Abstract
Maternal inflammation during pregnancy can alter the trajectory of fetal brain development and increase risk for offspring psychiatric disorders. However, the majority of relevant research to date has been conducted in animal models. Here, in humans, we focus on the structural connectivity of frontolimbic circuitry as it is both critical for socioemotional and cognitive development, and commonly altered in a range of psychiatric disorders associated with intrauterine inflammation. Specifically, we test the hypothesis that elevated maternal concentration of the proinflammatory cytokine interleukin-6 (IL-6) during pregnancy will be associated with variation in microstructural properties of this circuitry in the neonatal period and across the first year of life.
Pregnant mothers were recruited in early pregnancy and maternal blood samples were obtained for assessment of maternal IL-6 concentrations in early (12.6±2.8 weeks [S.D.]), mid (20.4±1.5 weeks [S.D.]) and late (30.3±1.3 weeks [S.D.]) gestation. Offspring brain MRI scans were acquired shortly after birth (N=86, scan age=3.7±1.7 weeks [S.D.]) and again at 12-mo age (N=32, scan age=54.0±3.1 weeks [S.D.]). Diffusion Tensor Imaging (DTI) was used to characterize fractional anisotropy (FA) along the left and right uncinate fasciculus (UF), representing the main frontolimbic fiber tract. In N=30 of the infants with serial MRI data at birth and 12-mo age, cognitive and socioemotional developmental status was characterized using the Bayley Scales of Infant Development. All analyses tested for potentially confounding influences of household income, prepregnancy Body-Mass-Index, obstetric risk, smoking during pregnancy, and infant sex, and outcomes at 12-mo age were additionally adjusted for the quality of the postnatal caregiving environment.
Maternal IL-6 concentration (averaged across pregnancy) was prospectively and inversely associated with FA (suggestive of reduced integrity under high inflammatory conditions) in the newborn offspring (bi-lateral, p<0.01) in the central portion of the UF proximal to the amygdala. Furthermore, maternal IL-6 concentration was positively associated with rate of FA increase across the first year of life (bi-lateral, p<0.05), resulting in a null association between maternal IL-6 and UF FA at 12-mo age. Maternal IL-6 was also inversely associated with offspring cognition at 12-mo age, and this association was mediated by FA growth across the first year of postnatal life.
Findings from the current study support the premise that susceptibility for cognitive impairment and potentially psychiatric disorders may be affected in utero, and that maternal inflammation may constitute an intrauterine condition of particular importance in this context.
Keywords: Longitudinal DTI, Newborn, Fractional Anisotropy, Uncinate Fasciculus, Interleukin-6, Inflammation
1. Introduction
Over the course of the last decade, there has been a dramatic improvement in the ability to non-invasively image the developing human brain using MRI-based techniques.1–4 This methodological advance holds great promise in increasing our understanding of the early origins of many neurodevelopmental and psychiatric disorders.5–7
Epidemiological findings have consistently associated maternal infection in pregnancy with suboptimal offspring cognitive and socio-emotional development and increased risk for schizophrenia and autism.8–16 Findings across studies of different maternal infections during pregnancy converge to suggest that it is the maternal inflammatory response to the infectious antigen, and not the presence of any specific antigen per se, that produces these observed effects in offspring17. Moreover, the presence during pregnancy of non-infectious clinical conditions associated with a proinflammatory state (e.g. high maternal BMI, 18–20 elevated social stress21 ) are also associated with an increased risk in offspring for psychiatric disorders. Maternal inflammation during pregnancy, therefore, may represent a common pathway that mediates the effects of a diverse set of inflammation-related states and conditions during pregnancy on offspring neurodevelopment and mental health outcomes.
The pro-inflammatory cytokine interleukin-6 (IL-6) likely constitutes a key biological mediator by acting as a sensor, transducer, and effector of environmental conditions on the developing fetal brain.22 Several studies have established elevated IL-6 concentrations among women with obstetric states and conditions that have been shown to increase the risk for neurodevelopmental and psychiatric disorders in the offspring including obesity, infection and high psychosocial stress (sensor).21,23 These states and conditions are also characterized by higher IL-6 and other pro-inflammatory cytokines in the placental, intra- amniotic and fetal compartments, including the fetal brain and cord blood, with significant correlations between maternal and fetal IL-6 (transducers).24–27 While IL-6 plays a requisite role in fetal brain development,28 it is evident that inappropriately elevated levels may produce perturbations in cellular survival, proliferation and differentiation, axonal growth and synaptogenesis (effectors)29–32 Moreover, rodent models involving direct administration of IL-6 in the pregnant dam have demonstrated IL-6 as being both sufficient and necessary to evoke the pathway through which maternal inflammation negatively effects fetal outcomes33. For these reasons, we submit that characterization of maternal IL-6 concentration during pregnancy in the context of studying human fetal brain development represents a crucial first step towards understanding pathways by which maternal inflammation influences offspring risk for neurodevelopmental and psychiatric disorders. In support of this premise, we have recently published findings establishing a link between maternal systemic inflammation during pregnancy, indexed by IL-6 concentrations, and offspring (newborn) amygdala volume and functional connectivity, as well as the longer-term significance of this association in terms of child impulse control at 24 mo age (a phenotype that underlies many emotional and behavioral problems).34
In the brain, the uncinate fasciculus (UF) connects the anterior temporal lobe to the orbitofrontal cortex via limbic (e.g. amygdala) regions to facilitate a direct structural link between higher-order cognition and emotional regulation. Reduced integrity of the UF has been linked to a range of neurodevelopmental and psychiatric disorders (e.g., anxiety,35 autism,36–37 conduct disorder39, schizophrenia,40–43 and psychopathy44–46), all of which are characterized by common underlying symptoms of socioemotional and cognitive deficits. Additional evidence of the importance of UF integrity in socioemotional and cognitive phenotypes comes from of a diverse set of studies in which socioemotional and cognitive deficits are preceded by structural insults to frontolimbic circuitry (e.g., traumatic brain injury,47 frontotemporal lobar degeneration,48 acute ischemic stroke,49 early life stress,50 and preterm birth51). For these reasons, we suggest examination of the UF in the context of maternal inflammation during pregnancy is of interest for understanding potential neurodevelopmental pathways through which maternal inflammation during pregnancy may increase risk for poor developmental outcomes and psychiatric disorders.
The present study extends our previous work34 focusing on limbic functional connectivity by examining whether maternal IL-6 concentrations during pregnancy are also relevant for the structural development of offspring frontolimbic circuitry. We here focus on the microstructural integrity of the UF as indexed by Fractional Anisotropy (FA), an MRI-based direct measure of the anisotropy of water diffusing in the brain, and thereby an indirect measure of microstructural properties including axonal coherence, oligodendrocyte proliferation, and myelination. FA was characterized shortly after birth and at 12-mo age in order to characterize interindividual variation in UF microstructural integrity. The importance of selecting the newborn brain as a starting point derives from the logic that brain circuitry at this time has not yet been influenced by postnatal environmental conditions. However, because brain connectivity changes rapidly over the first 12 months of postnatal life, we further tested whether maternal inflammation during pregnancy was associated with maturational change in UF during the first 12 months of postnatal life, after adjusting for variation in the postnatal caregiving environment. Finally, in consideration of UF variation as a potential neurodevelopmental pathway through which maternal inflammation increases risk for poor neurodevelopmental and psychiatric outcomes, we examined associations between UF development and socioemotional and cognitive functioning at 12-mo age.
2. Methods and Materials
2.1 Sample
Mother-child dyads were part of an ongoing, longitudinal study, conducted at the University of California, Irvine, for which mothers (N=147) were recruited during the first trimester of pregnancy. Exclusionary criteria were as follows: maternal use of psychotropic medications or systemic corticosteroids during pregnancy; infant birth before 34 weeks gestation; and infant congenital, genetic, or neurologic disorder. Demographic characteristics are presented in Table 1 . MR imaging was successfully performed shortly after birth in offspring (N=86, range: gest. Age=34.6–41.8 weeks, postnatal age at scan=0.7–8.2 weeks) and again at 12-mos (N=32 longitudinally, range: postnatal age at scan=51.3–56.1 weeks). Socioemotional and cognitive developmental status at 12-mo age was available for all but two children with longitudinal (newborn and 12-mo age) brain imaging data (N=30 with longitudinal imaging and behavioral data). There were no significant differences in demographic variables between the sample of mother-child pairs enrolled in this study (N=147) versus the sample with available diffusion-weighted MRI data near birth (N=86, all p>0.1) or with the subset of infants with available longitudinal neuroimaging data and behavioral data (N=30, all p>0.1). All procedures were approved by the Institutional Review Board at the University of California, Irvine, and written informed consent was obtained from all mothers.
Table 1. Demographic Information.
Demographic information of the participants included in the analyses (N=86 MRI scans at birth) and (N=32 MRI scans at 12-mo age).
| Maternal Age [years (SD)] | 28.2 (5.5) |
| Infants | |
| Gestational Age at Birth [N=86,weeks (SD)] | 39.3 (1.5) |
| Age at Newborn MRI Scan [N=86, weeks (SD)] | 3.73 (1.7) |
| Age at 12-mo MRI Scan [N=32, weeks (SD)] | 54.0 (3.1) |
| Sex (male, frequency %) | 55.8 |
| Infant Race/Ethnicity (%) | |
| White non-Hispanic | 37.5 |
| White Hispanic | 35.0 |
| Asian | 8.75 |
| Other | 18.75 |
| Household Highest Level of Maternal Education (%) | |
| High-School or Test Equivalent | 22.1 |
| Vocational School or Some College | 41.9 |
| Associates Degree | 4.65 |
| Bachelors or Graduate Level Degree | 31.4 |
| Gross Annual Household Income (%) | |
| < $15,000 | 10.7 |
| $15,000 – 29,999 | 22.6 |
| $30,000 – 49,999 | 23.8 |
| $50,000 – 100,000 | 35.7 |
| > $100,000 | 7.1 |
2.2 Measurement of Maternal IL-6 Concentration during Pregnancy
Maternal antecubital venous blood samples were collected in serum tubes (BD Vacutainer) in the first, second and third trimester. Serum IL-6 concentration was determined using a commercial high sensitivity ELISA (eBioscience) with a sensitivity of 0.03 pg/ml. IL-6 concentration was moderately stable across pregnancy (ICC=0.45) and averaged across pregnancy to minimize error stemming from intra-individual physiological variation. The average was base 2 logarithm transformed to bring outliers closer to the mean and normalize the distribution. Maternal IL-6 concentration during pregnancy was not associated with offspring: gestational age at birth, postnatal age at scan or postmenstrual age at scan (all p>0.1, Supplementary Figure 1).
2.3 Characterization of Infant Socioemotional and Cognitive Developmental Status
Infant socioemotional and cognitive development at 12-mo age was assessed with the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III; Bayley, 2006). This is a widely used, standardized measure of infant development. The Social- Emotional Scale is based on caregiver report and designed to evaluate emerging social skills, self-regulation, emotional expression and communication of needs. The Cognitive Scale includes developmentally appropriate assessment of emerging sensorimotor, attention and memory skills, as well as interest in and understanding of the environment. Higher scores indicate more developmentally advanced functioning in these areas. The distributions of the Socio-Emotional and Cognitive Scales in the current sample were in line with expectations for the general population (Social-Emotional: Mean=94.2±14.3 [S.D.]; Cognitive: Mean=106.1±11T [S.D.]).
2.4 Characterization of the Postnatal Caregiving Environment
The Home Observation for Measurement of the Environment (HOME) Inventory52 was used to index the quality of the caregiving environment when the infant was 6- months-of-age. Measurement is based on in-home observation and a semi-structured interview with mothers. Observers were trained, and achieved reliability with a certified administrator of this inventory (95% agreement on two consecutive videos). We used a summary measure that incorporates assessment of interactions between infants and caregivers (responsivity and acceptance), and the extent to which the physical environment is organized and supportive of infant development (learning materials and organization); thus this measure can be considered as an estimate of the quality of the caregiving environment (Mean=34.9±3.9 [S.D.], Range=23–39).
2.5 Neuroimaging Data Acquisition
MRI scans were acquired during natural sleep using a 12-channel head receive coil at 3T field strength on a Siemens Tim Trio system in N=86 neonates, of whom, N=32 were successfully rescanned at 12-mo age. After feeding and soothing to the point of sleep, neonates were placed in a CIVCO beaded pillow (www.civco.com). The pillow covered the neonates’ body and head, became rigid under vacuum, and provided a comforting swaddle, motion prevention and hearing protection when used in conjunction with standard foam earplugs. The longitudinal visit at 12-months-of-age was also conducted during natural sleep. A pediatric specialist observed participants throughout the duration of scans, monitoring for heart rate and oxygen saturation via a pulse-oximeter attached to the foot. Imaging parameters described below were matched between the neonatal and 12-mo age scans. High-resolution anatomical scans including T1-weighted (MPRAGE, TR/TE/TI=2400/3.16/1200ms, Flip Angle=8 degrees, Matrix=256×256×160, Resolution=1×1×1mm, 6m18s) and T2-weighted (TSE, TR/TE=3200/255ms, Matrix=256×256×160, Resolution=1×1×1mm, 4m18s) images were acquired. The 42- direction diffusion weighted protocol (EPI, TR/TE=8900/83ms, FoV=256×224×150mm, Resolution=2×2×2mm, Partial Fourier=6/8, GRAPPA Phase Encoding Acceleration Factor=2, 42 unique directions at b=1000s/mm2, 7 at b=0) was 7 minutes and 43 seconds in duration.
2.6 Data Preprocessing
Diffusion profile measurements were generated via the NA-MIC atlas-based fiber analysis toolkit.53 In brief, diffusion datasets were first rigorously checked for appropriate quality,54 which included slice-wise and gradient-wise artifact detection, as well as eddy current and motion correction. Participants (NNewbom,removed/remaining=20/86; N12- mos,removed/remaining=2/32) were removed from analysis if they had less than 28 of 42 remaining good directions after the initial QC process to ensure similar signal-to-noise ratio. Remaining participants had bad directions (NNewborn=36.5+/−3.6 remaining directions; N12-mos=39.4+/−1.8) censored prior to the derivation of diffusion metrics (e.g. FA). This was followed by weighted least square tensor estimation, skull stripping via prior brain mask from co-registered structural T2-weighted images, and unbiased study specific DTI atlas building. The sample-based atlas specific to the current studies acquisition parameters and age range was built using DTIAtlasBuilder (www.nitric.org/projects/dtiatlasbuilder) via a three-step registration: 1) affine registration, 2) unbiased diffeomorphic atlas computation,55 and 3) refinement with a symmetric diffeomorphic registration via Advanced Normalization Tools (ANTS56). Fiber tract streamline DTI tractography was performed via 3D Slicer (version 4.3.0) (http://www.slicer.org) in the DTI atlas space followed by fiber cleaning with FiberViewerLight (www.nitric.org/projects/fwlight). Tractography was performed bilaterally for the UF and tracked according to the definition in the UNC pediatric DTI atlas (www.nitrc.org/projects/uncebds_neodti). The spatial extent of the UF was defined anteriorly by the medial fronto-orbital gyrus and posteriorly as the temporal pole. Fiber profiles were sampled at a 1mm step size along both directions away from the fiber’s intersection with the origin plane (plane that intersects the fiber bundle orthogonally at the median fiber location). Fiber profiles of FA were extracted after fiber parameterization for profile analysis. In supplementary analyses (Supplementary Section S.5), the set of observed diffusion properties was extended to include axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD).
Fiber profiles at 12-mo age were aligned with their neonatal counterparts using a linear registration with two degrees of freedom (a global scale factor applied to the arc-length and a bias applied to the origin across all participants) via cross-correlation of the group average as the cost function. The transformation (shift in origin, resampling via spline interpolation) from newborn to 12-mo space was applied uniformly to all newborn profiles. The output was visually confirmed to be in spatial correspondence (Supplementary Figure 2).
2.6.1 Analytical Approach
The methods outlined below (Sections 2.6.2–2.6.4) reflect a top-down analytical approach to elucidating associations between average maternal IL-6 concentrations across pregnancy and early life UF development. To summarize, we first characterized FA across the entire UF in the neonate (using whole-tract measures, Section 2.6.2), and tested for an association with maternal IL-6 concentration. We chose a Principal Components Analysis (PCA) approach in order to capture a whole-tract measure while also allowing for spatial heterogeneity in the UF. In order to establish the specificity of the effect on the UF, we extended the analyses to other major white matter pathways in the newborn brain and tested for an association with maternal IL-6 concentration during pregnancy using methods identical to the main analyses (Supplementary Materials Section S.4). We then conducted analyses allowing a finer parcellation of the UF to test for region-specific associations between maternal IL-6 concentration during pregnancy and FA along the UF (using single points along the tract, Section 2.6.3). Finally, using regions-of-interest (ROIs) identified in the prior analyses, we characterized change in UF integrity during the first 12 months of postnatal life (Section 2.6.4) and examined this metric in relation to maternal IL-6 during pregnancy.
When considering the approach for testing the effect of maternal IL-6 concentration on fronto-limbic white matter circuitry, three primary considerations were made with respect to heterogeneity within the UF tract. First, there exists anatomical variation within the tract. For example, central UF fibers are tightly bound and coherently organized, whereas at the peripheral ends they begin to diverge towards their terminal endpoints. The consequences of this are a greater potential for measurement error in the peripheral ends (more spatial variation, lower signal-to-noise-ratio). By treating the points on the tract as independent observations, tract-points are statistically naive to other points along the tract. Alternatively, taking the mean of the whole tract potentially averages out anatomical-specific variation. Second, there exists spatiotemporal variation in gestational exposure to maternal inflammation across the UF. The UF is developing in utero through rapid neurogenesis, axonal organization, oligodendrocyte proliferation and even limited myelination. Therefore, the life history of a cell within the UF with respect to state, extent, duration, and timing of exposure to the inflammatory milieu varies greatly based on its age and position within the tract. By allowing a level of statistical independence to each point across the tract, one can model the relationship between maternal IL-6 concentration during pregnancy and FA, specific to a given position. Finally, because the peripheral ends will be less mature and therefore likely exhibit more maturation related changes in the age range of our sample, there will exist spatial variation in the strength of association with postmenstrual age (e.g., being more pronounced at the peripheral ends of the tract). A tract-based approach allows the model to have a spatially varying effect size with respect to subject age parameters, thereby increasing statistical precision.
While the tract-based approach used here addresses spatial heterogeneity in a simple to interpret linear regression model, it does not speak to the UF as a single entity beyond significance at the tract-level after a multiple comparisons correction. For this reason, the PCA-based approach addresses this by spatially collapsing across the tract using weights that orthogonally maximize variation.
2.6.2 Characterization of PCA-based Newborn Fractional Anisotropy along the Uncinate Fasciculus
Whole tract analysis was performed using a PCA data reduction technique, reducing on the tract dimension (from N=86 participants x M tract locations, to N=86 participants x P=2 selected principal components). PCA allowed us to transform highly correlated observations along the tract into linearly uncorrelated observations of reduced dimensionality, thereby increasing statistical power (fewer comparisons) while retaining important information about interindividual and regional variation. We used power analysis to define component selection criteria with the aim of minimizing comparisons (components) while optimizing variance explained (minimum effect size=8% variance explained for n=0.8, atwo-tailed=0.05, N=86). The threshold criteria were verified to be visually consistent with standard retention criteria (being above the knee in the scree plot, Figure 1). The first two principal components for left and right UF were retained using these criteria and were tested for an association with maternal IL-6 during pregnancy using the analytic approach described below (Section 2.7). In order to justify data reduction, intra-tract Pearson correlation was used to demonstrate redundancy in interindividual variation along the UF (Figure 1).
Figure 1. Intratract Principal Components Analysis (PCA).
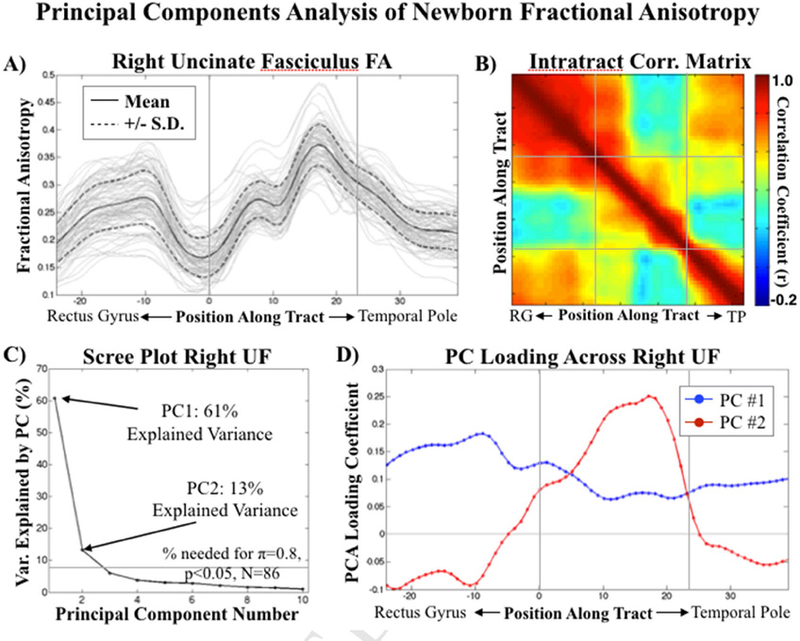
Intratract PCA reveals two, spatially distinct, principal components. A) Distribution (individual profiles in gray) of Fractional Anisotropy (FA) values along the right Uncinate Fasciculus (UF) spanning from the frontal to the temporal lobe. B) Heat map demonstrating high FA intratract correlation structure, separated by medial and lateral (frontal and temporal) portions of the UF. C) Scree plot showing PC selection criteria. D) PC1 loads uniformly across the tract relative to PC2, which loads primarily in the medial portion of the UF. Left UF statistics and mappings were consistent with those shown here for the right UF. Markers of spatial correspondence between figures 1A/D (vertical gray lines), and 1B (horizontal/vertical gray lines) are overlaid.
2.6.3 Characterization of Tract-based Newborn Fractional Anisotropy along the Uncinate Fasciculus
Tract-based profile analysis considered each individual point along the UF (N=86 participants x M tract locations) using the analytic approach described below (Section 2.7). Clusters (ROIs) significant after considering the multiple comparisons made along the length of the UF were identified using a Monte Carlo approach by replacing the sign of the demeaned IL-6 values with randomly assigned signs (+1/−1) and simulating 10,000 times to identify necessary cluster properties (extent and effect size) for significance at a corrected threshold of p<0.05.
2.6.4 Characterization of ROI-based Fractional Anisotropy at 12-mo age and Change from Birth Until 12-mo age
To test the association between maternal inflammation during pregnancy and longitudinal change in UF integrity, we restricted 12-mo age FA and serial change in FA to the ROIs identified in the above-described neonatal analyses in order to conserve statistical power by reducing degrees of freedom. Specifically, we used the linear regression analyses described below (Section 2.7) to test for an association between average maternal IL-6 concentration across pregnancy and ROI-extracted FA at birth, at 12-mo age, and change in FA from birth until 12-mo age. Despite its apparent redundancy, the newborn time point was included because of the necessity for the longitudinal analyses to first residualize FA values for gestational age at birth and postnatal age at scan before testing for IL-6 associations (as opposed to a single linear model including gestational age at birth, postnatal age at scan, and maternal IL-6 concentration as predictors as described below for the characterization of the associations between maternal IL-6 concentration and newborn FA). The age residualization step is necessary to generate FA variables that represent change in FA from shortly after birth until 12-mo age that are independent of age at scan and the time between the two scans.
2.7 Statistical Approach
For statistical modeling we used the most parsimonious model possible in conjunction with two additional models that considered: 1) sex-specific associations between maternal IL-6 concentration during pregnancy and UF FA outcomes, and 2) whether potentially confounding covariates changed the association between maternal IL- 6 concentration during pregnancy and UF FA outcomes. The parsimonious model reflected the strong age dependence of early life FA by including gestational age at birth and postnatal age at scan in the same linear model as maternal IL-6. As a separate test of sex-specific effects of maternal IL-6 on FA outcomes, we added infant sex and an IL-6 x sex interaction term to the parsimonious model. Finally, in an omnibus model we further considered household income, prepregnancy BMI, obstetric risk, smoking during pregnancy, and infant sex as potentially confounding variables. The set of confounding factors included in the omnibus model were selected on the basis of their established association with maternal inflammation and/or offspring neurodevelopment. While they may themselves be effectors of maternal IL-6 concentration (with the exception of sex), we tested their potential as hidden confounds due to their known associations with physiological processes unrelated to inflammation (e.g., BMI and altered glucose sensitivity) that have the potential to influence fetal brain development. All analyses examining associations between maternal IL-6 concentrations during pregnancy, UF development over the first year of life, and socioemotional and cognitive developmental outcomes at 12-mo also included the total HOME score as a covariate to account for the influence of the postnatal environment on infant brain development during this period.
2.8 Characterization of Maternal IL-6-associated Change in Fractional Anisotropy from Birth until 12-Mo Age and Socioemotional and Cognitive Development at 12-Mo Age
The association between region specific change in FA (from birth until 12-mo age) and the behavioral outcomes (socioemotional and cognitive development at 12-mo age) was tested via linear regression. To limit the number of comparisons and because it has been suggested that neurodevelopmental trajectories are more informative than specific neurophenotypic endpoints in neurodevelopmental disorders (e.g. autism57), we focused on longitudinal change in UF FA as the main predictor of 12-mo socioemotional and cognitive development. These analyses were adjusted for total HOME score, in acknowledgment of the impact the caregiving environment can have for brain, and subsequently socioemotional and cognitive, development.
Statistical mediation of the association between maternal IL-6 concentration during pregnancy and infant behavior at 12-mo age via variation in UF FA (longitudinal change in FA) was assessed using the Baron and Kenny method.58 This was followed by formal testing of the indirect pathway from maternal IL-6 concentrations to infant behavior via longitudinal change in FA within a structural equation modeling framework and employing bootstrapping (N=1,000) (Mplus, Version 7(37)).
3. Results
3.1 Interleukin-6 Concentration during Pregnancy
Median IL-6 across pregnancy (prior to base 2 logarithm transformation to normal distribution) was 0.76 pg/ml with a lower and upper quartile of 0.47 and 1.34 pg/ml, respectively. The base 2 logarithm values of mean IL-6 across pregnancy were −0.36 ± 1.05 (S.D.) on average and normally distributed. The base 2 logarithm values were highly correlated across pregnancy trimesters (r1st,2nd=0.70; r2nd,3rd=0.59; r1st,3rd=0.54).
3.2 PCA-based Associations between Maternal IL-6 Concentration During Pregnancy and Newborn Fractional Anisotropy along the Uncinate Fasciculus
PCA-based results showed that the spatial intra-correlation structure was consistent with the known anatomical subdivisions of the UF59: composed of a frontal, isthmus and temporal segment (Figure 1B). Intra-tract correlation coefficients were large (r>0.6 within the three tract sub-regions), demonstrating a large degree of redundancy in interindividual FA variation across the tract, thus supporting data reduction. The first principal component explained 60%/61% (left/right) of interindividual variation in FA (Figure 1C), was uniformly loaded across the entirety of the UF (Figure 1D), and strongly associated with gestational age at birth (left UF: 95% CI [0.45 0.74], p<10−4; right UF: 95% CI [0.51 0.77], p<10−4) and postnatal age at scan (left UF: 95% CI [0.49 0.78], p<10- 4; right UF: 95% CI [0.53 0.79], p<10−4) but not maternal IL-6 concentration during pregnancy (left and right UF: p>0.4, Supplementary Table 1). The second principal component explained 9%/13% (left/right) of interindividual variation in FA (Figure 1C), was loaded primarily in the central isthmus portion of the UF (Figure 1D), and associated with average maternal IL-6 concentration across pregnancy but not gestational age at birth or postnatal age at scan (Table 2). The main effect of maternal IL-6 concentration during pregnancy on the second principal component was bi-lateral in the parsimonious model correcting for gestational at birth and postnatal age at scan. Sex-specific interactions with maternal IL-6 concentration during pregnancy were not significant in either the left or right UF (Table 2). Brain-wide analyses suggested that the association between maternal IL-6 concentration during pregnancy and newborn white matter FA is particularly salient to the UF (see Supplementary Materials Section S.4). The right UF survived post-hoc testing for potential prenatal confounding factors including household income, prepregnancy BMI, obstetric risk, smoking during pregnancy, and infant sex (Table 2). When including these additional covariates, the association between maternal IL-6 concentration and the left UF was reduced to a tendency (pIL-6, omnibus=0.082); however, none of the potential confounding factors were significantly (p<0.05) associated with the UF FA outcome in the full model, thereby supporting the appropriateness of the parsimonious model (Table 2).
3.3 Tract-based Associations between Maternal IL-6 Concentration During Pregnancy and Newborn Fractional Anisotropy along the Uncinate Fasciculus
Tract-based association analyses were conducted by treating points along the tract as independent observations in order to characterize regional effects of maternal IL-6 concentration during pregnancy on newborn UF FA. Consistent with the PCA-based findings, gestational age at birth and postnatal age at scan were significantly and positively associated (p<0.05) with FA bi-laterally across large extents of the UF (95% of left tract locations; 98% right). After accounting for the effects of gestational age at birth and postnatal age at scan, maternal IL-6 concentration during pregnancy was bi-laterally and negatively associated with FA along the isthmus of the UF (Figure 2). The regional variation in effect size (beta coefficients) of maternal IL-6 (bottom row, Figure 2) mirrored the second principal component loading coefficients (Figure 1D), identified using the PCA data reduction approach. Significant clusters of association were identified using Monte Carlo methods (p<0.05) and served as ROIs (via spatial masking) for the post-hoc testing. The identified clusters were bilateral, along the isthmus of the UF, and in a region proximal to the amygdala (Figure 3). There was no significant main effect of infant sex or an interaction effect between maternal IL-6 concentration during pregnancy and infant sex on either left or right ROI-extracted UF FA (Table 3). Further, the association between maternal IL-6 concentration during pregnancy and FA in the identified clusters survived, bi-laterally, post-hoc testing for the potentially confounding variables household income, prepregnancy BMI, obstetric risk, smoking during pregnancy, and infant sex (Table 3).
Figure 2. Tract-based Associations Between Early Life Fractional Anisotropy and Average Maternal IL-6 Concentration Across Pregnancy.
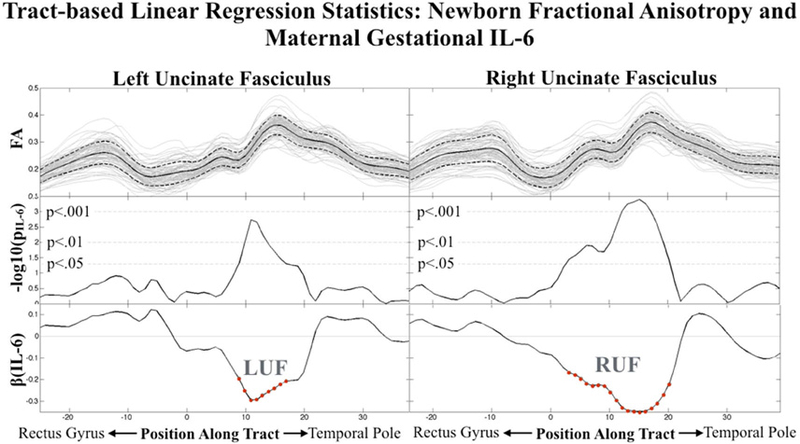
Full sample Fractional Anisotropy (top row), tract-based significance for maternal gestational IL-6 associations (middle row) and effect size for maternal gestational IL-6 associations (bottom row) are shown bi-laterally for the uncinate fasciculus. Clusters used in post-hoc analyses are denoted by red circle indicators and a label: (LUF=Left Uncinate Fasciculus, RUF=Right Uncinate Fasciculus).
Figure 3. Negative Association Between Newborn Fractional Anisotropy and Average Maternal IL-6 Concentration Across Pregnancy.
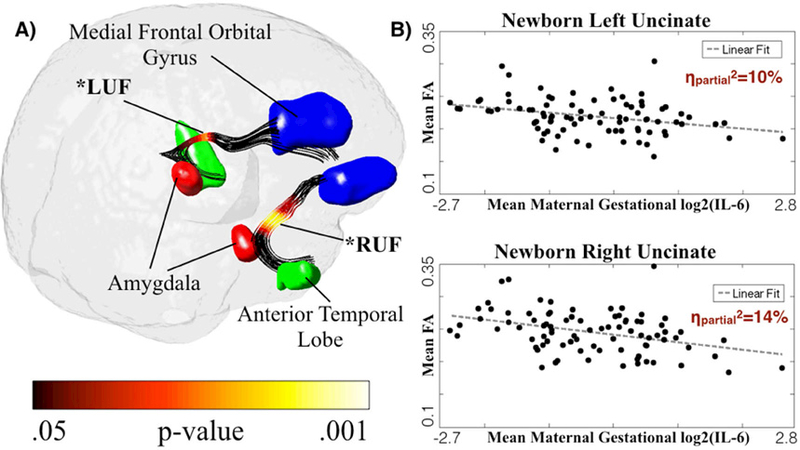
A) Tract-based significance as projected onto the uncinate fasciculus in template space demonstrates a bilateral pattern of significance along the middle portion of the UF and proximal to the amygdala. B) Linear regression-derived scatter plots of gestational and postnatal age-corrected FA versus mean maternal IL-6 concentration across pregnancy are shown. Clusters used in post-hoc analyses are denoted by an asterisk and a label: (LUF=Left Uncinate Fasciculus, RUF=Right Uncinate Fasciculus).
3.4 ROI-based Associations between IL-6 Concentration and Fractional Anisotropy at 12-Mo Age and Change from Birth Until 12-Mo Age
For these analyses, FA at 12-mo age and longitudinal change in FA from birth until 12-mo age were restricted to the significant clusters (ROIs) identified in the neonatal analyses (described above) in order to conserve statistical power by reducing degrees of freedom. Average maternal IL-6 concentration across pregnancy was positively associated with change in FA in the UF ROI from birth until 12-mo age in the UF (Figure 4, p<0.05), but not with FA in the UF ROI at 12-mo age (Table 4). Thus, higher maternal IL-6 concentration across pregnancy was reflective of lower UF FA at birth, but accelerated maturation during the first year of life, resulting in no association between maternal IL-6 concentration during pregnancy and FA in the UF ROI at 12-mo age.
Figure 4. Positive Association Between Change in Uncinate Fasciculus (UF) FA from Birth Until 12-mo Age and Average Maternal IL-6 Concentration Across Pregnancy.
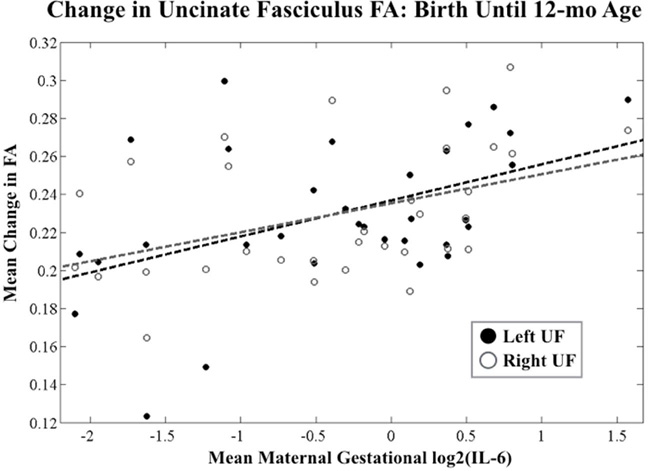
Linear regression-derived scatter plots of gestational and postnatal age- corrected change in FA versus mean maternal IL-6 concentration across pregnancy are shown. Scatter points reflect ROI-based extracted FA values from central UF.
3.5 Maternal IL-6-associated Change in Fractional Anisotropy from Birth until 12-Mo Age and Socioemotional and Cognitive Development at 12-Mo Age
Average maternal IL-6 concentration across pregnancy was associated with Bayley cognitive development scores (95% CI [−8.7, −0.3], p=0.036), but not socioemotional development scores (95% CI [−5.2, 6.1], p=0.88) at 12-mo age after adjustment for interindividual variation in the postnatal caregiving environment. ROI-based longitudinal FA change from birth to 12-mo age in the left UF (95% CI [−263.1, −35.5], p=0.012), but not the right UF (95% CI [−111.1, 122.5], p=0.92), was associated with Bayley cognitive development scores at 12-mo age, after adjustment for the postnatal caregiving environment. Neither left (95% CI [−271.1, 53.5], p=0.18) nor right (95% CI [−192.5, 76.3], p=0.38) UF FA were associated with Bayley socioemotional scores at 12-mo age, after adjustment for the postnatal caregiving environment.
Based on the consideration that maternal IL-6 concentration during pregnancy was prospectively associated with both change in left UF FA from birth until 12-mo age and Bayley cognition scores at 12-mo age, we tested whether change in FA (newborn until 12-mo age) mediated the association between maternal IL-6 concentration during pregnancy and cognitive developmental status at 12-mo age. Using the Baron and Kenny method for testing mediation, there was evidence for the change in UF FA from newborn until 12-mo age mediating the association between maternal IL-6 concentration during pregnancy and cognitive development at 12-mo age (full model including IL-6 and change in FA as predictors of cognitive developmental status at 12-mo: pIL-6=0.18, Pchange,FA=005; Figure 5). The mediation effect remained significant in a formalized, bias-corrected, bootstrap analysis (95%CI [−4.1 −.12], p=0.05).
Figure 5. Mediation Model.
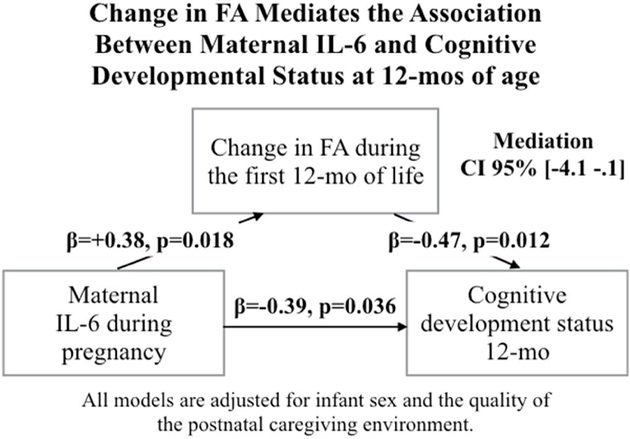
Maternal gestational IL-6 concentration is positively associated with longitudinal change in left UF FA during the first 12 mos of life and negatively associated with cognitive developmental status at 12-mo. Longitudinal change in left UF FA is negatively associated with cognitive developmental status at 12-mo. Longitudinal change during the first year of life in the left UF FA mediates the association between maternal gestational IL-6 concentration and cognitive developmental status at 12-mo age (p<0.05).
4. Discussion
The current study provides strong supporting evidence that the maternal inflammatory state during pregnancy is prospectively associated with offspring frontolimbic white matter microstructural properties. Further, we provide evidence that maternal inflammatory-related maturational changes in frontolimbic white matter microstructural properties during the first 12 months of postnatal life are associated with early cognitive development. Specifically, using a prospective longitudinal study design, we made the following three observations: 1) maternal IL-6 concentration across pregnancy was inversely associated with newborn UF FA, 2) maternal IL-6 concentration across pregnancy was positively associated with UF FA growth during the first 12 months of postnatal life, and 3) maternal inflammatory-related accelerated UF FA growth during the first 12 months of postnatal life mediated the negative association between maternal IL-6 concentration during pregnancy and offspring cognitive functioning at 12-mo age. Thus, maternal inflammation during pregnancy (as indicated by elevated IL-6 concentrations) appears to have implications both for the status of the offspring newborn brain and for subsequent postnatal brain development and cognitive functioning.
The magnitude of the association between maternal IL-6 concentration during pregnancy and offspring UF FA in the neonate was substantial, accounting for up to 14% of interindividual variation in newborn FA. In addition, this association persisted after correcting for household income, prepregnancy BMI, obstetric risk, smoking during pregnancy, and infant sex. Finally, in support of the generalizability of the current findings to within and across the UF, the observed association between maternal IL-6 concentration during pregnancy and newborn UF FA was found to be consistent across two complimentary methodological approaches (PCA-based and tract-based).
The strongest associations between maternal IL-6 concentration during pregnancy and offspring FA (newborn and change from near birth until 12-mo age) were in the central portion (isthmus) of the UF, a region proximal to limbic structures including the amygdala and hippocampus. The effect observed in the central UF is spatially consistent with FA reductions previously reported in high functioning ASD patients.60 However, it remains unclear how this region is uniquely affected by a higher inflammatory state during gestation or if the less coherent organization of the peripheral ends of the UF obfuscates the effect. While the inferior fronto-occipital fasciculus (IFOF) runs superior along the UF past the temporal stem, no major white matter tracts cross the UF in its short path from the temporal lobe to the frontal cortex, suggesting that the regional specificity is likely not a result of an interaction with crossing fibers. Interestingly, maternal depression during pregnancy has been shown to be associated with lower FA in the newborn amygdala (proximal to central UF),61 whereas maternal depression during pregnancy was positively associated with amygdala FA by 4 years of age.62 This supports the notion that the influence of the prenatal environment may be more salient to the dynamic process of brain development, as opposed to specific developmental end-points.
The observed pattern between maternal inflammation during pregnancy and early life UF FA can be summarized as two phases: 1) retarded growth during gestation (negative association between maternal IL-6 concentration and offspring UF FA), and 2) accelerated growth postnatally (positive association between maternal IL-6 concentration and offspring UF FA growth). Accelerated postnatal growth can be considered compensatory (i.e. a regression towards the mean in response to growth selectively restricted in utero) or reflective of a more general postnatal overgrowth pattern (i.e. the 12-month time point is where the growth trajectories of infants exposed to high versus low gestational inflammation cross paths, resulting in a null association at 12-mo age). Interestingly, the latter is consistent with the abnormal patterns of postnatal brain growth observed in Autism Spectrum Disorder (ASD)63–67, for which prenatal inflammation is a risk factor, that have been characterized as reduced at birth but increasing in an accelerated manner during the first year of life.68 Certainly, while our current study is not enriched for autism-risk, and therefore not positioned to meaningfully inform ASD disease related processes, the overlapping patterns and hypothesized links between maternal inflammation and offspring ASD-risk highlight the need for more comprehensive studies in humans characterizing the association between an inflammatory gestational milieu and postnatal brain growth trajectories in mother-child dyads at high risk for psychiatric disorders.
Our observation that accelerated change in UF FA from birth until 12-mo age is related to poorer cognitive development at 12-mo age suggests that this observed catchup growth may not be beneficial. This furthermore supports the notion that the dynamic process of brain development (i.e., change in UF integrity within the first year of life) may be more important and informative for the establishment of later behavioral phenotypes than the outcome at a certain point in time (i.e., UF integrity at 12-mo age), further emphasizing the need for prospective longitudinal studies aimed at characterizing brain developmental trajectories. While we again emphasize that the current study is not positioned to unravel ASD disease related processes, it is worth noting that in addition to maternal IL-6 concentration during pregnancy being associated with increased postnatal growth, the direction of effects observed here (neg. association between postnatal UF FA growth and cognitive development at 12-mo) are consistent with the cognitive deficits commonly seen in ASD at an early age.69,70 Finally, the association between UF FA maturation during the first year of life and cognitive developmental status at 12-mo age was significant in the left, but not the right, UF. This is consistent with empirical evidence for alterations in the left UF being especially relevant in the context of early social emotional deprivation,71 and cognitive deficits found in clinical conditions.43
Based on the consideration that the structural connectivity of the UF is known to play a role in a variety of neurodevelopmental and psychiatric disorders in humans, of which many share common underlying socioemotional deficits, we hypothesized that altered UF FA development would be associated with socioemotional development at 12-mo age. While our findings did not support such a hypothesis, we cannot rule out the possibility that the combination of the reduced sample size in longitudinal data with behavioral outcomes (N=30), and the high degree of rater variability that accompanies maternal self-reporting of a single nuanced dimension of behavior, was insufficiently powered for identifying a meaningful effect of frontolimbic development on socioemotional outcomes. Given the strong presence of evidence for our hypothesis, we submit that longitudinal behavioral follow-up of this sample is warranted in order to more deeply characterize socioemotional outcomes in childhood.
It should be noted that common measures of diffusion, including FA, are an aggregate measure of a number of concomitant structural attributes at the microscopic level including axonal diameter, compaction, water content and fiber coherence,72 thereby complicating the specification of underlying microstructural properties associated with maternal inflammation during pregnancy. However, given that white matter maturation in fetal and early postnatal life is characterized by rapid neurogenesis, axonal organization, oligodendrocyte proliferation and myelination,73–75 it is plausible that in utero exposure to maternal inflammation has affected these developmental processes. Because FA is largely insensitive to the isotropic process of oligodendrocyte proliferation and because the UF is one of the last structures in the developing brain to fully myelinate, based on our findings, the most likely processes to be influenced by maternal IL-6 concentration during pregnancy are pre- and postnatal neurogenesis and changes in axonal coherence.76–78 This is further supported by an observed correspondence between FA and AD findings (Supplementary Table 2 and Figure 4) when considering existing models of developmental changes in which axonal coherence and neurogenesis are uniquely accompanied by changes in FA and AD.73,75 However, given the speculative nature of interpreting microscopic properties from macroscopic DTI observations72, future efforts in animal models aimed at elucidating the microstructural correlates of macroscopic diffusion measures are warranted, particularly in the context of developmental programming via maternal inflammation.
Limitations of the current study include the use of post-hoc statistics aimed at accounting for potentially confounding factors, the narrow focus on the UF, and the limited longitudinal sample size. Because testing for potential confounds occurred in a post-hoc fashion by selecting regions significantly associated with maternal IL-6 concentration during pregnancy in a parsimonious model, subsequent testing of confounding factors was biased towards IL-6 significance.79 Therefore, the bias towards IL-6 does not fully allow the omnibus model to generalize how other aspects of the prenatal environment relate to newborn UF FA. However, the lack of significant associations with UF FA across the potential confounding factors, and the stability of the association between maternal IL-6 concentration and offspring UF FA after their inclusion in the statistical model, supports the parsimonious model as being the most appropriate choice. While supplementary analyses (Section S.4) suggested that the effects of maternal IL-6 concentration during pregnancy are highly salient to fronto-limbic white matter pathways (UF), less significant unilateral effects were detected on other frontally connected pathways (e.g. inferior fronto-occipital fasciculus). Therefore, we cannot fully exclude a more diffuse association between maternal IL-6 and frontal white matter. Future efforts employing complimentary strategies (e.g. amygdala-specific connectivity80) aimed at characterizing a more refined specificity of offspring brain alterations in association with maternal inflammation during pregnancy would be beneficial. Finally, complete longitudinal data (with newborn and 12-mo measures) were available in only a subset (N=30) of the neonatal population. While this limits the generalizability of the longitudinal findings, we submit that this study sample represents the largest, to date, with serial characterization of maternal inflammation during pregnancy and of offspring longitudinal brain development. In addition, it should be noted that the effect size (R2=22%) of the negative association between maternal IL-6 concentration during pregnancy and newborn FA was consistent between the subset of individuals with serial imaging (N=32) and those with newborn imaging (N=86), suggesting the subsample as being representative of the larger sample.
The findings of the current study contribute towards a continually growing body of evidence that supports maternal inflammation during pregnancy as having an influence on fetal brain development with potential implications for psychiatric disease risk. The magnitude of the association between maternal IL-6 concentration during pregnancy and offspring neonatal FA along the UF was large (up to 14% of interindividual variation explained). Put into a clinical context, one recent study demonstrated a regionally similar reduction in UF FA of ~16% in high functioning ASD individuals,60 roughly equivalent to the observed difference between offspring in the lowest and highest quintiles of maternal IL-6 concentration during pregnancy in the current sample. While our data could be interpreted as a normalization of UF FA in infants exposed to a high inflammatory milieu by 12-mo age, this is achieved by an accelerated growth trajectory (i.e. prenatal undergrowth is followed by early postnatal overgrowth) that is predictive of poorer cognitive performance at 12-mo age.
5. Conclusions
Findings from the current study support the premise that susceptibility for cognitive impairment (and potentially neurodevelopmental and psychiatric disorders) may, in part, be programmed in utero, and that such programming may be mediated through changes in frontolimbic white matter growth trajectories in early life. Thus, the current study adds to the growing awareness of the role the intrauterine environment plays for interindividual variation in offspring developmental outcomes and further suggests that the origins of cognitive and mental health problems may have their origins very early in life. Because the results of the current study highlight the gestational inflammatory milieu as a plausible etiological pathway, and because the gestational inflammatory milieu is a feasible intervention target, we further assert that this study provides a clinically-relevant basis for an improved understanding of the developmental origins of psychiatric disease, early identification of at-risk pregnancies, and thereby targets for the primary prevention of disease risk.
Supplementary Material
Table 2. Parsimonious, Sex-Specific and Full Models for Uncinate Fasciculus (UF) Fractional Anisotropy (FA) Principal Component 2.
The association between average maternal Interleukin-6 (IL-6) concentration across pregnancy and FA in the right UF remained significant (p<0.05) after adjusting for confounding variables. Sex-specific effects of maternal gestational IL-6 concentration were not significant. The significance of the association in the left UF was attenuated to a tendency (p=0.08) in the full model. However, none of the confounding factors in the full model were significantly (p<0.05) associated with FA suggesting the parsimonious model as the most appropriate choice.
| Dependent Var. |
Fiber Tract |
Independent Var. | r- squared |
p- statistic |
|
|---|---|---|---|---|---|
| Parsimonious | L UF | Gestational Age | 1.1% | 0.303 | |
| FA (N=86) | Postnatal Age | 0.1% | 0.802 | ||
| Avg. Maternal IL-6 | 8.1% | 0.008 | ** | ||
| R UF | Gestational Age | 0.6% | 0.384 | ||
| Postnatal Age | 1.5% | 0.213 | |||
| Avg. Maternal IL-6 | 12.2% | 0.001 | ** | ||
| Sex-Specific | L UF | Gestational Age | 1.1% | 0.352 | |
| FA (N=86) | Postnatal Age | 0.1% | 0.728 | ||
| Avg. Maternal IL-6 | 6.5% | 0.016 | * | ||
| Sex | 0.0% | 0.950 | |||
| Sex x IL-6 | 0.9% | 0.370 | |||
| R UF | Gestational Age | 0.6% | 0.387 | ||
| Postnatal Age | 1.0% | 0.246 | |||
| Avg. Maternal IL-6 | 9.5% | 0.003 | ** | ||
| Sex | 1.3% | 0.392 | |||
| Sex x IL-6 | 1.8% | 0.248 | |||
| Full Model | L UF | Gestational Age | 2.3% | 0.162 | |
| FA (N=86) | Postnatal Age | 0.1% | 0.690 | ||
| Avg. Maternal IL-6 | 3.4% | 0.082 | |||
| Sex | 0.0% | 0.368 | |||
| Obstetric Risk | 2.3% | 0.099 | |||
| Pre-preg. BMI | 3.2% | 0.676 | |||
| Family Income | 1.3% | 0.279 | |||
| Maternal Smoking | 0.2% | 0.837 | |||
| R UF | Gestational Age | 0.8% | 0.380 | ||
| Postnatal Age | 0.9% | 0.368 | |||
| Avg. Maternal IL-6 | 8.9% | 0.006 | ** | ||
| Sex | 0.8% | 0.235 | |||
| Obstetric Risk | 3.5% | 0.670 | |||
| Pre-preg. BMI | 0.3% | 0.911 | |||
| Family Income | 0.0% | 0.990 | |||
| Maternal Smoking | 0.0% | 0.473 |
Table 3. Parsimonious, Sex-Specific and Full Models for Regional Fractional Anisotropy (FA) in the Uncinate Fasciculus (UF).
The association between average maternal Interleukin-6 (IL-6) concentration across pregnancy and FA in the central UF ROI remained bi-laterally significant (p<0.05) after adjusting for confounding variables. Sex-specific effects of maternal gestational IL-6 concentration were not significant. None of the confounding factors in the full model were significantly (p<0.05) associated with FA suggesting the parsimonious model as the most appropriate choice.
| Dependent Var. |
Fiber Tract |
Independent Var. | r- squared |
p- statistic |
|
|---|---|---|---|---|---|
| Parsimonious | L UF | Gestational Age | 6.7% | 0.016 | * |
| FA (N=86) | Postnatal Age | 14.2% | <0.001 | *** | |
| Avg. Maternal IL-6 | 10.1% | 0.003 | ** | ||
| R UF | Gestational Age | 6.3% | 0.017 | * | |
| Postnatal Age | 8.2% | 0.009 | ** | ||
| Avg. Maternal IL-6 | 14.3% | 0.001 | *** | ||
| Sex-Specific | L UF | Gestational Age | 5.8% | 0.015 | * |
| FA (N=86) | Postnatal Age | 11.4% | 0.001 | ** | |
| Avg. Maternal IL-6 | 6.7% | 0.009 | ** | ||
| Sex | 0.0% | 0.953 | |||
| Sex x IL-6 | 0.9% | 0.317 | |||
| R UF | Gestational Age | 5.7% | 0.016 | * | |
| Postnatal Age | 6.9% | 0.008 | ** | ||
| Avg. Maternal IL-6 | 10.2% | 0.001 | ** | ||
| Sex | 0.8% | 0.369 | |||
| Sex x IL-6 | 1.5% | 0.214 | |||
| Full Model | L UF | Gestational Age | 4.6% | 0.033 | * |
| FA (N=86) | Postnatal Age | 10.4% | 0.002 | ** | |
| Avg. Maternal IL-6 | 4.9% | 0.028 | * | ||
| Sex | 0.0% | 0.457 | |||
| Obstetric Risk | 1.5% | 0.504 | |||
| Pre-preg. BMI | 0.4% | 0.968 | |||
| Family Income | 0.1% | 0.747 | |||
| Maternal Smoking | 0.0% | 0.860 | |||
| R UF | Gestational Age | 4.6% | 0.032 | * | |
| Postnatal Age | 7.0% | 0.009 | ** | ||
| Avg. Maternal IL-6 | 10.1% | 0.002 | ** | ||
| Sex | 0.4% | 0.462 | |||
| Obstetric Risk | 1.5% | 0.929 | |||
| Pre-preg. BMI | 0.0% | 0.340 | |||
| Family Income | 0.0% | 0.840 | |||
| Maternal Smoking | 0.9% | 0.523 |
Table 4. Correlation Between Maternal Gestational Interleukin-6 (IL-6) Concentration and Age-Corrected Offspring Regional Fractional Anisotropy (FA) in Early Life.
Average maternal IL-6 concentration during pregnancy was negatively associated with age-corrected FA bi-laterally in the newborn Uncinate Fasciculus (UF). Maternal gestational IL-6 concentration and UF FA at 12-mo age were not significantly associated. However, elevated maternal gestational IL-6 concentration was associated with accelerated UF FA increase during the 12-mos of life.
| Dep. Var. | Fiber Tract | Age Assessment | r [95% CI] | p-statistic |
|---|---|---|---|---|
| FA (N=86) | L UF ROI | Newborn | -0.32 [−0.5 −0.1] | 0.004** |
| R UF ROI | Newborn | -0.34 [−0.52 −0.13] | 0.002** | |
| FA (N=32) | L UF ROI | 12-mo | 0.09 [−0.27 0.42] | 0.643 |
| R UF ROI | 12-mo | 0.03 [−0.32 0.37] | 0.875 | |
| FA (N=32) | L UF ROI | 12-mo Change | 0.42 [0.09 0.67] | 0.013* |
| R UF ROI | 12-mo Change | 0.47 [0.14 0.7] | 0.007** |
Acknowledgements
Support for this work was provided by National Institute of Child Health and Human Development [grant number R01 MH091351]; and National Institute of Mental Health [grant number R01 MH091351].
Footnotes
Declarations of interest: None.
References
- 1.Qiu A, Mori S & Miller MI Diffusion tensor imaging for understanding brain development in early life. Annu. Rev. Psychol 66, 853–876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makropoulos A, Counsell SJ & Rueckert D A review on automatic fetal and neonatal brain MRI segmentation. NeuroImage (2017). doi:10.1016/j.neuroimage.2017.06.074 [DOI] [PubMed] [Google Scholar]
- 3.Gao W, Lin W, Grewen K & Gilmore JH Functional Connectivity of the Infant Human Brain: Plastic and Modifiable. Neuroscientist (2016). doi:10.1177/1073858416635986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham AM et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci 18, 12–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buss C, Entringer S & Wadhwa PD Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Science signaling (2012). doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham AM et al. Developmental Cognitive Neuroscience. Accident Analysis and Prevention 12, 12–39 (2015). [Google Scholar]
- 7.Graham AM, Pfeifer JH, Fisher PA, Carpenter S & Fair DA Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol. & Psychiat 56, 1212–1222 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandaker GM, Zimbron J, Lewis G & Jones PB Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol. Med 43, 239–257 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown AS et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry 61, 774–780 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Brown AS & Patterson PH Maternal infection and schizophrenia: implications for prevention. Schizophr Bull 37, 284–290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AS & Derkits EJ Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. American Journal of Psychiatry 167, 261– 280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buka SL et al. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry 58, 1032–1037 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Fang S-Y, Wang S, Huang N, Yeh H-H & Chen C-Y Prenatal Infection and Autism Spectrum Disorders in Childhood: A Population-Based Case-Control Study in Taiwan. Paediatr. Perinat. Epidemiol 29, 307–316 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Machón RA, Mednick SA & Huttunen MO Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 54, 322– 328 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Cannon M et al. Prenatal exposure to the 1957 influenza epidemic and adult schizophrenia: a follow-up study. Br. J. Psychiatry 168, 368–371 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Meyer U, Feldon J & Dammann O Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res 69, 26R– 33R (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knuesel I et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol 10, 643–660 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Li Y-M et al. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J. Autism Dev. Disord 46, 95–102 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Krakowiak P et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129, e1121–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds LC, Inder TE, Neil JJ, Pineda RG & Rogers CE Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J Perinatol 34, 688–692 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coussons-Read ME, Okun ML, Schmitt MP & Giese S Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom. Med 67, 625–631 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Entringer S, Buss C & Wadhwa PD Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 62, 366–375 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madan JC et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine 47, 61–64 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Gayle DA et al. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol 286, R1024–9 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Urakubo A, Jarskog LF, Lieberman JA & Gilmore JH Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res 47, 27–36 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Meyer U et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. Journal of Neuroscience 26, 4752–4762 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal M, Marzouk AC, Donnelly R & Ponzio NM Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain , Behavior, and Immunity 25, 863–871 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Burns TM, Clough JA, Klein RM, Wood GW & Berman NE Developmental regulation of cytokine expression in the mouse brain. Growth Factors 9, 253–258 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Mehler MF & Kessler JA Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci 20, 357–365 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Deverman BE & Patterson PH Cytokines and CNS development. Neuron 64, 61–78 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Boulanger LM Immune proteins in brain development and synaptic plasticity. Neuron 64, 93–109 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Zhao B & Schwartz JP Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J. Neurosci. Res 52, 7–16 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Smith SEP, Li J, Garbett K, Mirnics K & Patterson PH Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience 27, 10695–10702 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham AM et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biological Psychiatry (2017). doi:10.1016/j.biopsych.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindner P, Flodin P, Larm P, Budhiraja M, Savic-Berglund I, Jokinen J, Tiihonen J, Hodgins S. Amygdala-orbitofrontal structural and functional connectivity in females with anxiety disorders, with and without a history of conduct disorder. Scientific reports. (2018). Jan 18;8(1):1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Martino A et al. Aberrant striatal functional connectivity in children with autism. Biological Psychiatry 69, 847–856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinstein I et al. Disrupted neural synchronization in toddlers with autism. Neuron 70, 1218–1225 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schipul SE, Keller TA & Just MA Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci 5, 10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Passamonti L et al. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS ONE 7, e48789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns J et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry 182, 439–443 (2003). [PubMed] [Google Scholar]
- 41.Kubicki M et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. NeuroImage 26, 1109–1118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voineskos AN et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 133, 1494–1504 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitis O et al. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin. Neurosci 66, 34–43 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Craig MC et al. Altered connections on the road to psychopathy. Mol. Psychiatry 14, 946–53– 907 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Sundram F et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex 48, 216–229 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Motzkin JC, Newman JP, Kiehl KA & Koenigs M Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience 31, 17348–17357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan NP et al. White matter microstructure predicts longitudinal social cognitive outcomes after paediatric traumatic brain injury: a diffusion tensor imaging study. Psychol. Med 1–13 (2017). doi:10.1017/S0033291717002057 [DOI] [PubMed] [Google Scholar]
- 48.Downey LE et al. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. Neuroimage. Clin 8, 640–651 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oishi K et al. Critical role of the right uncinate fasciculus in emotional empathy. Ann. Neurol 77, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho TC et al. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc. Cogn. Affect. Neurosci 12, 1460–1469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Counsell SJ et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 131, 3201–3208 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Bradley RH et al. The HOME inventory: a new scale for families of pre- and early adolescent children with disabilities. Res. Dev. Disabil 13, 313–333 (1992). [DOI] [PubMed] [Google Scholar]
- 53.Verde AR et al. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Frontiers in Neuroinformatics (2014). doi:10.3389/fninf.2013.00051/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oguz I, Farzinfar M, Matsui J, Budin F & Liu Z DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics (2014). doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. NeuroImage 2004. Jan 1;23:S151-60. [DOI] [PubMed] [Google Scholar]
- 56.Pineda RG, Neil J, Dierker D, Smyser CD, Wallendorf M, Kidokoro H, Reynolds LC, Walker S, Rogers C, Mathur AM, Van Essen DC. Alterations in brain structure and neurodevelopmental outcome in preterm infants hospitalized in different neonatal intensive care unit environments. The Journal of pediatrics 2014. January 1;164(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courchesne E et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57, 245–254 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Baron RM & Kenny DA The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol 51, 1173–1182 (1986). [DOI] [PubMed] [Google Scholar]
- 59.Ebeling U, von Cramon D Topography of the uncinate fascicle and adjacent temporal fiber tracts, Acta Neurochir (Wien). (1992), vol. 115 (pg. 143–8) [DOI] [PubMed] [Google Scholar]
- 60.Samson AC et al. White matter structure in the uncinate fasciculus: Implications for socio-affective deficits in Autism Spectrum Disorder. Psychiatry Research 255, 66–74 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Rifkin-Graboi A et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biological Psychiatry 74, 837–844 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Wen DJ et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry 7, e1103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zielinski BA et al. Longitudinal changes in cortical thickness in autism and typical development. Brain 137, 1799–1812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chomiak T & Hu B Alterations of neocortical development and maturation in autism: insight from valproic acid exposure and animal models of autism. Neurotoxicol Teratol 36, 57–66 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Courchesne E, Campbell K & Solso S Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Research 1380, 138– 145 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chomiak T, Karnik V, Block E & Hu B Altering the trajectory of early postnatal cortical development can lead to structural and behavioural features of autism. BMC Neurosci 11, 102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben Bashat D et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. NeuroImage 37, 40–47 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Redcay E & Courchesne E When is the brain enlarged in autism? A metaanalysis of all brain size reports. Biological Psychiatry 58, 1–9 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Torras Mañá M, Gómez Morales A, González Gimeno I, Fornieles Deu A, Brun Gasca C Assessment of cognition and language in the early diagnosis of autism spectrum disorder: usefulness of the Bayley Scales of infant and toddler development. Journal of Intellectual Disability Research (2016). May 1;60(5):502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höglund Carlsson L, Norrelgen F, Kjellmer L, Westerlund J, Gillberg C & Fernell E Coexisting disorders and problems in preschool children with autism spectrum disorders. The Scientific World Journal (2013), 1–6. DOI: 10.1155/2013/213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eluvathingal TJ et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics 117, 2093–2100 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Jones DK, Knösche TR & Turner R White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage 73, 239–254 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen JM et al. A novel maturation index based on neonatal diffusion tensor imaging reflects typical perinatal white matter development in humans. Int. J. Dev. Neurosci 56, 42–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubois J et al. Microstructural correlates of infant functional development: example of the visual pathways. Journal of Neuroscience 28, 1943–1948 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubois J et al. Review the early devlopment of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience 1–24 (2014). doi:10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 76.Miranda-Dominguez O et al. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. Journal of Neuroscience 34, 5552–5563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grayson DS & Fair DA Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage 160, 15–31 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stafford JM et al. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. U.S.A 111, 18745–18750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kriegeskorte N, Simmons WK, Bellgowan PSF & Baker CI Circular analysis in systems neuroscience: the dangers of double dipping. Nature Publishing Group 12, 535–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saygin ZM, Osher DE, Koldewyn K, Martin RE, Finn A, Saxe R, Gabrieli JD, Sheridan M Structural connectivity of the developing human amygdala. PloS one (2015);10(4):e0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


