Abstract
Introduction
Lipoma is a benign mesenchymal tumor originating from adipose tissue cells. In the head and neck, this tumor is not common. The occurrence of lipomas in the parotid gland is extremely rare, ranging between 0.6 and 4%.
Case Reports
In this article, we present two clinical cases of lipomas of the superficial lobe of the parotid gland.
Discussion
Clinical diagnosis of parotid gland lipomas is difficult, while usually, they are asymptomatic and presented as a painless swelling of the parotid gland. Tomographic imaging modalities are very accurate in preoperative diagnosis in contrast to FNA or FNAB which is not helpful in the case of lipoma. This pathological entity should be considered in the differential diagnosis of parotid gland’s mass lesions. The usual indication for surgical intervention is cosmetic appearance. Meticulous surgical excision should be performed to avoid disturbing adverse events.
Keywords: Lipoma, Parotid gland, Superficial lobe, Parotidectomy
Introduction
Lipoma is a benign mesenchymal tumor originating from adipose tissue cells and usually occurs in the abdomen, back, and shoulder. Head and neck area is less frequently affected, and its involvement has ranged between 15 and 20% [1]. Parotid gland lipoma is, even more, rare ranging between 0.6 and 4% [2, 3]. Clinical diagnosis is difficult, but usually, they are asymptomatic and presented as a painless swelling of the parotid gland, very soft during palpation in contrast to a hard consistency of the pleomorphic adenomas, the most usually encountered tumors of the parotid gland [4].
The CT scan and MRI are the diagnostic modalities of choice to differentiate pathologic entities of the parotid gland. CT scanning is extremely valuable in preoperative diagnosis [2], but MRI is preferred by most authors because of better imaging of soft tissue [6]. FNA or FNAB even though is valuable to investigate parotid gland masses, in the case of lipoma is not helpful [6, 7].
Most of the reported cases were located at superficial parotid gland, and the maximum dimension was found to be less of 8 cm. In our first case, we describe a patient with a large lipoma of the superficial lobe of the parotid gland measuring about 12 cm without clinical symptoms except cosmetic problems. The dimensions of the tumor even though are not critical for surgical excision, the bigger the dimension the tumor the greater the involvement of the facial nerve and its expected weakness.
Case One
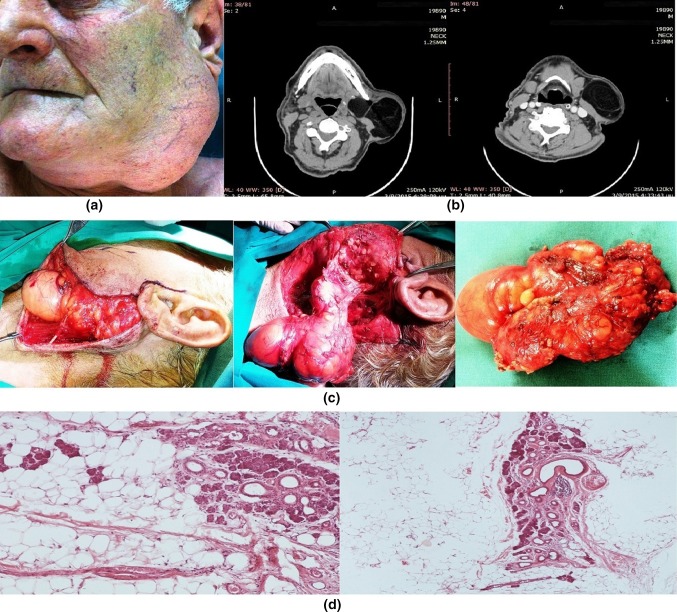
An 81-year-old man presented with a mass of the left parotid region. He had been aware of a slow-growing, painless swelling for 5 years. Clinical examination confirmed a mobile, soft, non-tender, intraparotid mass that measured about 7 × 12 cm. The function of the facial nerve was intact (Fig. 1a).
Fig. 1.
a Mass of the left parotid region, b CT scans of parotid gland lipoma, c surgical approach: superficial parotidectomy with facial nerve preservation, under general anesthesia, using a classic Blair’s incision and d histological section of parotid gland lipoma (Hematoxylin–Eosin)
An intragranular well-defined lesion of the left parotid with features of lipoma was observed on the CT of the face and neck. The lesion extended from the superficial to the deep lobe. The sternocleidomastoid muscle was highly repelled by the mass (Fig. 1b). Under general anesthesia using a classic Blair’s incision, a superficial parotidectomy was performed. The distal tumor extended deep to the plane of the facial nerve. The mass was removed while the facial nerve was preserved. The specimen was a soft, yellowish, well-circumscribed mass, measuring 12.5 × 8 × 4.5 cm (Fig. 1c). Histological sections showed lobules of adipose tissue separated by a thin core of connective tissue stroma. Features were consistent with that of a lipoma (Fig. 1d). There were no signs of recurrence at the time of surveillance performed 2 years after surgery.
Case Two
A 59-year-old female presented in our department with a right parotid mass. The patient had been aware of the swelling for 6 months, but she had not experienced any pain or tenderness. Physical examination revealed about 2 cm in diameter soft lump with a smooth surface in the right parotid. The tumor did not adhere to the skin. The parotid papilla was intact, and no facial palsy was observed.
T1- and T2-weighted axial magnetic resonance images (MRIs) showed a well-defined lesion in the superficial lobe of the right parotid gland. This produced a strong signal on both T1- and T2-weighted sequences (Fig. 2a). The margin of the tumor was clearly delineated. These findings suggested a superficial lobe parotid lipoma.
Fig. 2.
a MRI image of parotid gland lipoma, b surgical approach: superficial parotidectomy with facial nerve preservation, under general anesthesia, using a classic Blair’s incision and c histological section of parotid gland lipoma (Hematoxylin–Eosin)
A superficial parotidectomy was performed under general anesthesia using a classic Blair’s incision. The facial nerve was preserved. A glistening, partially encapsulated, yellowish tumor resembling adipose tissue originated in the superficial lobe of the parotid. It was difficult to distinguish the tumor from the surrounding adipose tissue and parotid gland itself, in part because the appearance and softness of the tumor were similar to the surrounding tissue (Fig. 2b). Histological examination confirmed the diagnosis of lipoma (Fig. 2c). No recurrence was seen 15 months after surgery.
Discussion
Lipoma of the head and neck region is benign mesenchymal tumor, accounting for 15–20% of total lipoma of the body, where they account for 0.1–5% of all benign tumors [1]. According to a large study of lipomas affecting the oral and maxillofacial area [7] in a total of 125 cases, the distribution of lipoma has been found to locate in the parotid and buccal area with almost similar percentages 23%, followed by the lips, submandibular area, tongue, palate and floor of the mouth. Extremely rare were lipomas of the vestibule (2 lipomas of the vestibule in a total of 125 cases). In another study of 638 parotidectomies, the authors found only eight cases (1.3%) to be lipomatous lesions [7].
The term lipomatous lesion is an extensive description of these kinds of lesions mainly including two histological types, focal lipoma and diffuse infiltrating lipomatosis. In the case of focal lipoma, histopathologic investigation reveals mature adipose tissue to be separated from parotid gland parenchyma with a fibrous capsule while in diffuse lipomatosis adipose tissue cells found to infiltrate parotid gland parenchyma [7]. In this study, five patients had focal lipoma and the remaining three patients had diffuse lipomatosis. Both of our cases were focal lipomatous lesions.
Lipomas of the parotid gland have a predilection to males, according to most studies with male to female ration 3:1 [6, 7] even though a greater gap (10:1) have been referred in another older study. The age distribution is wide even though lipomas of the parotid gland are found in the fifth and sixth decade. Those in deep lobe have been reported to be highest in the fourth decade. Few cases have been described in pediatric patients [7, 8].
Clinical diagnosis of the parotid gland lipoma is difficult, especially for tumors located at the deep lobe of the parotid gland. Those situated at superficial parotid lobe usually appear as a painless, asymptomatic and slow-growing swelling. Mean time of lipoma presence before its excision is 3.2 years [6]. In the first of our cases, the presence of lipoma before its surgical excision was 5 years and in the second case only 6 months.
According to the literature, lipomas can be caused by heredity, obesity, diabetes, radiation, endocrine disorders, insulin injection, corticosteroid therapy and trauma [9]. In both our cases, no trauma or endocrine disorders were reported in patients’ history.
The preoperative imaging has a crucial role to correctly diagnose the nature and the location of lesions. CT scan and MRI can be helpful in giving information about pathological features of the tumors, to evaluate its location and its relation to important anatomic structures. CT imaging shows a hypodense and homogeneous, well delineated mass with few septations and less than water density with − 50 to − 150 Hounsfield densities which is characteristic of lipoma. However, CT scan does not help much in differentiation of lipoma from surrounding adipose tissue. This information can be clearly collected from the MRI scan [5, 7].
MRI remains the best diagnostic technique that can accurately diagnose lipomas preoperatively by comparing the signal intensity on T1- and T2-weighted images. MRI image depicts a high signal on T1 sequence. MRI can also clearly define the limits of lipoma from normal adipose tissue (subcutaneous tissue) with a “black-rim” around the mass. In our first case, a high clinical suspicion of lipoma was established according to its characteristic clinical features and confirmed by CT scanning. In the second case, clinical diagnosis was difficult and the possibility of lipoma was based on MRI and confirmed during the operation [7]. FNA or FNACB even though is valuable in parotid gland tumors, in cases of lipoma it is of little diagnostic value [7–9].
Lipomas of the superficial parotid gland could develop freely and take long dimensions before its excision. Review of the literature revealed a maximum dimension less of 8 cm while in the deep parotid lobe; its extension is confined by surrounding tissue [10]. In our first case, the lipoma had an extended dimension with its maximum diameter measured about 12 cm by far the largest lipoma of the parotid gland that have been described in the English literature. Facial disfigurement was intense, and it was the main incentive for the patient to seek treatment.
Management of parotid gland lipoma is under controversy. Enucleation or excision with a small border of healthy parotid gland parenchyma for encapsulated intra- or paraparotid lipomas have been proposed [11]. Superficial parotidectomy, however, is preferred by most authors [12, 13]. As we noted in our cases, the lipoma was seated on the main trunk and its peripheral branches and the excision of the lesion performed through a classic Blair approach. Lipomas of the deep lobe of parotid gland encountered less frequently than the lipomas of the superficial lobe. Their management is challenging. In these cases, surgical management is total parotidectomy with full exposure of the main trunk of the facial nerve and its peripheral branches to avoid nerve damage [14].
Facial nerve dysfunction ranged between 8.2 and 65% after parotid gland surgery for benign tumors [15]. Not facial palsy or facial nerve weakening was recorded on both of our cases.
In conclusion, lipoma of the parotid gland is a rare benign pathology, which should be considered in the differential diagnosis of parotid gland’s mass lesions. Clinical suspicion combined with appropriate imaging leads to efficient diagnosis. Meticulous surgical excision should be performed to avoid disturbing adverse events.
References
- 1.Weiss SW, Goldblum JR. Chapter 16: “Benign Lipomatous Tumors”. In: Weiss SW, Goldblum JR, editors. Enzinger and Weiss’s soft tissue tumors. 4. St Louis: Mosby; 2001. p. 571. [Google Scholar]
- 2.Korentager R, Noyek AM, Chapnik JS, Steinhardt M, Luk SC, Cooter N. Lipoma and liposacoma of the parotid gland: high resolution preoperative imaging diagnosis. Laryngoscope. 1988;98:967–971. doi: 10.1288/00005537-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Dispenza F, De Stefano A, Romano G, Mazzoni A. Posttraumatic lipoma of the parotid gland: case report. Acta Otorhinolaryngol Ital. 2008;28:87–88. [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan V, Gamesan S, Premachandra J. Lipoma of the parotid gland presenting with facial palsy. J Laryngol Otol. 1996;110:93–95. doi: 10.1017/S0022215100132827. [DOI] [PubMed] [Google Scholar]
- 5.Som PM, Sacher M, Stollman MD, Biller HF, Lawson W. Common tumors of the parapharyngeal space: refined imaging diagnosis. Radiology. 1988;169:81–85. doi: 10.1148/radiology.169.1.2843942. [DOI] [PubMed] [Google Scholar]
- 6.Furlong MA, Fanburg-Smith JC, Childers ELB. Lipoma of the oral and maxillofacial region: site and subclassification of 125 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(4):441–450. doi: 10.1016/j.tripleo.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 7.Ethunandan M, Vura G, Anand R, Macpherson DW, Wilson AW. Lipomatous lesions of the parotid gland. J Oral Maxillofac Surg. 2006;64:1583–1586. doi: 10.1016/j.joms.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Esposito C, Califano L, D’Armiento M, Longo F. Lipomatosis of the parotid gland in a child. Br J Plast Surg. 2000;53:699–701. doi: 10.1054/bjps.2000.3437. [DOI] [PubMed] [Google Scholar]
- 9.Layfield IJ, Glasgow BJ, Goldstein N, et al. Lipomatous lesions of the parotid gland—potential pitfalls in fine needle aspiration biopsy diagnosis. Acta Cytol. 1991;35:553–556. [PubMed] [Google Scholar]
- 10.Starkman SJ, Olsen SM, Lewis JE, Olsen KD, Sabri A. Lipomatous lesions of the parotid gland: analysis of 70 cases. Laryngoscope. 2013;123:651–656. doi: 10.1002/lary.23723. [DOI] [PubMed] [Google Scholar]
- 11.Weiner GM, Pahor AL. Deep lobe parotid lipoma: a case report. J Laryngol Otol. 1995;109:772–773. doi: 10.1017/s0022215100131287. [DOI] [PubMed] [Google Scholar]
- 12.Malave DA, Ziccardi VB, Greco R, Patterson GT. Lipoma of the parotid gland: report of a case. J Oral Maxillofac Surg. 1994;52:408–411. doi: 10.1016/0278-2391(94)90451-0. [DOI] [PubMed] [Google Scholar]
- 13.Debnath SC, Saikia A. Lipoma of the parotid gland extending from the superficial to the deep lobe: a rarity. Br J Oral Maxillofac Surg. 2010;48(3):203–204. doi: 10.1016/j.bjoms.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Ulku CH, Uyar Y, Unaldi D. Management of lipomas arising from deep lobe of the parotid gland. Auris Nasus Larynx. 2005;32:49–53. doi: 10.1016/j.anl.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Mehle ME, Kraus DH, Wood BG, Benninger MS, Eliachar I, Levine HI, et al. Facial nerve morbidity following parotid surgery for benign disease: the Cleveland Clinic Foundation experience. Laryngoscope. 1994;104:1487–1494. doi: 10.1002/lary.5541030404. [DOI] [PubMed] [Google Scholar]