovered abrupt decline in weight based on whether the weight trajectory fit a quadratic equation with a relative maximum and weight loss continued to the end of weight trajectory (yes/no).
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
Includes age, clinic, calcium use, Year 20 weight, walking speed (quartiles), comorbidity score, smoking, self-reported health, and walks for exercise.
Includes age, clinic, and total hip BMD.
Includes age, clinic, walking speed (quartiles), and depression
HRs calculated using competing risk model (including death as a competing risk).
Table 3.
Physical Function: Odds or Poorer Physical Function According to Individual Characteristics of Weight Trajectory
| SPPB Score Category N=1323 |
||||||
|---|---|---|---|---|---|---|
| GOOD (SPPB 10–12, referent) n=331 (25.0) | ||||||
| POOR SPPB 0–3 n=262 (19.8) |
LOW INTERMEDIATE SPPB 4–6 n=344 (26.0) |
HIGH INTERMEDIATE SPPB 7–9 n=386 (29.2) |
||||
| Weight measurea | Age, clinic adjusted OR (95% CI) |
MV adjustede OR (95% CI) |
Age, clinic adjusted OR (95% CI) |
MV adjustede OR (95% CI) |
Age, clinic adjusted OR (95% CI) |
MV adjustede OR (95% CI) |
| Weight change baseline to Year 20b | ||||||
| No weight loss | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Minimal weight loss | 1.27 (0.85, 1.89) | 1.54 (0.96, 2.47) | 1.21 (0.85, 1.72) | 1.40(0.93, 2.10) | 1.20 (0.86, 1.68) | 1.34 (0.93, 1.93) |
| Moderate weight loss | 3.20 (1.82, 5.59) | 3.60 (1.86, 6.95) | 2.43 (1.43, 4.14) | 2.52 (1.38, 4.61) | 1.14 (0.65, 2.00) | 1.21 (0.66, 2.21) |
| Abrupt weight declinec | ||||||
| No | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Yes | 1.20 (0.81, 1.76) | 1.21 (0.80, 1.83) | 1.47 (1.03, 2.08) | 1.37 (0.95, 1.99) | 1.10 (0.78, 1.56) | 1.07 (0.75, 1.53) |
| Weight variability (RMSPE) quartiled | ||||||
| Q1 | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Q2 | 0.84 (0.51, 1.39) | 0.83 (0.48, 1.43) | 1.09 (0.71, 1.66) | 1.09 (0.69, 1.71) | 1.28 (0.86, 1.92) | 1.32 (0.87, 1.99) |
| Q3 | 1.42 (0.89, 2.29) | 1.22 (0.73, 2.04) | 1.10 (0.71, 1.69) | 0.89 (0.56, 1.42) | 1.27 (0.84, 1.91) | 1.17 (0.76, 1.79) |
| Q4 | 2.69 (1.67, 4.32) | 2.26 (1.34, 3.8) | 1.77 (1.14, 2.76) | 1.56 (0.97, 2.5) | 1.45 (0.94, 2.25) | 1.39 (0.88, 2.19) |
Each model includes only the weight measure of interest and the non-weight covariates.
Categories are relative to population change SD (8.99 kg); no weight loss signifies weight change >=0; minimal weight loss indicates weight change was <=1 SD but <0; moderate weight loss indicates weight change was >1 SD.
Presence of an unrecovered abrupt decline in weight based on whether the weight trajectory fit a quadratic equation with a relative maximum and weight loss continued to the end of weight trajectory (yes/no).
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
Includes age, clinic, baseline height, Year 20 weight, comorbidity score, self-reported health, and depression.
Statistical analyses were completed using SAS v9.4 (SAS, Inc., Cary, North Carolina). We considered p<0.05 to be significant.
RESULTS
Sample characteristics
The average weight change between baseline and Year 20 was a loss of 5.38 kg (SD=8.99; median=−4.8; range: loss of 42.50 kg to gain of 27.50 kg). Most women (72%) experienced weight loss. Those who lost weight were older, had higher baseline BMIs but lower Year 20 weight, had higher baseline BMDs, and were less likely to have ever used estrogen than those who had no weight loss; at Year 20, they also had slower walking speed, more comorbidities, poorer self-reported health, more depression, and less alcohol use (Table 1). Almost two-thirds (63%) died and 10% suffered an incident hip fracture during an average follow-up of approximately 5 years after Year 20. Thirty-one percent experienced 2 or more falls over 1.5 years after Year 20. At Year 20, 25% (n=331) were in the highest (referent) physical performance category (SPPB score 10–12) and almost 20% (n=262) were in the lowest (SPPB score 0–3).
Health Outcomes
Absolute change in weight
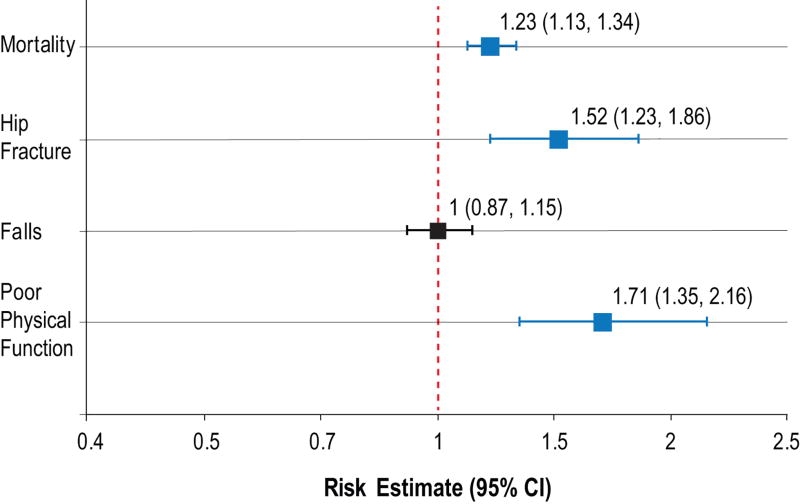
Every 10 kg decrease in weight over 20 years was associated with a 23% (HR=1.23 [95% CI: 1.13, 1.34]) increased risk of death and 52% (HR=1.52 [95% CI: 1.23, 1.86]) increased risk of hip fracture during average follow-up of approximately 5 years (Figure 2). When weight loss was examined categorically, women with moderate weight loss (>1 SD [9kg]) over 20 years had a 74% increased risk of death (HR=1.74 [95% CI: 1.37, 2.20]) and a nearly 3-fold increased risk of hip fracture (HR=2.56 [95% CI: 1.39,4.70]) compared with those with no weight loss. Women with minimal weight loss also had an increased risk of death (HR=1.22 [95% CI: 1.02, 1.47]) but no increased risk of hip fracture (HR=1.44 [95% CI: 0.91, 2.26]). There was no association between weight change and odds of experiencing 2 or more falls during approximately 18 months of follow-up.
Figure 2. Risk of developing health outcome per 10 kg decrease in weight from baseline to year 20.
Falls refers to 2 or more falls.
Poor physical function is a score of 0–3 on SPPB compared to a score of 10–12
Risk estimates are HRs for mortality and fracture and ORs for falls and poor physical function. A >10kg weight loss over 20 years (>0.5 kg/yr) was associated with a significantly higher risk of mortality, hip fracture, and poor physical function (denoted in red) but not falls (denoted in black).
Slope of weight change
Slope of weight change was associated with several health outcomes (Supplementary Table 1). For every 0.5 kg/year decrease in weight, risk of death increased by over 20% (HR=1.22 [95% CI: 1.12, 1.33] and risk of hip fracture increased by over 50% (HR=1.52 [95% CI: 1.23, 1.89]). This translated to women with the most negative slopes of weight loss (termed “moderate negative slope”) having a 70% (HR=1.70 [95% CI: 1.34, 2.15]) increased risk of death and a nearly 3-fold (HR=2.69 [95% CI: 1.47, 4.93]) increased risk of hip fracture compared to women with positive weight slopes. Weight slopes that were negative but of less magnitude (termed “minimal negative slope”) were not associated with risk of death but were associated with increased risk of hip fracture (HR=1.65 [95% CI: 1.04, 2.61]). There was no association between slope of weight change and risk of suffering 2 or more falls.
Abrupt weight decline and weight variability
Neither presence of an abrupt weight decline nor weight variability was associated with risk of developing any health outcome (Table 2 and Supplementary Table 1).
Physical Performance
Every 10 kg decrease in weight over 20 years was associated with a 71% (OR=1.71 [95% CI: 1.35, 2.16]) and 43% (OR=1.43 [95% CI: 1.15, 1.78]) increased risk of being in the poor and low intermediate physical performance categories, respectively, compared to the good category (Figure 2). Those with moderate weight loss were 3.6 times (OR=3.60 [95% CI: 1.86, 6.95]) more likely to be in the poor physical performance category and 2.5 times (OR=2.52 [95% CI: 1.38, 4.61]) more likely to be in the low intermediate category than those at the good level. For every 0.5 kg/year of weight loss, there was a 75% (OR=1.75 [95% CI: 1.38, 2.22]) and 44% (OR=1.44 [95% CI: 1.15, 1.79]) increased risk of being in the poor and low intermediate physical performance categories, respectively (Supplementary Table 2). Those with the most negative slopes of weight loss were 5.9 times (OR=5.87 [95% CI: 3.03, 11.36]) more likely to have poor than good physical performance scores; they were 3 times (OR=3.14 [95% CI: 1.71, 5.79]) more likely to have low intermediate than good scores.
Abrupt weight decline was not associated with physical performance scores (Table 3). In terms of weight variability, for every 1% average deviation from the predicted weight curve, women had a 15% (OR=1.15 [95% CI: 1.04, 1.28]) increased risk of poor physical performance (Supplementary Table 2). When examined categorically, those in the highest quartile of variability were 2.3 times (OR=2.26 [95% CI: 1.34, 3.80]) more likely to have a poor SPPB score (Table 3).
Sensitivity analysis
We conducted a sensitivity analysis of hip fracture using conventional Cox proportional hazard regression where mortality is treated as uninformative censoring. Results were similar, although risk estimates were stronger (Supplementary Table 3). When we excluded cancer deaths or those with MCI or dementia, results for the association between weight trajectory components and overall mortality were essentially unchanged (Supplementary Table 4).
DISCUSSION
This study offers a unique perspective into women’s weight trajectories in the 20 years leading up to a mean age of 88 and the impact of those trajectories on health outcomes and physical performance. Independent of weight at the year 20 visit, greater than average weight loss over time (approximately >10 kg over 20 years or 0.5 kg/year) was associated with increased risk of hip fracture, poor physical performance, and death, but not falls. Although weight variability was associated with poor physical performance, it was not associated with other health outcomes. An abrupt decline in weight was not associated with any outcome.
Previous studies have noted an association between weight loss and mortality and physical function. However, these studies were primarily conducted in men6, 7, 26, 27 or middle-aged persons,12, 26–28 or had only 3–4 years of follow-up.5, 9–11 In the Dubbo Osteoporosis Epidemiology Study, whose population and follow-up time most closely matches our own (1,059 women, mean age of 70), weight loss of ≥1% per year was associated with 2.2-fold increased risk of mortality over 13 years.13 Because weight trajectories could differ by cause of death, we excluded cancer deaths and those with MCI or dementia. However, the association between weight trajectory components and overall mortality was essentially unchanged, suggesting our findings are not related to cause of death.
We previously reported that SOF women with 5% weight loss over an average of 5.7 years had 1.8 times greater risk of hip fracture over the subsequent 6.6 years.29 In this study, we examine the association between weight change over 20 years and hip fracture in the subsequent 5 years in women surviving into their 80s and 90s. The association between weight loss and hip fracture is even stronger over this extended period and in this older population.
As women age, they risk weight loss because of changes in taste and smell, impaired digestion, and malabsorption/poor usage of nutrients due to chronic diseases or drug-nutrient interactions. In addition, social-environmental factors (e.g., solitude, institutionalization), psychological factors (e.g., depression), and a limited level of independence (e.g. difficulty purchasing/preparing foods) can lead to weight loss.30 Our findings suggest that such weight loss may contribute to the pathogenesis of health decline. Altered adipose tissue function and structure may be associated with impaired cardiovascular autoreparative potential.31 Adipokines secreted from adipose tissue may influence cancer risk.32, 33 Sarcopenia and low bone density, potential correlates of weight loss, have been associated with increased risk of all-cause mortality and disability.34–36 Alternatively, altered adipogenesis may impair both health and physical function, leading to decreased weight.31
We did not find an association between weight trajectory and falls. This was unexpected given the association between weight change and fracture risk. Although most fractures occur because of a fall, not all falls lead to fractures. We captured any fall regardless of whether injury was sustained, and it may be weight loss predisposes to falls that are more likely to result in fractures.
We had hypothesized that greater weight variability would be associated with poorer health outcomes and physical function. Although we did not find weight fluctuations were associated with mortality, previous studies have shown conflicting results.1, 13, 37, 38 These studies differ from our work by relying on subjective weight history1, 38 or following measured weights for only 7–13 years.37 We did find, consistent with another study,37 greater weight variability was associated with increased risk of poor physical performance. We hypothesize that variability in weight may reflect episodic declines in physical function.
Although previous studies have noted weight declines in the years preceding death,39 we did not find an association between abrupt weight decline and mortality, poorer health, or physical performance. Our study’s population was different as we examined women who had survived into their 80s and beyond; we also examined weight trajectories after age 65, while previous studies followed subjects from midlife.12, 26–28
Our study has several strengths. We studied a large cohort of women with multiple weight measurements over 20 years. The mean age at study’s end was 88, so our observation period covered the period of greatest risk of physical function and health decline. Measurements were rigorous,15 including detailed physical performance evaluation at Year 20 and adjudication of health outcomes every 4–6 months. One limitation was that women had to be weighed for 20 years, potentially leading to survival bias. We did not have sufficient data on voluntary weight loss to distinguish between intentional and unintentional weight loss, and making such distinctions in this older age group is particularly challenging (i.e., women who are lifelong dieters may believe they are losing weight due to their efforts rather than subclinical illness). Smoking and alcohol exposure from the year 20 examination was used and women may have changed these behaviors due to declining health during the follow-up period. Because we did not have SPPB scores at baseline, we cannot exclude early, preclinical physical function decline contributed to greater weight loss over time. Finally, our findings may not be generalizable to men or non-white racial/ethnic groups.
Conclusions
Our results suggest long-term weight loss in older women may be a marker for increased risk of poor health outcomes. Therefore, particular attention should be given to women who have survived into their 80s and 90s but have experienced moderate weight loss (≥10 kg), regardless of whether there was an abrupt weight decline. Targeting nutritional status, social-environmental factors, and/or adipose tissue function and structure in such women could help to preserve health and physical function into old age,34, 40 but additional research is needed.
Supplementary Material
Supplemental material: Table 1 and 2 describe the risk of falls, fractures, survival, and physical function according to slope of weight loss and weight variability. Table 3 describes the risk of hip fracture according to weight trajectory characteristics using conventional Cox proportional hazards regression (mortality is treated as uninformative censoring). Table 4 describes risk of mortality according to individual weight trajectory characteristics after cxcluding cancer deaths or those with mild cognitive impairment (MCI) or dementia.
Impact Statement.
We certify the work is novel. The Study of Osteoporotic Fractures (SOF) measured weights of elderly women for 20 years, offering a unique opportunity to examine the association between long-term weight trajectory and health outcomes with aging.
Acknowledgments
Funding Source: This work was supported by the National Institutes of Health. The National Institute on Aging provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
We thank Cassie Angus of the Kaiser Permanente Center for Health Research for help with manuscript preparation. We thank Katherine K. Essick of the Kaiser Permanente Center for Health Research for editorial assistance.
ESL’s institution has received grant funding from Merck Inc., Amgen, Astrazeneka, and Bristol Meyer Squibb for unrelated projects on which she was PI or Co-I.
Footnotes
Conflict of Interest: This work does not represent a conflict of interest. The other authors have no conflicts of interest.
Author Contributions: All authors participated in study concept and design, acquisition of subjects and/or data, analysis and/or interpretation of data, and preparation of manuscript.
References
- 1.Taing KY, Ardern CI, Kuk JL. Effect of the Timing of Weight Cycling During Adulthood on Mortality Risk in Overweight and Obese Postmenopausal Women. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.207. [DOI] [PubMed] [Google Scholar]
- 2.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40–64 years. Am J Epidemiol. 1995;141:1128–1141. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. Journal of epidemiology and community health. 2005;59:134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138:383–389. doi: 10.7326/0003-4819-138-5-200303040-00007. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involuntary weight loss in older outpatients: incidence and clinical significance. J Am Geriatr Soc. 1995;43:329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 7.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165:1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:857–863. [PubMed] [Google Scholar]
- 9.Payette H, Coulombe C, Boutier V, Gray-Donald K. Weight loss and mortality among free-living frail elders: a prospective study. J Gerontol A Biol Sci Med Sci. 1999;54:M440–445. doi: 10.1093/gerona/54.9.m440. [DOI] [PubMed] [Google Scholar]
- 10.Locher JL, Roth DL, Ritchie CS, et al. Body mass index, weight loss, and mortality in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1389–1392. doi: 10.1093/gerona/62.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie CS, Locher JL, Roth DL, McVie T, Sawyer P, Allman R. Unintentional weight loss predicts decline in activities of daily living function and life-space mobility over 4 years among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63:67–75. doi: 10.1093/gerona/63.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen ND, Center JR, Eisman JA, Nguyen TV. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22:1147–1154. doi: 10.1359/jbmr.070412. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc ES, Rizzo JH, Pedula KL, et al. Weight Trajectory over 20 Years and Likelihood of Mild Cognitive Impairment or Dementia Among Older Women. J Am Geriatr Soc. 2017;65:511–519. doi: 10.1111/jgs.14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh J, J Y. Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. 1986. pp. 165–173. [Google Scholar]
- 17.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. American journal of optometry and physiological optics. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:155–160. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 19.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- 20.Ensrud KE, Lui LY, Paudel ML, et al. Effects of Mobility and Cognition on Risk of Mortality in Women in Late Life: A Prospective Study. J Gerontol A Biol Sci Med Sci. 2016;71:759–765. doi: 10.1093/gerona/glv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24.Rothman KJ, Greenland S contributors w. Modern Epidemiology. Second. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 25.Kleinbaum DG, Kupper LL, Morgenstern H. Confounding. In: Kleinbaum DG, Kupper LL, Morgenstern H, editors. Epidemiologic Research: Principles and Quantitative Methods. Belmont, CA: Lifetime Learning Publications; 1982. pp. 242–265. [Google Scholar]
- 26.Yaari S, Goldbourt U. Voluntary and involuntary weight loss: associations with long term mortality in 9,228 middle-aged and elderly men. Am J Epidemiol. 1998;148:546–555. doi: 10.1093/oxfordjournals.aje.a009680. [DOI] [PubMed] [Google Scholar]
- 27.Iribarren C, Sharp DS, Burchfiel CM, Petrovitch H. Association of weight loss and weight fluctuation with mortality among Japanese American men. N Engl J Med. 1995;333:686–692. doi: 10.1056/NEJM199509143331102. [DOI] [PubMed] [Google Scholar]
- 28.Gregg EW, Cauley JA, Stone K, et al. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289:2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 30.Inelmen EM, Sergi G, Coin A, Girardi A, Manzato E. An open-ended question: Alzheimer's disease and involuntary weight loss: which comes first? Aging Clin Exp Res. 2010;22:192–197. doi: 10.1007/BF03324796. [DOI] [PubMed] [Google Scholar]
- 31.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocrine-related cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 33.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine & growth factor reviews. 2009;20:193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- 36.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 37.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881–886. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zawada ET., Jr Malnutrition in the elderly. Is it simply a matter of not eating enough? Postgrad Med. 1996;100:207–208. doi: 10.3810/pgm.1996.07.17. 211-204, 220-202 passim. [DOI] [PubMed] [Google Scholar]
- 40.Smith KGC, Payette H, Alibhai S. An Approach to the Nonpharmacologic and Pharmacologic Management of Unintentional Weight Loss Among Older Adults. Geriatrics and Aging. 2007;10:91–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material: Table 1 and 2 describe the risk of falls, fractures, survival, and physical function according to slope of weight loss and weight variability. Table 3 describes the risk of hip fracture according to weight trajectory characteristics using conventional Cox proportional hazards regression (mortality is treated as uninformative censoring). Table 4 describes risk of mortality according to individual weight trajectory characteristics after cxcluding cancer deaths or those with mild cognitive impairment (MCI) or dementia.