Abstract
Effective coordination of the biological stress response is integral for the behavioral well-being of an organism. Stress reactivity is coordinated by an interplay of the neuroendocrine system and the sympathetic nervous system. The hypothalamus-pituitary-adrenal (HPA) axis plays a key role in orchestrating bodily responses to stress, and activity of the axis can be modified by a wide range of experiential events. This review will focus on several factors that influence subsequent HPA axis reactivity. Some of these factors include early life adversity, exposure to chronic stress, immune activation, and traumatic brain injury. The central premise is that each of these experiences serve as general vulnerability factors that accelerate future HPA axis reactivity in ways that make individuals more sensitive to stress challenges, and therefore feed forward into exacerbation of ongoing, or greater susceptibility toward, future stress-related disease states, especially as they pertain to negative affect and overall brain health.
Keywords: stress, oxytocin, inflammation, glucocorticoids, depression, anxiety, TBI
Introduction
The ability to successfully adapt to environmental challenges, including stressful circumstances, is central to organism survival and has been a key topic in biomedical research for decades. Indeed, Walter Cannon first introduced the concept of “fight or flight” to describe an organism’s response to acute stress challenges that threaten physiological function [1]. In doing so, he coined the term “homeostasis” [2] to extend Claude Bernand’s concept of “milieu intérieur”, which generally refers to the tendency to maintain constancy of the interior environment [3]. Thus, Cannon defined homeostasis as “The coordinated physiological processes which maintain most of the steady states in the organism…”[2]. In contrast, Hans Selye expanded the definition of stress by introducing the concept of the general adaption syndrome as the organism’s pathology in response to a chronic stressor [4]. These pioneers of the stress field built the conceptual framework for how most researchers view stress challenges, how an organism adapts physiologically to those threats, and best practices for understanding the diverse nature of threats to organism health. This work also highlighted the need to further understand mechanisms underlying the stress response [5], revealing the complexity of communication between the autonomic, immune, nervous and endocrine systems. Perturbations in the homeostasis of the stress network may hinder psychological and behavioral well-being. This review discusses various ways in which an organism’s stress response may be altered including early-life stress, chronic stress, and brain injury.
The generalized stress response
The generalized physiological response to stress, whether psychogenic or systemic in nature, consists of activation of the sympathetic motor system and the hypothalamus-pituitary-adrenal (HPA) axis. Both systems are controlled centrally from the hypothalamus, the master command center for autonomic and neuroendocrine systems. The stress response writ large consists of engaging multiple cellular and physiological processes, marshaling metabolic resources to support the mobilization of those processes, and restoring the different systems to baseline levels upon termination of the stress exposure. The stress activation of sympathetic motor and HPA neuroendocrine outputs is coordinated at the level of the hypothalamus to engineer these different components of the stress response within prescribed time points following the stress presentation. Thus, the sympathetic system is activated initially to assign salience and to effectuate fight or flight by increasing arousal, augmenting blood flow, increasing blood oxygenation, and mobilizing cellular energy processes, with obvious acute selective benefits for survival. The HPA response is slower in onset and longer in duration due to its neuroendocrine nature, its protracted kinetics being timed for maintenance of tissue fitness following sympathetic activation and for termination of the response upon cessation of the stress exposure (Figure 1). Glucocorticoids are an end secretory product of the HPA axis activation, and are a main effectuator of the comparably slow HPA component of the stress response.
Figure 1.
Relative time courses of major components of the generalized stress response. Stress exposure stimulates excitatory input to the hypothalamus, which triggers a rapid activation of the sympathetic motor system, followed by a delayed activation of the HPA axis.
The hypothalamus-pituitary-adrenal (HPA) axis
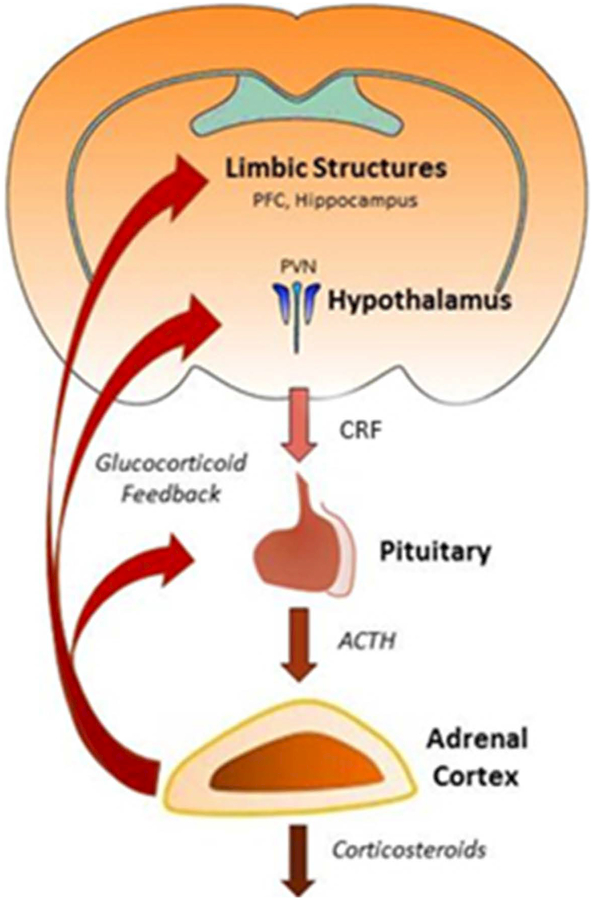
Among the many stress-responsive systems, the HPA axis remains one of the most studied indexes of stress reactivity and adaptation. This cascade is initiated by the release of neuropeptides and hormones from the parvocellular paraventricular nucleus of the hypothalamus (pPVN). Afferent stress signals converge in space and time to produce a neuroendocrine output in the form of corticotropin-releasing factor (CRF) secretion into the portal circulation of the anterior pituitary [6–8] (Figure 2). CRF stimulates corticotrophic cells within the anterior pituitary to release adrenocorticotropic hormone (ACTH). ACTH travels through systemic circulation and binds to receptors within the adrenal gland where it elicits glucocorticoid (CORT; cortisol in humans, corticosterone in rodents) synthesis and secretion [9]. Stress-induced activation of the HPA axis, therefore, leads ultimately to a surge of corticosteroids into the systemic bloodstream. Glucocorticoids have a plurality of cellular consequences that act throughout the body by binding to mineralocorticoid (MR) and glucocorticoid receptors (GR). In addition to influencing most physiological systems, MR and GR are both widely distributed in key brain regions that regulate the stress response, and participate in negative feedback regulation, which ultimately curtails further activity of the axis [10, 11]. The neuroendocrine nature of the HPA axis, which involves a multi-stage sequence of hormone secretion, blood transport, receptor activation, and corticosteroid synthesis and secretion (Figure 2), makes this a relatively slow and protracted process. The HPA component of the stress response, therefore, lags behind the sympathetic activation, and is phased to complement in function and in timing the acute activational properties of the sympathetic component of the response.
Figure 2.
HPA axis. HPA axis activation consists of CRF release from the hypothalamus, which stimulates ACTH secretion from the anterior pituitary and corticosteroid secretion from the adrenal cortex. Glucocorticoids feedback at multiple levels of the HPA axis and limbic system.
Although this canonical description of HPA axis reactivity creates the impression of a simplistic response to stress, each level of the axis can be modified by numerous other factors that are influenced by recent (or distal) stress history. The interaction and communication of many systems coordinate the propensity for homeostasis. In addition to glucocorticoid feedback, HPA axis-relevant limbic structures are activated in response to stress and, in turn, provide excitatory and inhibitory projections to the PVN, regulating the stress response [12]. Various neuropeptides may accompany activation of the HPA axis. For example arginine vasopressin (AVP), acts synergistically with CRF to activate downstream HPA axis responses [13]. Basal and stress induced HPA axis reactivity are also altered by oxytocin (OT) that is synthesized and released from the PVN, where OT attenuates activity within the axis [14]. Ultimately, output of the HPA axis has a profound influence on the immune system, autonomic regulation and behaviorally-relevant limbic regions [15]. Thus, the HPA axis is a highly dynamic, stress responsive system that is both causally activated by stressful circumstances and crucial for orchestration of organism-wide responses to stress.
Cellular mechanisms of rapid glucocorticoid negative feedback
Glucocorticoid receptors
Glucocorticoids are steroid hormones whose actions follow the canonical rules of nuclear signals by binding to intracellular receptors and regulating gene transcriptional activity. The classical nuclear glucocorticoid receptor belongs to the nuclear receptor superfamily of ligand-dependent transcription factors. However, while transcriptional regulation by glucocorticoids may influence long-term expression patterns and signaling capacity of glucocorticoid-sensitive target cells, transcriptional regulation is unlikely to play a defining role in the immediate glucocorticoid regulation of target tissues engaged in the acute stress response. Transcriptional glucocorticoid signaling is implicated in repeated or chronic stress exposure, but rapid, non-canonical glucocorticoid signaling mechanisms are required for glucocorticoids to mediate the acute HPA response to stress. A growing body of research has focused recently on rapid glucocorticoid actions that are mediated by the activation of one or more membrane-associated glucocorticoid receptors and downstream signal transduction mechanisms.
Crucial to the homeostatic function of the generalized stress response is the termination of the HPA response and the return to baseline of the HPA signals following cessation of the stress exposure. A critical role for glucocorticoids, among others, is to mediate feedback inhibition of HPA axis activation. Negative glucocorticoid feedback can occur at multiple levels of the HPA neuroendocrine axis, including at the CRF neurons [16] and the pituitary corticotrophs [17], as well as at upstream structures integrated into the broader limbic stress circuitry (e.g., hippocampus [18] and prefrontal cortex [19]) (Figure 2). While intuitively obvious from the rapid rate of glucocorticoid suppression of HPA activation, it was not until relatively recently that the rapid negative feedback effects of glucocorticoids on the HPA axis were understood to be mediated by non-transcriptional mechanisms via the activation of membrane-associated receptors [16, 20].
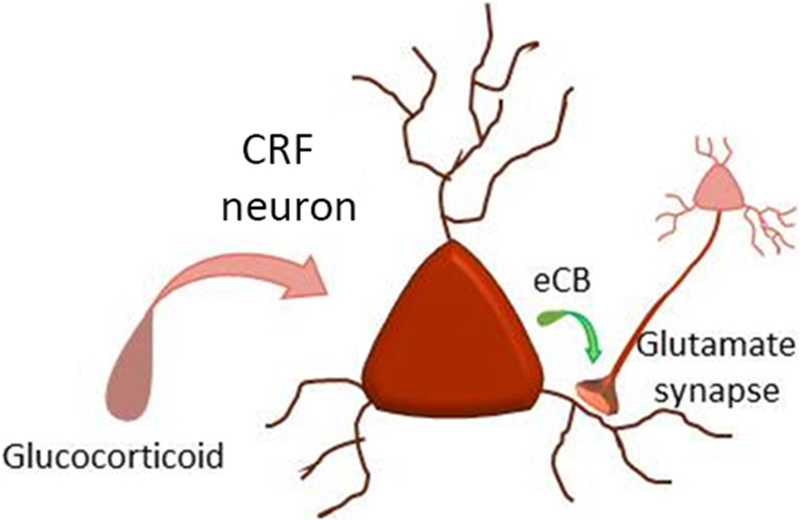
Intra-cerebral administration of glucocorticoids in the bilateral PVN rapidly suppresses stress-induced HPA activation via a membrane-associated receptor and endocannabinoid-dependent mechanism [16]. Glucocorticoids stimulate the synthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG) in PVN CRF neurons via the activation of a membrane-associated receptor [20–22] and a cAMP signaling pathway [22, 23]. The glucocorticoid-induced 2-AG is released at excitatory synapses onto the CRF neurons and is transmitted retrogradely to presynaptic glutamatergic axon terminals, where it suppresses glutamate release by binding to CB1 receptors [20, 24] (Figure 4). This endocannabinoid-induced suppression of synaptic excitation in the CRF neurons dampens the excitatory drive to the CRF neurons, which provides a circuit mechanism for the rapid negative feedback regulation of the HPA axis by glucocorticoids. The rapid glucocorticoid-induced, endocannabinoid-mediated suppression of synaptic excitation has also been found in magnocellular neuroendocrine cells [21, 22] and in preautonomic neurons of the PVN [25], suggesting these signaling effects may more broadly influence neuropeptides such as AVP and OT. The spatial restriction of the glucocorticoid-induced, as well as activity-dependent, endocannabinoid actions to excitatory synapses in PVN neuroendocrine cells is controlled by astroglial regulation of extracellular 2-AG trafficking [24].
Figure 4.
Glucocorticoid-induced suppression of synaptic excitation. Glucocorticoids stimulate the synthesis of the endocannabinoid 2-AG (eCB), which acts as a retrograde messenger to inhibit glutamate release from excitatory synapses via presynaptic CB1 receptor activation.
The membrane-associated glucocorticoid receptor in neurons has not been identified. Several pharmacological studies have indicated that intracellular signaling by the receptor is G protein- and protein kinase-dependent [22, 23], and that its activity is inhibited by intracellular immunoneutralization of the Gs, but not Gq, subtype of G protein [22]. This points to the possibility that the membrane glucocorticoid receptor is a G protein-coupled receptor. However, electron microscopic studies have revealed the nuclear glucocorticoid receptor located in membranes of hypothalamic neurons [26], neurons of the basolateral amygdala (BLA) [27], hippocampal neurons, medial PFC neurons. Biochemical data [28] suggest that its membrane localization may be regulated in pituitary cells by glucocorticoids [29]. Additionally, conditional genetic knockdown of the nuclear glucocorticoid receptor in PVN neurons blocks the rapid glucocorticoid modulation of excitatory synaptic transmission in PVN CRF and magnocellular neuroendocrine cells [30] and the glucocorticoid negative feedback regulation of the HPA axis [31], indicating that the rapid glucocorticoid effects on the CRF neurons are dependent on the nuclear glucocorticoid receptor. These findings together suggest the possibility that the membrane glucocorticoid receptor may be the nuclear glucocorticoid receptor localized to the membrane. However, because the nuclear glucocorticoid receptor regulates thousands of genes (see [32]), the loss of the rapid glucocorticoid effect in CRF neurons following the genetic knockdown of the nuclear receptor does not mean that the membrane receptor is the nuclear receptor per se, because the loss of the rapid glucocorticoid effect may be due to the loss of a signal in the membrane receptor signaling pathway that depends on transcriptional regulation by the nuclear glucocorticoid receptor. Therefore, the existing data do not indicate conclusively whether the membrane glucocorticoid receptor in the CRF neurons and magnocellular neuroendocrine cells of the PVN is the nuclear glucocorticoid receptor located at the membrane, or a distinct G protein-coupled receptor, or a membrane transport protein that shuttles the glucocorticoid into the cell and triggers a G protein signaling cascade in the process.
Rapid glucocorticoid regulation of pre-hypothalamic limbic structures
Glucocorticoid-induced retrograde endocannabinoid suppression of synaptic transmission has also been found to exert rapid feedback synaptic regulation in the hippocampus, prefrontal cortex (PFC), and amygdala. In the hippocampus, stress exposure in vivo and corticosterone application to in-vitro hippocampal slices caused an increase in hippocampal 2-AG levels and enhanced the endocannabinoid-dependent suppression of synaptic inhibition induced by depolarization or cholinergic activation in CA1 pyramidal neurons [33]. The hippocampus/subiculum exerts an inhibitory influence on the HPA axis, relayed via an inhibitory relay in the bed nucleus of the stria terminalis [34]. The glucocorticoid-induced suppression of inhibition in the hippocampus, therefore, is thought to activate this pathway to contribute to the glucocorticoid suppression of the HPA axis. However, rapid glucocorticoid facilitation of inhibitory synaptic inputs to CA1 pyramidal neurons via a nitric oxide-dependent activation of local inhibitory interneurons has also been reported [35, 36]. The glucocorticoid disinhibition of the CA1 neurons via endocannabinoid suppression of GABA release would be expected to oppose the glucocorticoid-induced, nitric oxide-dependent excitation of local inhibitory circuits, rendering the net effect of rapid glucocorticoid modulation of inhibitory synaptic inputs to CA1 pyramidal neurons unclear. However, corticosterone has also been shown to rapidly increase excitatory synaptic inputs to hippocampal pyramidal cells via activation of presynaptic MR [37], suggesting that the overall rapid glucocorticoid action in hippocampal pyramidal neurons is likely to be excitatory, and may contribute to the rapid feedback inhibitory effect of glucocorticoids on the HPA axis.
Stress exposure also causes a glucocorticoid receptor-dependent increase in 2-AG content in the medial PFC in vivo [38]. Corticosterone pretreatment of PFC slices leads to the suppression of GABAergic inhibitory synaptic inputs to layer V pyramidal neurons, which is dependent on 2-AG activation of CB1 receptors. Like the hippocampus, the PFC exerts an inhibitory influence on HPA activation via an inhibitory relay in the bed nucleus of the stria terminalis [34, 39], such that disinhibition of the PFC output neurons by stress-induced glucocorticoids would also be expected to contribute to the negative feedback regulation of the HPA axis. This may be a delayed effect compared to the direct negative glucocorticoid feedback occurring at the PVN, and may serve to prolong or sustain the feedback inhibition of the HPA axis. It should be noted, however, that glucocorticoids also rapidly modulate inhibitory synaptic inputs to pyramidal neurons in layer II/III of the medial PFC, but rather than suppressing GABA release through retrograde presynaptic endocannabinoid signaling, glucocorticoids rapidly increase inhibitory synaptic inputs by activating upstream local inhibitory interneurons [36]. How the rapid glucocorticoid-induced increase in inhibition of layer II/III neurons and decrease in inhibition of layer V neurons integrate to create a net PFC output to the HPA axis is not known.
In the basolateral amygdala (BLA), stress-induced glucocorticoids also modulate inhibitory synaptic transmission via an endocannabinoid-dependent mechanism [40]. Glucocorticoids stimulate the synthesis and retrograde release of 2-AG at GABA synapses onto principal cells in the BLA, and this stress-induced rise in intra-BLA endocannabinoid contributes to the anxiety-like behavior caused by acute stress. Excitatory synapses in the BLA also respond to glucocorticoids with an endocannabinoid-induced suppression of glutamate release, although this is seen only following in-vivo priming with a prior stress exposure or in-vitro priming of the BLA excitatory synapses with prior corticosterone treatment [41], suggesting that this endocannabinoid mechanism at excitatory synapses may play a role in chronic stress adaptation or sensitization. The endocannabinoid-dependent suppression of synaptic excitation may be purposed to restrain the increase in excitatory synaptic inputs to the BLA principal cells caused by acute stress and rapid corticosteroid actions at MR [41, 42].
Importantly, experiential factors have the ability to disrupt many levels of this circuitry, thereby creating vulnerabilities to expression of stress-related disorders. To better understand vulnerability factors that promote HPA axis dysregulation and its relation to stress-related disorders, a session was formed at the International Workshop in Neuroendocrinology in August 2017. Thus, the present review article was prepared to highlight three distinct experiential events that serve as vulnerability factors for later changes in stress reactivity, and the fundamental neurobiological mechanisms that subserve them. As this review will highlight, the adult stress response is sensitive to disruptors such as early life stress, as well as, chronic stress, immune activation and traumatic brain injury (TBI) experienced in adulthood. These environmental threats have feed-forward influences on physiological outcomes of the stress response. Dysregulation of stress reactivity can result in the development of neuropsychiatric illnesses, inflammation and other stress-related disease states (Figure 3). In this way, the following review will utilize an exemplar-driven approach to better understand mechanisms that might be common to diverse threats (early life stress, chronic stress in adulthood, and mild TBI) that contribute to stress dysregulation.
Figure 3.
Schematic summary of feed-forward influences of external threats on subsequent stress reactivity (TBI = traumatic brain injury, HPA = hypothalamus-pituitary-adrenal axis, SNS = sympathetic nervous system).
In sum, the HPA axis is regulated by a wide range of intrinsic and extrinsic mechanisms, offering a multitude of points at which HPA axis regulation can be disrupted when stress challenges are encountered repeatedly, during periods of developmental vulnerability, or in adult individuals with a history of traumatic experiences. These will be addressed in the sections that follow.
Chronic stress alters limbic signaling to the HPA axis
The adult brain displays a high degree of plasticity, a property that is essential for functional adaptation to a constantly changing environment. From a broad standpoint, brain areas involved in the stress response include the hypothalamus, hippocampus (learning and memory), the PFC (executive function) and the amygdala (threat appraisal) [43]. These structures play a key role in integrating physiological and behavioral responses during stress and coping with subsequent stressful events [44]. On the other hand, repeated exposure to stressors negatively influences the physiology of organ systems. Repeated stress sensitizes the stress system to the development of complex disorders such as autoimmune and cardiovascular diseases, cancer, obesity and mental diseases such as anxiety and depressive disorder [45]. Interestingly, epidemiological studies have revealed a strong comorbidity among these diseases [46] Recent molecular analysis identified a common gene expression signature, providing a potential shared mechanism for these stress-related pathologies [47].
Chronic stress causes dysfunction within multiple brain structures, resulting in a feed-forward impact on stress reactivity and the associated behavioral adaptation. Several stress paradigms in rodents have been used to model the structural, functional and neuroendocrine (enhanced HPA axis activity) abnormalities in the brain of depressed patients. These models use chronic exposure to a single type of stressor (mild or strong) [48] or unpredictable and variable stressors [49]). Chronic restraint in male rats reduced the responsiveness to reward (sucrose consumption) [50, 51], analogous to anhedonia, which is a core symptom of depressive disorders [52]. Chronic stress also promotes an incapacity to cope with a stressful stimuli, as demonstrated by reduced escape-oriented behaviors in the forced swim test (swimming and struggling) [50, 51, 53, 54]. The activity of dopaminergic nuclei may be inhibited by chronic stress. In mice, selective inhibition of dopaminergic neurons in the ventral tegmental area triggered depressive-like behaviors in chronically stressed animals (reduced sucrose consumption and tail suspension struggling behavior) [55].
The influence of chronic stress is not restricted to alteration of hedonic processes, but also extends to cognitive function. Chronically stressed animals displayed reduced spatial learning, suggestive of disruption in hippocampal functioning [56]. Furthermore, postmortem studies have revealed a reduction in the volume of the hippocampus and PFC in depressed patients [57]. Postmortem studies have also suggested that depressed patients present poor glutamate clearance, suggesting increased activation of glutamate receptors [58], an effect also observed in chronically stressed rodents [59, 60]. Thus, changes in levels of glutamate may affect the balance of excitatory and inhibitory cues, resulting in alterations of stress reactivity.
Glutamate activates both metabotropic and ionotropic (AMPA and NMDA) receptors. Variation in cell surface exposure of these receptors determines changes in excitatory synaptic efficacy, such as long-term depression (LTD) or long-term potentiation (LTP). Chronic stress reduced LTP in CA1 neurons and dentate gyrus of the hippocampus [61, 62]. Studies have examined the expression of glutamate receptors to understand this variation in LTP. In the dorsal hippocampus, chronic stress reduced NMDA receptor 1 (NR1; obligatory subunit) and resulted in a negative shift in NMDA receptor 2A/NMDA receptor 2B (NR2A/NR2B) [63]. Given that the subunit configuration of NMDA receptors influences deactivation kinetics of the channel and the affinity for glutamate [64], these variations may explain the reduction in LTP in chronically stressed animals [65]. Collectively, these studies suggest that chronic stress alters the strength of hippocampal excitatory synapses, probably through the variation in synaptic contact, a mechanism that may be important for stress-induced disruptions in cognitive function.
In addition to changes in GABA-glutamate signaling, chronic stress also produces morphological changes in the central nervous system (CNS), including dendritic atrophy and reduced spine density in neurons of hippocampus and PFC [51, 53, 66–68]. These findings suggest that processes implicated in the formation and stabilization of synaptic connections can be altered under chronic stress and depressive disorder. Some studies have found that the stress-induced reduction in spine density is associated with a reduction of hippocampal N-cadherin levels [51], an adhesion molecule required for spine enlargement and LTP at mature CA1 synapses of the hippocampus [69]. Morphological changes of axonal growth, dendritic arborization and dendritic spines during development and in the adult brain are driven by fast modifications of F-actin dynamics. These modifications are under the control of Rho-family small GTPases [70–73]. For instance, active RhoA diminishes spine length and density in cultured neurons [71, 72]. A downstream effector of RhoA is the Rho kinase (ROCK) [72, 73], which in non-muscular cells, stimulates the myosin-dependent contractibility of F-actin by inactivating myosin light chain (MLC) phosphatase through the phosphorylation of its regulator MYPT1, an exclusive target of ROCK [74]. Interestingly, a recent study revealed that chronic stress triggered a rise in phospho-MYPT1 levels in the hippocampus, suggesting activation of the RhoA-ROCK pathway, which may explain the reduction in spine density in hippocampal CA1 neurons [75]. More recently, it has been described that systemic administration of a ROCK inhibitor (Fasudil) prevented both the stress-induced increase in phospho-MYPT1 and the reduction in spine density of CA1 hippocampal neurons [53]. Interestingly, the administration of the ROCK inhibitor prevented stress-induced immobility observed in the forced swimming test [53], similar to the effects of antidepressant drugs [50, 76]. Structural changes in neurons within the hippocampus and the PFC are associated with the pathophysiology of depression [77]. A reduction of spine densities in dendrites of CA1 neurons has been associated with depression-like behaviors in several animal models of chronic stress, suggesting altered glutamatergic excitatory neurotransmission. Studies have shown that antidepressant drugs with different pharmacological targets reverse the stress-induced behavioral alterations and spine loss in hippocampal neurons [78]. Furthermore, recent evidences suggest that the disruption of the Rho-ROCK pathway and/or inhibition of other kinases by Fasudil seem to exert antidepressant-like actions and prevent hippocampal spine loss. Further studies are necessary to evaluate whether chronic stress alters Rho-ROCK pathways, to determine mechanisms for pharmacological tools to ameliorate depressive symptomology. Altered stress reactivity may result in depressive behaviors, however, the mechanisms by which chronic stress and the associated synaptic and dendritic changes result in this need to be examined.
In sum, repeated exposure to stress during adulthood is associated with multiple adaptations in neural circuits that govern the HPA axis and contribute to behavioral and neuropsychiatric problems later in life. However, vulnerability to the adverse consequences of stress is not consistent across the lifespan, with early life adversity, adolescence, and late aging all being associated with unique regulatory changes within the HPA axis and ultimately in the expression of stress-related pathology. With that in mind, we will now turn our attention to early life adversity and its long term impact on social processes, since disruption of social processes is a core feature of many neuropsychiatric conditions.
Perinatal environmental interventions have long-term consequences on stress reactivity
The interplay between neuroendocrine systems and the OT system are related to social and stress coping behaviors. For example, the OT system regulates the HPA stress response at baseline and during threat exposure. OT can act at the level of the hypothalamus, or throughout the limbic circuitry, providing direct and indirect modulation of stress and coping behaviors [79, 80]. OT has also been vastly associated with social behaviors, especially those related to bonding (mother-infant; pair bonding, monogamy). Environmental events early in life can change the OT system and thus be the basis for changes in adult social behaviors [81, 82], subsequent stress reactivity, and ultimately portend the development of stress-related mental illness later in life. Indeed, the pioneering work of Levine and others [83] has shown that even an apparently non-noxious environmental intervention on a newborn can induce long-lasting behavioral and neuroendocrine dysregulation. Epigenetic studies highlight the importance of environment of development and adult stress reactivity [84, 85]. Developing animals and humans are surrounded by all kinds of stimuli, with different nature, intensity, frequency, timing, pattern, and most importantly salience to the animal. Early developmental experiences, during the perinatal age (before or shortly after the time of birth) have the ability to produce life-long changes in organism health, underscoring the importance of early life adversity [86]. At least two important variables come into play in developing animals: the animal’s capacity to process stimuli; and the specific stimulus salience to the animal at that moment in life. An animal is vulnerable to long-lasting disturbances during this “critical period” of heightened plasticity, and stress-responsive systems such as the HPA axis are no exception.
A variety of approaches have been used to study the impact of perinatal environment and maternal behavior on the OT system, and the subsequent development of social behaviors in rodents. These include rodent OT knockout (OTKO) models and examination of the natural OT amino acid sequence changes after maternal and paternal offspring interaction. Here, we summarize results from the following mild environmental interventions used in laboratory rodents: (1) the impact of neonatal handling; (2) partial handling (split litter); (3) naturally occurring variations in maternal care; (4) litter overlapping; and (5) prenatal stress. Although other approaches have been implemented (e.g. maternal separation and maternal deprivation), we will focus on mild environmental interventions and the interplay between neuropeptides on the development of social behaviors and stress reactivity.
The early life mild interventions, such as neonatal rearing experience and social environment can have a profound impact on neuroendocrine and socially-relevant processes later in life. The effects of the neonatal handling procedure, which consists of brief, repeated manipulation of the whole litter have been previously reviewed (see [87]). Briefly, early life mild intervention reduced non-aggressive social interactions, in pups (reduction in maternal odor preference), juvenile (reduction in play behavior) and adulthood (reduction in sexual behavior and increased aggressiveness). Neonatal exposure to brief, repeated litter splitting, reduces sexual behaviors in aged (90–100 day old) males [88]. In addition to neonatal behaviors, natural variations in maternal care (high and low licking mothers) affect sexual receptiveness in female rats. Adult females reared by high licking mothers show reduced lordosis [89]. Similarly, the litter overlapping procedure (pups+2 juveniles from a previous pregnancy) reduced sexual receptiveness in female rats [90].
To this end, OT has been implicated in the expression of social processes, aggression and sexual behaviors as a result of prenatal and neonatal manipulations in rodent models. For instance, neonatal handling reduced the number of OT-positive cells in the pPVN in adult male and female rats [91]. Adult male rats stressed during gestation and reared by stressed mothers show a reduction in hypothalamic OT and AVP cells. Separately, the interventions (gestational stress and cross fostering) did not change OT expression [92]. The involvement of the OT system in social and stress coping behaviors has also benefited from the use of OTKO that allow for examination of effects arising from reduced OT early in life. OTKO males showed reduced social behaviors, but no change in sexual activity [93]. OTKO females displayed a reduction in sexual receptiveness and ovulation [94, 95]. OTKO females showed reduced OT receptors and AVP receptors (AVPR) in the hippocampus. V1aR are also reduced in the hypothalamus. Moreover, OTKO reduced estrogen receptor alpha gene expression in the PFC [95]. Similar effects have been observed in genetic variants of OT. For instance, the OT variant (Pro in position 8) in monkeys is related to males displaying paternal behavior. In male rats, the administration of OT variants (Val3Pro8OXT and Pro8OXT) tended to increase pup-related behaviors. Thus, OT appears to play an important role in programming later expression of social interactions.
Commensurate with this, exposure of the developing organism to active stress challenges such as gestational stress (restraint) plus cross-fostering induced changes in social behaviors of the offspring. Adult male rats stressed during gestation and reared by mothers that were also stressed showed reduced social interaction (but increased aggressive behaviors). Male pups stressed during gestation but reared by non-stressed mothers showed no dysregulation of later social behaviors [92] (females not studied). Overall, there is a complex interplay between mild early life interventions and exposures and long-lasting influences on the adult stress reactivity.
Overall, mild early life environmental interventions can affect several systems, resulting in long-lasting behavioral effects and stress-relevant outcomes. The neonatal neuroendocrine system seems to be “open” to those interventions; however, the messenger(s) between the environment and the effector systems remains to be established. OT could exert that role. The challenge that remains is to understand the processes and mechanisms involved in the development of social behaviors during that early period. What would be the salience of a stimulus to an animal that encounters it for the first time? Since there was no previous adaptation and other central processing of the stimulus, how would it be perceived by the neonate? Would the stimuli during infancy induce stable memories? How would they “build” future sociability later in life? Considering the evolutionary importance of social behavior and the fact that social behavior disruption is a core feature of multiple mental health problems later in life, understanding the influence of early life stress on adult social processes and the neurohormonal and neuropeptide mechanisms underlying such changes is critical.
Traumatic brain injury (TBI) induces long-lasting alteration in stress response circuitry
In addition to early developmental perturbations, traumatic experiences later in life can also have a profound influence on stress reactivity. Exposure to TBI is one such example. TBI refers to an external force impacting the brain and resulting in functional dysregulation and/or impairment. Different forms of TBI include concussion, closed head injury, diffuse axonal injury, blast over pressurization, penetrating wound, laceration, hemorrhage, contusion and anoxia. TBI is categorized into one of three clinically defined categories: mild, moderate or severe based on the prevalence and persistence of post-injury symptoms. Mild TBI (m)TBI is the most prevalent (~75%) level of TBI sustained [96], and symptoms of mTBI may be transient or even go unnoticed.
TBI results in detrimental physical, cognitive, emotional, social and stress coping behaviors. Immediately following mTBI, symptoms may appear mild; however, persistent symptoms may manifest as life-long impairments. Approximately 15% of people diagnosed with mTBI experience persistent neurobehavioral problems [97]. TBI exhibits a high comorbidity diagnosis with psychiatric disorders such as depression, anxiety disorders, panic disorder, substance abuse, and post-traumatic stress disorder. The subsequent development of neuropsychiatric disorders after TBI may be a result of secondary injury mechanisms in brain structures that play a role in behavioral coordination, but there is increasing recognition that mTBI may also exacerbate emotional reactivity to circumstances, reflecting greater vulnerability to stress challenges later in life.
Experimental evidence connects TBI and the subsequent development of detrimental anxiety and fear-related behaviors, potentially mediated by altered stress reactivity. Acute and chronic increases of anxiety and PTSD-like behaviors were observed in various rodent TBI models. For instance, in the open field test and elevated plus maze, both of which are used to measure anxiety-like behaviors in rodents, increased levels of anxiety-related behaviors persisted months after injury [98]. Similarly, TBI induced hyper-responsivity and enhanced fear retention in the acoustic startle and Pavlovian fear conditioning tests, respectively [99, 100]. Importantly, these outcomes in rodent models mimic symptoms observed in PTSD patients, underscoring the translational value of rodent models in delineating neurobiological mechanisms by which mTBI influence later stress reactivity.
Most endocrine axes are prone to TBI-induced dysfunction. Clinically, chronic hypopituitarism is observed in a large population of TBI patients [101]. Clinical and experimental evidence shows that the HPA axis was vulnerable to dysregulation as a result of injury [102, 103]. ACTH and CORT insufficiencies were observed in the acute phase (less than 2 weeks) post injury [104]. Although HPA axis disruption recovered post injury, neuropsychiatric symptoms persisted as much as 6 months later [105]. Preclinical data also provides supporting evidence of TBI-induced HPA axis dysregulation. Increased plasma ACTH and CORT [106] levels were present in the acute phase of injury. CORT remained dysregulated up to 70 days post injury [103]. mTBI decreased CORT in the sub-acute phase (up to 21 days post injury) after restraint and forced swim stressors. Conversely, in the chronic phase of injury, stress-induced CORT levels are elevated [103].
Alteration in central regulation by the PVN and/or feedback mechanisms (MR and GR) often results in HPA axis disruption. After diffuse TBI, cell death was not evident in the PVN; however neuronal plasticity was observed. In pPVN neurons, TBI resulted in increased neuronal process branch point number and complexity, potentially as a result of secondary injury mechanisms [107]. A second outcome of TBI was a transient effect on MR and GR levels. TBI increased GR levels (up to 3 days post injury) and decreased MR (1 day post injury) levels in the hippocampus [108, 109]. MR and GR levels returned to baseline at 7 days post injury [108]. Administration of GR antagonists was shown to decrease TBI-induced hippocampal cell death [110] and anxiety-like behaviors [111].
There is also evidence that TBI can alter neural regulation of HPA axis activity by affecting CNS sites that project to the PVN. Clinically, alterations in the PFC, amygdala and hippocampus were apparent after TBI [112, 113]. These limbic structures provide direct and indirect projections to CRF neurons in the pPVN to regulate the behavioral stress response [12, 114]. This communication is either excitatory (glutamatergic) from the amygdala or inhibitory (GABAergic) from the PFC, hippocampus and BNST. In experimental models, cell death also occurs throughout these limbic structures [115, 116]. Changes in glutamate and GABA are also observed after TBI [117]. For example, a loss of inhibitory neurons (measured by GAD-67 and parvalbumin immunohistochemistry) was observed in the amygdala and hippocampus after TBI [118].
Taken together, these findings suggest that TBI can have a profound influence on subsequent activity of the HPA axis when stress challenges are encountered post-injury. In this way, individuals with a history of acute or repeated TBI may be more sensitive to the adverse consequences of stress later in life, an effect that may persist for months to years post-injury. Thus, ongoing studies are required to better understand the neurobiological mechanisms by which TBI influences stress reactivity later in life.
Interplay of the stress and immune axes
As noted above, a variety of insults including early life adversity, chronic stress exposure, and TBI can all have a profound influence on cognitive function, with many of these effects being mediated by CORT or having long-lasting effects on HPA axis and sympathetic nervous system. Indeed, the PFC and hippocampus are highly sensitive to the deleterious effects of CORT [119, 120] and endogenous CORT can have detrimental effects on behavior. However, the downstream mechanisms by which CORT exerts its effects on brain function remain an active area of study. It can be noted, however, that both stress and CORT have powerful influences through downstream modulation of inflammatory processes. Glucocorticoids, in general, typically have anti-inflammatory properties, since they act by inhibiting the gene expression of pro-inflammatory mediators (cytokines and reactive oxygen and nitrogen species) in the periphery and CNS. These effects are particularly evident with use of exogenous, pharmacological concentrations of glucocorticoids or in cases in which high stress levels of endogenous glucocorticoids have been abrogated. Despite these well-documented anti-inflammatory actions of glucocorticoids, there is increasing evidence suggesting that glucocorticoids are not solely anti-inflammatory and in some cases, may even increase certain aspects of the inflammatory response, mainly in the CNS [121].
The ability of glucocorticoids to exert either pro-inflammatory or anti-inflammatory effects is dependent on timing, concentration and duration of glucocorticoid exposure. The specifics of the inflammatory actions of glucocorticoids are not well understood. Chronic unpredictable stress increases circulating CORT [122, 123]. In the PFC and hippocampus, elevated CORT increased the expression of pro-inflammatory genes (iNOS, IL-1β, TNF-α). Additionally, elevated CORT decreased anti-inflammatory genes including IL-1ra, IL-10, and MKP-1 in these limbic structures [122, 123]. Pre-treatment with the GR antagonist, RU486, reduced this pro-inflammatory effect, suggesting that this was indeed GR mediated [122, 123].
The PFC and hippocampus are prone to detrimental effects by glucocorticoids, potentially mediated by the inflammatory roles of microglia and astrocytes. The pro-inflammatory role of glucocorticoids after kainic acid insult, increased cell death [124]. Blocking microglial activation reduced the glucocorticoid-induced worsening of neuron survival [124]. Administration of high levels of glucocorticoids decreased anti-inflammatory gene expression in the hippocampus, but increased the pro-inflammatory gene, IL-1β [124]. After injury (kainic acid or focal ischemia), glucocorticoids decreased neuronal survival in GR knockout animals [125]. To this end, glucocorticoids may activate microglia, leaving them in a “primed” state [126].
In immune disorders, such as multiple sclerosis (MS), synthetic glucocorticoids, such as methylprednisolone (MP) and dexamethasone (DEX; high affinity for GR) are the standard treatment for acute disease symptomatology. In an experimental model of MS, the experimental autoimmune encephalomyelitis (EAE), Wurst et al (2012) [127] showed that MP administration prior to immunization, resulted in increased leukocyte infiltration into the CNS. A worsening of the symptoms was also observed, adding more evidence in a disease model, that glucocorticoids are not uniformly anti-inflammatory.
Overall, this suggests a bidirectional communication between stress reactivity and inflammation. The emerging, pro-inflammatory influence of stress challenges broadly, and CORT specifically, might help explain the general paradox that inflammatory disease states are typically exacerbated (rather than ameliorated) during times of chronic stress. This is particularly important given the large number of disorders that involve inflammation, and that are known to be exacerbated by stress, including obesity, asthma, diabetes, cardiovascular and neurodegenerative diseases, and cancer to name a few. Thus, a major path for the future is to better understand the mechanistic relationship between classic stress-responsive systems such as the HPA axis, inflammation, and subsequent disease states.
Overall conclusions
The over-arching goal of this brief review was to identify how a range of common, yet disparate, experiential events influences activity of the HPA axis to stress challenges (Figure 3), as discussed at the International Workshop in Neuroendocrinology in Concon, Chile in August, 2017. Early life adversity, chronic stress and TBI during adulthood were all discussed as established or emergent experiences that alter stress responses, and all of which tended to produce feed-forward activity within the axis. Although the mechanisms underlying responses to each of these experiences are likely to be unique, the long-lasting influence on the stress system (HPA axis and sympathetic nervous system) certainly influences stress reactivity, including downstream effects that may be mediated by alterations in neuropeptide signaling (OT, AVP), and inflammatory signaling pathways. Because, disruption of normal social processes and increased prevalence of negative affect (anxiety, depression) form core features of many neuropsychiatric disorders, these experiences may also magnify the psychological and physiological consequences of future stress challenges, contributing to a feed-forward vulnerability rooted in stress axis dysfunction and reflected by poor stress coping behaviors. Thus, understanding points of articulation in the mechanisms of stress adaptation across diverse model systems may provide important insight for the development of novel therapeutics to treat neuropsychiatric disorders.
Highlights.
The stress response is regulated by a complex central and peripheral network that relies on effective coordination among systems.
Environmental disruptors, such as chronic stress, immune activation, early life adversity and traumatic brain injury alter stress reactivity.
Dysregulated stress reactivity may manifest in the development of neuropsychiatric illnesses and/or inflammatory diseases.
Acknowledgements:
Dr. Ashley Russell is supported by the 2017 International Workshop in Neuroendocrinology Young Investigator Travel Award and the Center for Neuroscience and Regenerative Medicine Pre-doctoral Fellowship (308049-18.01-60855). Dr. Jeffrey Tasker is supported by the NIH grants MH104373 and MH066958. Dr. Carolina Munhoz is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: 2008/55178–0, 2012/24727–4, and 2016/03572–3) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 479153/2009–4). Dr. T. John Wu is supported by the Center for Neuroscience and Regenerative Medicine and the Office of Naval Research. Dr. Terrence Deak is currently supported by NIH grants R01AG043467 and P50AA017823.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
References
- 1.Cannon WB, Bodily Changes in Pain, Hunger, Fear and Rage. 1915, New York: Applegate and Co. [Google Scholar]
- 2.Cannon WB, Wisdom of the Body. 1932, New York: Norton. [Google Scholar]
- 3.Bernard C, An Introduction to the Study of Experimental Medicine, ed. D. ed. 1865: Macmillan and Co., Ltd. [Google Scholar]
- 4.Selye H, Stress and the general adaptation syndrome. Br Med J, 1950. 1(4667): p. 1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS, Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev, 2007. 87(3): p. 873–904. [DOI] [PubMed] [Google Scholar]
- 6.Antoni FA, Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev, 1986. 7(4): p. 351–78. [DOI] [PubMed] [Google Scholar]
- 7.Whitnall MH, Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol, 1993. 40(5): p. 573–629. [DOI] [PubMed] [Google Scholar]
- 8.Rivier C and Vale W, Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology, 1983. 113(3): p. 939–42. [DOI] [PubMed] [Google Scholar]
- 9.Dallman MF and Jones MT, Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone ssecretion on subsequent stress responses in the rat. Endocrinology, 1973. 92(5): p. 1367–75. [DOI] [PubMed] [Google Scholar]
- 10.Reul JM and de Kloet ER, Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology, 1985. 117(6): p. 2505–11. [DOI] [PubMed] [Google Scholar]
- 11.Reul JM and de Kloet ER, Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem, 1986. 24(1): p. 269–72. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, et al. , Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry, 2005. 29(8): p. 1201–13. [DOI] [PubMed] [Google Scholar]
- 13.Scott LV and Dinan TG, Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci, 1998. 62(22): p. 1985–98. [DOI] [PubMed] [Google Scholar]
- 14.Neumann ID, et al. , Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept, 2000. 96(1–2): p. 31–8. [DOI] [PubMed] [Google Scholar]
- 15.Hueston CM and Deak T, The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav, 2014. 124: p. 77–91. [DOI] [PubMed] [Google Scholar]
- 16.Evanson NK, et al. , Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology, 2010. 151(10): p. 4811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker JJ, et al. , The origin of glucocorticoid hormone oscillations. PLoS Biol, 2012. 10(6): p. e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman JP, Adams D, and Prewitt C, Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology, 1995. 61(2): p. 180–90. [DOI] [PubMed] [Google Scholar]
- 19.Diorio D, Viau V, and Meaney MJ, The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci, 1993. 13(9): p. 3839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di S, et al. , Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci, 2003. 23(12): p. 4850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di S, et al. , Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology, 2005. 146(10): p. 4292–301. [DOI] [PubMed] [Google Scholar]
- 22.Di S, et al. , Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci, 2009. 29(2): p. 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malcher-Lopes R, et al. , Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci, 2006. 26(24): p. 6643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di S, Popescu IR, and Tasker JG, Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci, 2013. 33(46): p. 18331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boychuk CR, et al. , Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons. Front Neurosci, 2013. 7: p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liposits Z and Bohn MC, Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J Neurosci Res, 1993. 35(1): p. 14–9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson LR, et al. , Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience, 2005. 136(1): p. 289–99. [DOI] [PubMed] [Google Scholar]
- 28.Nahar J, et al. , Further evidence for a membrane receptor that binds glucocorticoids in the rodent hypothalamus. Steroids, 2016. 114: p. 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Q, et al. , Rapid Glucocorticoid Feedback Inhibition of ACTH Secretion Involves Ligand-Dependent Membrane Association of Glucocorticoid Receptors. Endocrinology, 2015. 156(9): p. 3215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahar J, et al. , Rapid Nongenomic Glucocorticoid Actions in Male Mouse Hypothalamic Neuroendocrine Cells Are Dependent on the Nuclear Glucocorticoid Receptor. Endocrinology, 2015. 156(8): p. 2831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon MB, et al. , Neuroendocrine Function After Hypothalamic Depletion of Glucocorticoid Receptors in Male and Female Mice. Endocrinology, 2015. 156(8): p. 2843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakley RH and Cidlowski JA, The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol, 2013. 132(5): p. 1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, et al. , Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol, 2012. 26(1): p. 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radley JJ and Sawchenko PE, A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci, 2011. 31(26): p. 9683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W, et al. , Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology, 2010. 35(8): p. 1693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng Z, et al. , Glucocorticoid exerts its non-genomic effect on IPSC by activation of a phospholipase C-dependent pathway in prefrontal cortex of rats. J Physiol, 2013. 591(13): p. 3341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karst H, et al. , Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A, 2005. 102(52): p. 19204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill MN, et al. , Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci, 2011. 31(29): p. 10506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radley JJ, Gosselink KL, and Sawchenko PE, A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci, 2009. 29(22): p. 7330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di S, et al. , Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J Neurosci, 2016. 36(32): p. 8461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karst H, et al. , Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A, 2010. 107(32): p. 14449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karst H and Joels M, Severe stress hormone conditions cause an extended window of excitability in the mouse basolateral amygdala. Neuropharmacology, 2016. 110(Pt A): p. 175–180. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS, Nasca C, and Gray JD, Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 2016. 41(1): p. 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korte SM, Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev, 2001. 25(2): p. 117–42. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S, Janicki-Deverts D, and Miller GE, Psychological stress and disease. JAMA, 2007. 298(14): p. 1685–7. [DOI] [PubMed] [Google Scholar]
- 46.Marx P, et al. , Comorbidities in the diseasome are more apparent than real: What Bayesian filtering reveals about the comorbidities of depression. PLoS Comput Biol, 2017. 13(6): p. e1005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo L, Du Y, and Wang J, Network analysis reveals a stress-affected common gene module among seven stress-related diseases/systems which provides potential targets for mechanism research. Sci Rep, 2015. 5: p. 12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willner P, et al. , Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl), 1987. 93(3): p. 358–64. [DOI] [PubMed] [Google Scholar]
- 49.Katz RJ, Roth KA, and Carroll BJ, Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev, 1981. 5(2): p. 247–51. [DOI] [PubMed] [Google Scholar]
- 50.Bravo JA, et al. , Desipramine prevents stress-induced changes in depressive-like behavior and hippocampal markers of neuroprotection. Behav Pharmacol, 2009. 20(3): p. 273–85. [DOI] [PubMed] [Google Scholar]
- 51.Castaneda P, et al. , Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J Neurosci Res, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Willner P, Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl), 1997. 134(4): p. 319–29. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Rojo G, et al. , The ROCK Inhibitor Fasudil Prevents Chronic Restraint Stress-Induced Depressive-Like Behaviors and Dendritic Spine Loss in Rat Hippocampus. Int J Neuropsychopharmacol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucki I, The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol, 1997. 8(6–7): p. 523–32. [DOI] [PubMed] [Google Scholar]
- 55.Tye KM, et al. , Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature, 2013. 493(7433): p. 537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moser E, Moser MB, and Andersen P, Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci, 1993. 13(9): p. 3916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drevets WC, Price JL, and Furey ML, Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct, 2008. 213(1–2): p. 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanacora G, Treccani G, and Popoli M, Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology, 2012. 62(1): p. 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popoli M, et al. , The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci, 2011. 13(1): p. 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moghaddam B, et al. , Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res, 1994. 655(1–2): p. 251–4. [DOI] [PubMed] [Google Scholar]
- 61.Alfarez DN, Joels M, and Krugers HJ, Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci, 2003. 17(9): p. 1928–34. [DOI] [PubMed] [Google Scholar]
- 62.Pavlides C, Nivon LG, and McEwen BS, Effects of chronic stress on hippocampal long-term potentiation. Hippocampus, 2002. 12(2): p. 245–57. [DOI] [PubMed] [Google Scholar]
- 63.Pacheco A, et al. , Chronic Stress Triggers Expression of Immediate Early Genes and Differentially Affects the Expression of AMPA and NMDA Subunits in Dorsal and Ventral Hippocampus of Rats. Front Mol Neurosci, 2017. 10: p. 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vicini S, et al. , Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol, 1998. 79(2): p. 555–66. [DOI] [PubMed] [Google Scholar]
- 65.Pavlides C, Watanabe Y, and McEwen BS, Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus, 1993. 3(2): p. 183–92. [DOI] [PubMed] [Google Scholar]
- 66.McEwen BS and Seeman T, Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci, 1999. 896: p. 30–47. [DOI] [PubMed] [Google Scholar]
- 67.Vyas A, et al. , Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci, 2002. 22(15): p. 6810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinto V, et al. , Differential impact of chronic stress along the hippocampal dorsal-ventral axis. Brain Struct Funct, 2015. 220(2): p. 1205–12. [DOI] [PubMed] [Google Scholar]
- 69.Bozdagi O, et al. , Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci, 2010. 30(30): p. 9984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Govek EE, Newey SE, and Van Aelst L, The role of the Rho GTPases in neuronal development. Genes Dev, 2005. 19(1): p. 1–49. [DOI] [PubMed] [Google Scholar]
- 71.Luo L, Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci, 2000. 1(3): p. 173–80. [DOI] [PubMed] [Google Scholar]
- 72.Nakayama AY, Harms MB, and Luo L, Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci, 2000. 20(14): p. 5329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tashiro A, Minden A, and Yuste R, Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex, 2000. 10(10): p. 927–38. [DOI] [PubMed] [Google Scholar]
- 74.Amano M, et al. , Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem, 1996. 271(34): p. 20246–9. [DOI] [PubMed] [Google Scholar]
- 75.Castañeda P, Efecto del estrés sobre proteínas asociadas a conectividad sináptica y citoesqueleto: relación con la acción antidepresiva de sertralina en la rata. 2010. [Google Scholar]
- 76.Ulloa JL, et al. , Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav, 2010. 97(2): p. 213–21. [DOI] [PubMed] [Google Scholar]
- 77.Pittenger C and Duman RS, Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology, 2008. 33(1): p. 88–109. [DOI] [PubMed] [Google Scholar]
- 78.Bessa JM, et al. , The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry, 2009. 14(8): p. 764–73, 739. [DOI] [PubMed] [Google Scholar]
- 79.Huber D, Veinante P, and Stoop R, Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science, 2005. 308(5719): p. 245–8. [DOI] [PubMed] [Google Scholar]
- 80.Olff M, et al. , The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 2013. 38(9): p. 1883–94. [DOI] [PubMed] [Google Scholar]
- 81.Caldwell HK, Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist, 2017: p. 1073858417708284. [DOI] [PubMed] [Google Scholar]
- 82.Feldman R, The neurobiology of mammalian parenting and the biosocial context of human caregiving. Horm Behav, 2016. 77: p. 3–17. [DOI] [PubMed] [Google Scholar]
- 83.Seymour Levine GCH, George G. Karas, Victor H. Denenberg, Physiological and Behavioral Effects of Infantile Stimulation. Physiology and Behavior, 1967. 2: p. 55–59. [Google Scholar]
- 84.Champagne FA, Epigenetic influence of social experiences across the lifespan. Dev Psychobiol, 2010. 52(4): p. 299–311. [DOI] [PubMed] [Google Scholar]
- 85.Meaney MJ, Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci, 2001. 24: p. 1161–92. [DOI] [PubMed] [Google Scholar]
- 86.Barker DJ, The fetal and infant origins of disease. Eur J Clin Invest, 1995. 25(7): p. 457–63. [DOI] [PubMed] [Google Scholar]
- 87.Raineki C, Lucion AB, and Weinberg J, Neonatal handling: an overview of the positive and negative effects. Dev Psychobiol, 2014. 56(8): p. 1613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benetti F, et al. , Effects of neonatal novelty exposure on sexual behavior, fear, and stress-response in adult rats. Dev Psychobiol, 2007. 49(3): p. 258–64. [DOI] [PubMed] [Google Scholar]
- 89.Uriarte N, et al. , Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev Psychobiol, 2007. 49(5): p. 451–62. [DOI] [PubMed] [Google Scholar]
- 90.Uriarte N, et al. , Effects of litter-overlapping on emotionality, stress response, and reproductive functions in male and female rats. Dev Psychobiol, 2009. 51(3): p. 259–67. [DOI] [PubMed] [Google Scholar]
- 91.Todeschin AS, et al. , Effects of neonatal handling on social memory, social interaction, and number of oxytocin and vasopressin neurons in rats. Horm Behav, 2009. 56(1): p. 93–100. [DOI] [PubMed] [Google Scholar]
- 92.de Souza MA, et al. , Prenatal stress produces social behavior deficits and alters the number of oxytocin and vasopressin neurons in adult rats. Neurochem Res, 2013. 38(7): p. 1479–89. [DOI] [PubMed] [Google Scholar]
- 93.Lazzari VM, et al. , Oxytocin modulates social interaction but is not essential for sexual behavior in male mice. Behav Brain Res, 2013. 244: p. 130–6. [DOI] [PubMed] [Google Scholar]
- 94.Becker RO, et al. , Sexual behavior and dendritic spine density of posterodorsal medial amygdala neurons in oxytocin knockout female mice. Behav Brain Res, 2013. 256: p. 95–100. [DOI] [PubMed] [Google Scholar]
- 95.Zimmermann-Peruzatto JM, et al. , The Impact of Oxytocin Gene Knockout on Sexual Behavior and Gene Expression Related to Neuroendocrine Systems in the Brain of Female Mice. Cell Mol Neurobiol, 2017. 37(5): p. 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Center for Injury Prevention and Control, in Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. 2003, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 97.Alexander MP, Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology, 1995. 45(7): p. 1253–60. [DOI] [PubMed] [Google Scholar]
- 98.Pandey DK, et al. , Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav Brain Res, 2009. 205(2): p. 436–42. [DOI] [PubMed] [Google Scholar]
- 99.Heldt SA, et al. , A novel closed-head model of mild traumatic brain injury caused by primary overpressure blast to the cranium produces sustained emotional deficits in mice. Front Neurol, 2014. 5: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reger ML, et al. , Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol Psychiatry, 2012. 71(4): p. 335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandez-Rodriguez E, et al. , Hypopituitarism following traumatic brain injury: determining factors for diagnosis. Front Endocrinol (Lausanne), 2011. 2: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lieberman SA, et al. , Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J Clin Endocrinol Metab, 2001. 86(6): p. 2752–6. [DOI] [PubMed] [Google Scholar]
- 103.Taylor AN, et al. , Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma, 2008. 25(4): p. 311–23. [DOI] [PubMed] [Google Scholar]
- 104.Tanriverdi F and Kelestimur F, Pituitary dysfunction following traumatic brain injury: clinical perspectives. Neuropsychiatr Dis Treat, 2015. 11: p. 1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sojka P, et al. , One-year follow-up of patients with mild traumatic brain injury: occurrence of post-traumatic stress-related symptoms at follow-up and serum levels of cortisol, S-100B and neuron-specific enolase in acute phase. Brain Inj, 2006. 20(6): p. 613–20. [DOI] [PubMed] [Google Scholar]
- 106.Gottesfeld Z, Moore AN, and Dash PK, Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J Neurotrauma, 2002. 19(3): p. 317–26. [DOI] [PubMed] [Google Scholar]
- 107.Rowe RK, et al. , Diffuse traumatic brain injury affects chronic corticosterone function in the rat. Endocr Connect, 2016. 5(4): p. 152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Griesbach GS, et al. , Effects of acute restraint-induced stress on glucocorticoid receptors and brain-derived neurotrophic factor after mild traumatic brain injury. Neuroscience, 2012. 210: p. 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCullers DL, et al. , Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res, 2002. 947(1): p. 41–9. [DOI] [PubMed] [Google Scholar]
- 110.McCullers DL, et al. , Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience, 2002. 109(2): p. 219–30. [DOI] [PubMed] [Google Scholar]
- 111.Fox LC, et al. , Differential effects of glucocorticoid and mineralocorticoid antagonism on anxiety behavior in mild traumatic brain injury. Behav Brain Res, 2016. 312: p. 362–5. [DOI] [PubMed] [Google Scholar]
- 112.Bigler ED, et al. , Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol, 1997. 18(1): p. 11–23. [PMC free article] [PubMed] [Google Scholar]
- 113.Han K, Chapman SB, and Krawczyk DC, Altered Amygdala Connectivity in Individuals with Chronic Traumatic Brain Injury and Comorbid Depressive Symptoms. Front Neurol, 2015. 6: p. 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jankord R and Herman JP, Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci, 2008. 1148: p. 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raghupathi R, et al. , Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience, 2002. 110(4): p. 605–16. [DOI] [PubMed] [Google Scholar]
- 116.Roth TL, et al. , Transcranial amelioration of inflammation and cell death after brain injury. Nature, 2014. 505(7482): p. 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guerriero RM, Giza CC, and Rotenberg A, Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep, 2015. 15(5): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor AN, Tio DL, and Sutton RL, Restoration of neuroendocrine stress response by glucocorticoid receptor or GABA(A) receptor antagonists after experimental traumatic brain injury. J Neurotrauma, 2013. 30(14): p. 1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cerqueira JJ, et al. , Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci, 2005. 25(34): p. 7792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Quervain D, Schwabe L, and Roozendaal B, Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci, 2017. 18(1): p. 7–19. [DOI] [PubMed] [Google Scholar]
- 121.Sorrells SF, et al. , The stressed CNS: when glucocorticoids aggravate inflammation. Neuron, 2009. 64(1): p. 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Munhoz CD, et al. , Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci, 2006. 26(14): p. 3813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Munhoz CD, et al. , Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci, 2010. 30(41): p. 13690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sorrells SF, et al. , Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology, 2014. 100(2–3): p. 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sorrells SF, et al. , Glucocorticoid signaling in myeloid cells worsens acute CNS injury and inflammation. J Neurosci, 2013. 33(18): p. 7877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frank MG, et al. , Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun, 2012. 26(2): p. 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wust S, et al. , Preventive treatment with methylprednisolone paradoxically exacerbates experimental autoimmune encephalomyelitis. Int J Endocrinol, 2012. 2012: p. 417017. [DOI] [PMC free article] [PubMed] [Google Scholar]