Abstract
Osteoporosis is a widespread disease among older peoples. The aim of this study is to estimate the prevalence of self-reported osteoporosis and assessing its association with socio-economic status. A population-based cross-sectional study was conducted in Tehran, Iran in 2011. Participants were 45,990 individuals aged above 20 years from 22 urban districts. Osteoporosis was measured by self-administrative questionnaire. Wealth index was constructed using principal component analysis based on household assets. Chi-square test, chi square test for trend, and crude odds ratio were used to assess associations in univariate analysis. Multiple logistic regression utilized to estimate adjusted associations between self-reported osteoporosis and socio-economic status.
The overall estimated prevalence of self-reported osteoporosis was 4% (95% CI 3.88–4.13), 1.19% in men, and 6.84% in women (P < 0.001). The prevalence increased considerably as age increased (P for trend < 0.001). In multivariable analysis, education and wealth status were negative, and smoking was positively associated with the prevalence of self-reported osteoporosis. No association was found between participants’ skill levels and Townsend deprivation index with the prevalence of self-reported osteoporosis.
The findings of the present study have improved understanding of the association between socioeconomic status and osteoporosis in the Iranian population. It is important to consider socioeconomic status in screening and prevention programs.
Keywords: Osteoporosis, Self-reporting, Prevalence, Socioeconomic status, Tehran
Introduction
Osteoporosis is one of the globally hidden health disorders that more frequently affects older adults. It is estimated 200 million women suffer from this problem worldwide [1]. Osteoporosis causes about 9 million fractures annually, whereupon an osteoporotic fracture every 3 s in the world [2]. It is predicted that the worldwide incidence of hip fracture in men increases by 310 and 240% in women by 2050 [3]. In addition, more than about 50% of all osteoporotic hip fractures will occur in Asia by the year 2050 [3]. In 2010, there were 50,000 osteoporotic fractures in Iran, and it is expected to increase to 62,000 fractures, by 2020 [4]. This serious health problem may have major burden on family, society, and health systems. A reason for this is that 0.83% of the global burden of non-communicable disease related to osteoporotic fractures [2]. Also, some evidences showed excess mortality up to 5 years following osteoporotic fractures in both sexes [5]. It is estimated that there were $20 billion and $30 billion annual cost of all osteoporotic fractures in the USA and in the European Union, respectively [5].
The impact of social inequality and socioeconomic status (SES) on all aspect of health is well established [6]. Little information exist about role of demographic factors including SES in developing osteoporosis [7]. Several studies have showed the role of social inequality in the osteoporosis and its outcome, osteoporotic fractures [7–10]. The other studies have indicated low income, low educational level, and being unmarried as risk factors for osteoporosis and osteoporotic fractures [11, 12]. One systematic review reveals that despite of several limited good quality researches that indicate the association between SES and the risk of osteoporosis, there is need for further research [7].
Certainly, to reduce osteoporosis frequency and incidence of osteoporotic fractures, knowing about magnitude of problem and identification at-risk peoples is necessary [6]. The purpose of this study was to estimate the prevalence of self-reported osteoporosis and determine the association between SES and self-reported osteoporosis. The results can lead health policy makers and decision makers to better planning and conducting preventive and screening programs.
Methods
The studied sample was drawn from second round of Urban HEART project. Urban HEART project was a large population-based cross-sectional study conducted in Tehran, Iran, in fall 2011. The participants were interviewed at their house. Al the interviewers were trained in a 2-day workshop prior to collecting data.
Sampling Method
A multistage cluster sampling was applied in the study design. Twenty-two districts were considered as stratum in the first stage. Then 200 clusters in each district and eight households in each cluster were selected using systematic random sampling method. Household persons were utilized as the primary sampling unit. All eligible members of the selected households were recruited for interview. Inclusion criteria included people aged 20 years and older, willing to participate in the study, and staying at least 1 year in the area of interest. People with complete disability or sever impairment in answering questions or with apparent psychological illness were excluded from the study. To estimate required sample size, each district was considered independently. Based on Cochrane formula, for each district, 1535 households were calculated. Two hundred blocks were selected in each district, equally. In each block, eight households were selected systematically. To collect samples at neighborhood level, method of the probability proportional to size of each district was used. The total sample size was 34,116 households covering 118,542 individuals from 22 districts and 368 neighborhoods. After excluding participants aged less than 20 years, the analysis was performed on data of the remaining 45,990 individuals, who were above 20 years. The overall response rate of this study was 72.8% which can be satisfactory.
Measures
SES Checklist
To calculate wealth index, as an individual-level of SES, the principal component analysis (PCA) was performed on household assets [13]. Fourteen assets including: owning a fridge, a personal computer, a telephone, a mobile phone, a washing machine, a microwave oven, a car, a motorcycle, a kitchen, a bathroom, a toilet, house ownership, number of rooms per capita (less than one vs. one and more), and area of the house (below the median vs. above the median) were considered. All of these 14 assets are originally binary or converted to binary variables via classification of a numerical variable. Finally, to estimate each household wealth status, based on the first principal component, the scores converted to five-ordered categories from poorest to richest. In addition, we used the Townsend deprivation index, as an area-level indicator of SES, and educational level and skill level as individual socio-economic indicators [14, 15].
Self-Report Osteoporosis
Whereas gold standard for measuring osteoporosis is bone mineral density (BMD), measuring BMD is not practical for many large-scale studies. In the present study, we used the self-reporting method to classify participants on osteoporosis status. Although, some studies showed poor agreement between self-reported osteoporosis and bone mineral density [16, 17], but Peeters et al. showed acceptable validity and reliability of self-reporting method for osteoporosis diagnosis so that both concurrent and construct validity was acceptable for self-reported prevalent osteoporosis. Sensitivity and specificity were at least 68.8 and 88.6%, respectively, for different age and sex groups for self-reported prevalent osteoporosis [18].
Statistical Analysis
Descriptive statistical analysis were used to describe the data. Principle component analysis was used to combine asset variables and construct the wealth index. Body mass index (BMI) was calculated using the formula weight (kg)/height (cm2). Chi-square test, chi square test for trend, and crude odds ratio were used to assess associations in univariate analysis. In multivariate analysis, adjusted odds ratios from unconditional logistic regression model were used as the measures of association between the study variables. Hosmer-Lemeshow test conducted to goodness of fit of model. All reported P values are based on two-sided tests and compared to a significance level of 0.05. STSTA version 12.0 was used for all the statistical calculations.
Results
Table 1 shows the characteristics of study participants; among 69,173 participants, 49% (n = 33,884) were female and the rest were male. The mean age ± SD of the participants was 41.5 ± 11.37 years. The overall weighted prevalence of self-reported osteoporosis in participants was 4% (95% CI 3.88–4.13). Among the females, lowest (0.34%) and highest (26.62%) weighted prevalence was observed in age group of 20–29 and 70–79 years, respectively. For males, the age group of 20–29 years has the lowest weighted prevalence (0.12%), and the age group of higher than of 80 years has the highest weighted prevalence (7.4%) (Table 1).
Table 1.
Prevalence rates of osteoporosis by sex and age groups
| Age group | Male | Female | P value | ||||
|---|---|---|---|---|---|---|---|
| n | Case | Prevalence (%) (95% CI) | n | Case | Prevalence (%) (95% CI) | ||
| 20–29 | 13,016 | 16 | 0.12 (0.07–0.19) | 12,473 | 42 | 0.34 (0.23–0.51) | 0.001 |
| 30–39 | 9221 | 16 | 0.17 (0.11–0.24) | 10,057 | 112 | 1.11 (0.92–1.43) | < 0.001 |
| 40–49 | 8256 | 47 | 0.57 (0.38–0.79) | 8909 | 409 | 4.59 (4.24–5.36) | < 0.001 |
| 50–59 | 7234 | 114 | 1.58 (1.31–1.92) | 7580 | 979 | 12.92 (12.21–13.72) | < 0.001 |
| 60–69 | 4655 | 143 | 3.07 (2.69–3.67) | 4191 | 913 | 21.79 (20.49–23.13) | < 0.001 |
| 70–79 | 2716 | 141 | 5.19 (4.32–6.24) | 1950 | 519 | 26.62 (24.72–28.62) | < 0.001 |
| + 80 | 892 | 66 | 7.40 (5.21–9.93) | 663 | 160 | 24.13 (20.87–27.59) | < 0.001 |
| Total | 45,990 | 543 | 1.19 (1.10–1.30) | 45,824 | 3134 | 6.84 (6.61–7.07) | < 0.001 |
Bivariate analysis showed that the prevalence of self-reported osteoporosis is different in level of age group, educational level, marital status, body mass index (BMI), wealth status, job, and Townsend index (P < 0.001). In addition, the overall weighted prevalence of self-reported osteoporosis was higher among females than males, significantly (P < 0.001). The result of trend analyses clearly indicates significantly an upward pattern for age group, BMI, Townsend index (P < 0.001), while this pattern was downward for educational level and wealth status (P < 0.001). The prevalence of self-reported osteoporosis was significantly higher among older than younger people. Osteoporosis prevalence increased with increasing of BMI category (P < 0.001), see Table 2 for more details. As shown in Figs. 1and 2, educational level and wealth status had a negative association with self-reported osteoporosis.
Table 2.
Prevalence of osteoporosis by demographic and socio-economic characteristics of study participants
| Variable | Total | Cases | Prevalence % (CI 95%) | P value | P value for trend | |
|---|---|---|---|---|---|---|
| Age group | 20–29 | 25,489 | 58 | 0.23 (0.18–0.28) | < 0.001 | < 0.001 |
| 30–39 | 19,278 | 128 | 0.66 (0.57–0.75) | |||
| 40–49 | 17,165 | 458 | 2.67 (2.38–2.86) | |||
| 50–59 | 14,814 | 893 | 6.03 (5.73–6.42) | |||
| 60–69 | 8846 | 660 | 7.46 (6.94–8.02) | |||
| 70–79 | 6221 | 920 | 14.79 (13.92–15.70) | |||
| + 80 | 1555 | 226 | 14.53 (12.82–16.39) | |||
| Sex | Male | 45,990 | 543 | 1.18 (1.08–1.28) | < 0.001 | |
| Female | 45,824 | 3134 | 6.84 (6.61–7.07) | |||
| Marital status | Married | 59,659 | 2654 | 4.45 (4.28–4.62) | < 0.001 | |
| Single | 24,599 | 93 | 0.38 (0.32–0.44) | |||
| Widow or Divorced | 5917 | 915 | 15.46 (14. 61–16.42) | |||
| Educational level | Illiterate | 6684 | 890 | 13.32 (12.49–13.97) | < 0.001 | < 0.001 |
| Primary school | 8171 | 756 | 9.25 (8.62–9.93) | |||
| Middle school | 10,867 | 609 | 5.60 (5.18–6.05) | |||
| High school | 37,633 | 1037 | 2.76 (2.59–2.93) | |||
| University | 27,914 | 356 | 1.28 (1.12–1.43) | |||
| BMI | Under Weight | 3130 | 73 | 2.33 (1.78–2.86) | < 0.001 | < 0.001 |
| Normal | 40,733 | 1144 | 2.81 (2.65–2.97) | |||
| Over Weight | 31,609 | 1427 | 4.52 (4.29–4.75) | |||
| Obese | 12,191 | 920 | 7.55 (7.24–7.98) | |||
| Current smoking | No Smoker | 84,603 | 3491 | 4.13 (3.99–4.26) | < 0.001 | |
| Smoker | 7227 | 187 | 2.59 (2.20–3.02) | |||
| Wealth status | Poorest | 17,550 | 1189 | 6.39 (6.38–7.18) | < 0.001 | < 0.001 |
| Poor | 16,543 | 719 | 4.35 (4.03–4.72) | |||
| Moderate | 17,187 | 622 | 3.62 (3.31–3.93) | |||
| Rich | 17,599 | 520 | 2.96 (2.71–3.22) | |||
| Richest | 16,772 | 422 | 2.52 (2.29–2.83) | |||
| Job | Skill Level I | 2662 | 49 | 1.84 (1.38–2.42) | < 0.001 | 0.006 |
| Skill Level II | 12,170 | 155 | 1.27 (1.08–1. 53) | |||
| Skill Level III | 2591 | 36 | 1.39 (1.03–1.87) | |||
| Skill Level IV | 3724 | 86 | 2.31 (1.93–2.84) | |||
| Townsend index | Most Affluent | 17,097 | 617 | 3.61 (3.32–3.91) | < 0.001 | < 0.001 |
| Affluent | 12,040 | 442 | 3.67 (3.28–4.03) | |||
| moderate | 17,325 | 655 | 3.78 (3.51–4.12) | |||
| Deprived | 20,734 | 875 | 4.22 (4.02–4.53) | |||
| Most Deprived | 20,006 | 923 | 4.61 (4.28–4.89) | |||
Fig. 1.
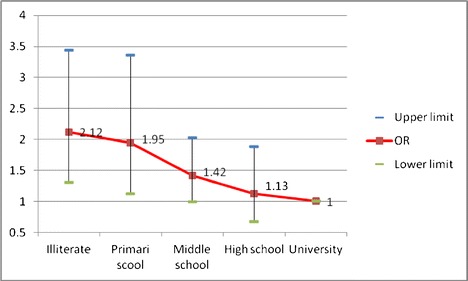
Adjusted odds ratios (95% CI) of osteoporosis according to levels of education
Fig. 2.
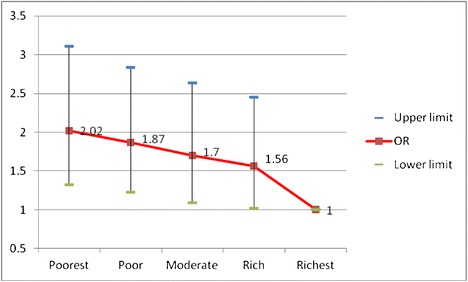
Adjusted odds ratios (95% CI) of osteoporosis according to wealth status
Multivariable Analysis
As shown in Table 3, multiple logistic regression revealed that report of osteoporosis had significant association with the age group, sex, educational level, current smoking, and wealth status. Hosmer-Lemeshow test showed that goodness of fit of model was good (Chi-square = 8.55, df = 8, P = 0.382). There were significant age group trends for odds ratio of self-reported osteoporosis (P < 0.001). The odds of report osteoporosis in the age group of 30–39 was 43% higher than the age group of 20–29 as a reference group (P < 0.001). The adjusted odds of self-reported osteoporosis in female was nearly 5.5 times males (P < 0.001). Although the marital status variable had no statistically significant association with the self-reported osteoporosis, but the odds of report osteoporosis in widow or divorced participant was nearly two times of single participants [OR = 1.96 (95% CI; 1.03 to 3.74)]. Level of education was the other variable that had statistically significant trends in odds ratio of self-reported osteoporosis (P < 0.001). This means that as the level of education increased, the odds of self-reported osteoporosis decreased. The odds ratio for report of osteoporosis in illiterate participant was 2.12 times higher than those with university degrees.
Table 3.
Crude and adjusted odds ratios using logistic regression model
| Variable | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Age group | 20–29 | 1 | 1 | < 0.001 |
| 30–39 | 2.96 (2.17–4.03) | 1.43 (0.56–3.68) | ||
| 40–49 | 11.97 (9.10–15.74) | 4.28 (1.79–10.24) | ||
| 50–59 | 34.94 (26.81–45.53) | 13.65 (5.86–31.81) | ||
| 60–69 | 59.44 (45.58–77.52) | 28.77 (12.21–67.78) | ||
| 70–79 | 74.20 (55.21–99.72) | 32.82 (20.56–80.69) | ||
| + 80 | 72.26 (55.14–94.71) | 55.54 (13.92–150.02) | ||
| Sex | Male | 1 | 1 | <0.001 |
| Female | 6.14 (5.60–6.74) | 5.19 (4.99–5.40) | ||
| Marital status | Single | 1 | 1 | 0.098 |
| Married | 12.27 (9.97–15.09) | 1.62 (0.79–3.34) | ||
| Widow or divorced | 48.20 (38.86–59.79) | 1.96 (1.03–3.74) | ||
| Educational level | Illiterate | 10.50 (9.26–11.90) | 2.12 (1.31–3.44) | 0.014 |
| Primary school | 4.78(4.21–5.43) | 1.95 (1.13–3.36) | ||
| Middle school | 3.33 (2.92–3.80) | 1.42 (0.99–2.03) | ||
| High school | 1.98 (1.68–2.13) | 1.13 (0.67–1.89) | ||
| University | 1 | 1 | ||
| BMI | Under weight | 0.27 (0.22–0.34) | 1.90 (0.92–3.82) | 0.153 |
| Normal | 1 | 1 | ||
| Over weight | 1.90 (1.75–2.05) | 1.30 (0.81–1.86) | ||
| Obese | 3.11 (2.85–3.40) | 0.99 (0.75–1.30) | ||
| Current smoking | No smoker | 1 | 1 | 0.021 |
| Smoker | 0.62 (0.53–0.72) | 1.54 (1.07–2.22) | ||
| Wealth status | Poorest | 2.82 (2.51–3.15) | 2.02 (1.32–3.11) | 0.008 |
| Poor | 1.76 (1.56–1.99) | 1.87 (1.23–2.84) | ||
| Moderate | 1.46 (1.28–1.65) | 1.70 (1.09–2.64) | ||
| Rich | 1.18 (1.04–1.34) | 1.56 (1.02–2.45) | ||
| Richest | 1 | 1 | ||
| Job | Skill level I | 0.69 (0.50–0.95) | 0.92 (0.64–1.31) | 0.482 |
| Skill level II | 0.49 (0.21–0.75) | 0.86 (0.53–1.39) | ||
| Skill level III | 1.28 (0.90–1.82) | 1.21 (0.76–1.94) | ||
| Skill level IV | 1 | 1 | ||
| Townsend index | Most affluent | 1 | 1 | 0.233 |
| Affluent | 1.02 (0.90–1.15) | 1.01 (0.68–1.51) | ||
| Moderate | 1.05 (1.05–1.17) | 0.87 (0.56–1.36) | ||
| Deprived | 1.18 (1.06–1.31) | 1.03 (0.72–1.47) | ||
| Most deprived | 1.29 (1.16–1.43) | 0.65 (0.42–1.01) | ||
After adjustment for all variables in the model, current smokers had significantly higher odds of self-reported osteoporosis compare with non-smoker [OR = 1.54 (95% CI; 1.07 to 2.22)].
Self-reported osteoporosis was significantly associated with lower wealth status adjusted for other variables in model. Participants in the poorest category of wealth had odds of self-reported osteoporosis 6.39 times that of those in the richest category (95% CI = 6.38 to 7.18).
Discussion
The two focuses of the present study were to obtain the prevalence estimate using a self-report measure and specify the adjusted association between SES and self-reported osteoporosis. The results from this study showed a relatively low prevalence of osteoporosis among general population. We estimated that prevalence of self-reported osteoporosis among the participants of this study was 4%. One study demonstrates that the overall prevalence of self-reported osteoporosis in the Australian population was 3.2% [19]. Another study shows that the prevalence of self-reported osteoporosis among 54,369 Brazilian people was 4.4% that is similar to our result [20]. In addition, Gill et al. reported the prevalence of self-reported osteoporosis among Australian aged 15 years and over, between 1995 and 2010, was 4.8% (95% CI 4.6–5.0). Our finding was consistent with these studies. However, the prevalence of self-reported osteoporosis was not similar in the other studies [16, 21, 22] .
The results indicated a reverse relationship between wealth status and the self-reported osteoporosis. Participants in lower wealth quintiles were significantly more likely to report osteoporosis compared to those within the highest wealth quintiles. To the best of our knowledge, there is no published report of the association between wealth status and the self-reported osteoporosis. Although, several studies show that the association between socioeconomic status and osteoporotic fracture, but Brennan et al. reported that there is conflicting evidence for the association between osteoporotic fracture and level of income and education [7, 9, 10, 23].
This population-based study revealed strong associations between several variable and self-reported osteoporosis. Based on trend analysis, as we expected, the prevalence of self-reported osteoporosis increased in higher age-group categories. This increasing trend was observed for job, BMI, and Townsend index. In addition, self-reported osteoporosis decreases as educational level increases from illiterate to group with a university degree.
In the present study, BMI did not show association with self-reported osteoporosis, while Asomaning et al. show that odds ratios for low, high, and obese compared with moderate BMI women were 1.8 (95% CI 1.2–2.7), 0.46 (95% CI 0.29–0.71), and 0.22 (95% CI 0.14–0.36), respectively [24].
Current smoking is the other variable that was significantly associated with reporting of osteoporosis. Although, crude OR for current smoking and self-reported osteoporosis was protective, the adjusted OR shows that current smoking increases odds of self-reported osteoporosis by 54% compare to no smoking. Kanis et al. report current smoking increased risk of osteoporotic fracture compared to non-smokers [25]. The other study shows that current smoking was associated with bone loss, and cigarette smoking is one of the important components of bone health [26].
A major strength of our study is the large sample size in relation to previous studies conducted in Tehran, Iran. This leads to provide more precise estimates as it can obviously be seen in the narrow confidence intervals for the estimated rates. Moreover, a high level of response rate was another major strength of this study. Our study had several limitations: first, the founded associations are not proof of causality because the study design was a cross-sectional survey and reverse causality bias could be occurred. Second, it was logistically difficult to use laboratory tests in this survey; hence, all measurements in this study were self-reported, so we could not present new and undiagnosed cases of osteoporosis in the study population. This can lead to both underestimating true osteoporosis prevalence and producing non-differential misclassification that can bias estimated associations toward the null although, self-reported measurements were frequently used in other chronic diseases [27, 28].
In summary, self- reported prevalence of osteoporosis among general population was relatively low. The finding of this study revealed that socioeconomic status is one of the strongest predictors for self-reported osteoporosis. Our results highlight the need to consider socioeconomic status in screening and prevention programs. The traditional public awareness programs about healthy-eating and physical activity can increase bone health in population. Future research is necessary for osteoporosis prevention and risk reduction.
Acknowledgments
This project is supported by Iran University of Medical Sciences.
References
- 1.Kanis J A. Assessment of osteoporosis at the primary health care level. World Health [Internet]. 2007;339. Available from: http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. Accessed 16 Nov 2016.
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. [Internet]. Springer-Verlag. 2006;17:1726–33. Available from: http://link.springer.com/10.1007/s00198-006-0172-4. Accessed 10 Nov 2016. [DOI] [PubMed]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide Projections for Hip Fracture. Osteoporos. Int. [Internet]. Springer-Verlag London Limited. 1997;7:407–13. Available from: http://link.springer.com/10.1007/PL00004148. Accessed 10 Nov 2016. [DOI] [PubMed]
- 4.Ahmadi-Abhari S, Moayyeri A, Abolhassani F. Burden of Hip Fracture in Iran. Calcif. Tissue Int. [Internet]. Springer-Verlag. 2007;80:147–53. Available from: http://link.springer.com/10.1007/s00223-006-0242-9. Accessed 10 Nov 2016. [DOI] [PubMed]
- 5.Holroyd C, Cooper C, Dennison E, Conference CD, Cooper AP, Cooper C, et al. Epidemiology of osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. [Internet]. Elsevier. 2008;22:671–85. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1521690X08000766. Accessed 10 Nov 2016. [DOI] [PubMed]
- 6.Graham H. Unequal lives: health and socioeconomic inequalities. Maidenhead, Berkshire: McGraw-Hill Education; 2007.
- 7.Brennan SL, Pasco JA, Urquhart DM, Oldenburg B, Hanna F, Wluka AE. The association between socioeconomic status and osteoporotic fracture in population-based adults: a systematic review. Osteoporos. Int. [Internet]. Springer-Verlag. 2009;20:1487–97. Available from: http://link.springer.com/10.1007/s00198-008-0822-9. Accessed 10 Nov 2016. [DOI] [PubMed]
- 8.Quah C, Boulton C, Moran C. The influence of socioeconomic status on the incidence, outcome and mortality of fractures of the hip. Bone Joint J. 2011;93–B. [DOI] [PubMed]
- 9.Zingmond DS, Soohoo NF, Silverman SL. The role of socioeconomic status on hip fracture. Osteoporos. Int. [Internet]. Springer-Verlag. 2006;17:1562–8. Available from: http://link.springer.com/10.1007/s00198-006-0161-7. Accessed 10 Nov 2016. [DOI] [PubMed]
- 10.Farahmand BY, Persson P-G, Michaëlsson K, Baron JA, Parker MG, Ljunghall S, et al. Socioeconomic status, marital status and hip fracture risk: a population-based case-control study. Osteoporos. Int. [Internet]. Springer-Verlag London Limited. 2000;11:803–8. Available from: http://link.springer.com/10.1007/s001980070060. Accessed 10 Nov 2016. [DOI] [PubMed]
- 11.Bacon WE, Hadden WC. Occurrence of hip fractures and socioeconomic position. J. Aging Health [Internet]. SAGE Publications. 2000;12:193–203. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11010696. Accessed 10 Nov 2016. [DOI] [PubMed]
- 12.Perez Cano R, Galan Galan F, Dilsen G. Risk factors for hip fracture in Spanish and Turkish women. Bone. 1993;14:69–72. doi: 10.1016/8756-3282(93)90353-C. [DOI] [PubMed] [Google Scholar]
- 13.Filmer D, Pritchett L. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in States of India. Demography [Internet]. Springer-Verlag. 2001;38:115–32. Available from: http://link.springer.com/10.1353/dem.2001.0003. Accessed 10 Nov 2016. [DOI] [PubMed]
- 14.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health [Internet]. BMJ Publishing Group Ltd. 2006;60:7–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16361448. Accessed 10 Nov 2016. [DOI] [PMC free article] [PubMed]
- 15.Galobardes B, Shaw M, Lawlor DA, Lynch JW, Smith GD. Indicators of socioeconomic position (part 2). J Epidemiol Community Heal [Internet]. 2006;60:95–101. Available from: http://www.jstor.org/stable/40795178. Accessed 16 Nov 2016. [DOI] [PMC free article] [PubMed]
- 16.Frost M, Wraae K, Gudex C, Nielsen T, Brixen K, Hagen C, et al. Chronic diseases in elderly men: underreporting and underdiagnosis. Age Ageing [Internet]. Oxford University Press. 2012;41:177–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22146130. Accessed 17 Nov 2016. [DOI] [PubMed]
- 17.Bergmann MM, Jacobs EJ, Hoffmann K, Boeing H. Agreement of self-reported medical history: comparison of an in-person interview with a self-administered questionnaire. Eur. J. Epidemiol. [Internet]. Kluwer Academic Publishers. 2003;19:411–6. Available from: http://link.springer.com/10.1023/B:EJEP.0000027350.85974.47. Accessed 17 Nov 2016. [DOI] [PubMed]
- 18.Peeters GMEE, Tett SE, Dobson AJ, Mishra GD. Validity of self-reported osteoporosis in mid-age and older women. Osteoporos. Int. [Internet]. Springer-Verlag. 2013;24:917–27. Available from: http://link.springer.com/10.1007/s00198-012-2033-7. Accessed 17 Nov 2016. [DOI] [PubMed]
- 19.Phillipov G, Phillips P, Leach G, Taylor A. Public perceptions and self-reported prevalence of osteoporosis in South Australia. Osteoporos. Int. [Internet]. 1998. Available from: http://www.springerlink.com/index/TY4FR7YCCXW5W6VG.pdf. Accessed 18 Feb 2017. [DOI] [PubMed]
- 20.Martini LA, Moura EC de, Santos LC dos, Malta DC, Pinheiro M de M. Prevalência de diagnóstico auto-referido de osteoporose, Brasil, 2006. Rev. Saude Publica [Internet]. Faculdade de Saúde Pública da Universidade de São Paulo. 2009;43:107–16. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-89102009000900014&lng=pt&nrm=iso&tlng=pt. Accessed 17 Nov 2016.
- 21.Baccaro LF, de Souza Santos Machado V, Costa-Paiva L, Sousa MH, Osis MJ, Pinto-Neto AM. Factors associated with osteoporosis in Brazilian women: a population-based household survey. Arch. Osteoporos. [Internet]. Springer London. 2013;8:138. Available from: http://link.springer.com/10.1007/s11657-013-0138-z. Accessed 17 Nov 2016. [DOI] [PubMed]
- 22.Werner P. Self-reported prevalence and correlates of osteoporosis: results from a representative study in Israel. Arch. Gerontol. Geriatr. [Internet]. 2003;37:277–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14511853. Accessed 17 Nov 2016. [DOI] [PubMed]
- 23.Crandall CJ, Han W, Greendale GA, Seeman T, Tepper P, Thurston R, et al. Socioeconomic status in relation to incident fracture risk in the study of women’s health across the nation. Osteoporos. Int. [Internet]. Springer London. 2014;25:1379–88. Available from: http://link.springer.com/10.1007/s00198-013-2616-y. Accessed 17 Nov 2016. [DOI] [PMC free article] [PubMed]
- 24.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J. Women’s Heal. [Internet]. Mary Ann Liebert, Inc. 2 Madison Avenue Larchmont, NY 10538 USA. 2006;15:1028–34 Available from: http://www.liebertonline.com/doi/abs/10.1089/jwh.2006.15.1028. Accessed 17 Nov 2016. [DOI] [PubMed]
- 25.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. Osteoporos. Int. [Internet]. Springer-Verlag. 2005;16:155–62. Available from: http://link.springer.com/10.1007/s00198-004-1640-3. Accessed 17 Nov 2016. [DOI] [PubMed]
- 26.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PWF, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis study. J. Bone Miner. Res. [Internet]. John Wiley and Sons and The American Society for Bone and Mineral Research (ASBMR). 2010;15:710–20. Available from: http://doi.wiley.com/10.1359/jbmr.2000.15.4.710. Accessed 16 Nov 2016. [DOI] [PubMed]
- 27.Manuel DG, Schultz SE, Kopec JA. Measuring the health burden of chronic disease and injury using health adjusted life expectancy and the health utilities index. J. Epidemiol. Community Health [Internet]. BMJ Publishing Group Ltd. 2002;56:843–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12388577. Accessed 16 Nov 2016. [DOI] [PMC free article] [PubMed]
- 28.Levesque J-F, Mukherjee S, Grimard D, Boivin A, Mishra S. Measuring the prevalence of chronic diseases using population surveys by pooling self-reported symptoms, diagnosis and treatments: results from the World Health Survey of 2003 for South Asia. Int. J. Public Health [Internet]. SP Birkhäuser Verlag Basel. 2013;58:435–47. Available from: http://link.springer.com/10.1007/s00038-013-0446-5. Accessed 16 Nov 2016. [DOI] [PubMed]


