Abstract
Background
The prophylactic extraction of third molars is a common practice in dental offices, but divergent opinions are found in the literature regarding the indication of this procedure. The aim of the present study was to determine the prevalence of pathological changes associated with the pericoronal tissue of asymptomatic impacted third molars that could justify prophylactic extraction.
Materials and Methods
A cross-sectional observational study was conducted in which 109 pericoronal tissues with no radiographic evidence of pathology were histopathologically analyzed. The specimens were fixed in 10% formalin, embedded in paraffin, stained with hematoxylin and eosin and analyzed individually by two pathologists.
Result
The frequency of inflammatory infiltrate in the dental follicle of patients older than 20 years of age was significantly higher than that of younger patients (p = 0.004), demonstrating an association between inflammation in the dental follicle and patient age. The occurrence of squamous metaplasia was also greater in patients older than 20 years (p = 0.042), demonstrating that the prevalence of squamous metaplasia increases with age. A significant association was also found between inflammation and squamous metaplasia (p < 0.001).
Conclusion
Pathological changes may be present in the dental follicle of impacted third molars even in the absence of clinical or radiographic signs of disease.
Keywords: Third molar, Tooth extraction, Pathology, Dental sac
Introduction
The extraction of third molars is the most frequent minor dental surgery performed in dental offices [1–4]. The US National Institutes of Health have established well-defined criteria for third molar extraction [5]. However, the removal of asymptomatic third molars remains a controversial topic in the literature [6].
Prophylactic third molar extraction is a common practice in dentistry and is the main indication for the surgical removal of asymptomatic impacted teeth [7–9]. Inflammatory, cystic or neoplastic tumors can develop in the tissue surrounding third molars even with no clinical or radiographic evidence of disease [10]. Thus, the absence of clinical or radiographic signs does not necessarily mean the absence of disease from the histological standpoint [6], and many consider this to be a justification for the indication of the removal of these teeth [7, 8, 11–13]. Nonetheless, there is insufficient evidence to justify the prophylactic extraction of asymptomatic, disease-free third molars [6, 9, 14].
The aim of the present study was to perform a histopathological analysis of the pericoronal follicle of impacted third molars in the mandible and maxilla with no clinical or radiographic evidence of pathological alterations in an attempt to identify histopathological changes that might justify the prophylactic removal of asymptomatic teeth.
Materials and Methods
This study received approval from the human research ethics committee of the Federal University of Santa Maria, Brazil (certificate number: 23081.017097/2009-89).
Histopathological analyses were performed on 109 pericoronal tissues from patients who visited the surgical clinic of the dental scholl for the extraction of impacted third molars after orthodontic indication. Patient data (sex, age and anatomic location of extracted teeth) were obtained from patients’ records. All patients were asymptomatic, had good general health, did not take any type of medication and had no radiographic evidence of any pathological changes associated with the third molars. The teeth were covered either completely or partially with bone tissue and completely covered with mucosa. The dental follicles analyzed were from teeth with a follicular space less than or equal to 3 mm [13, 15]. The panoramic radiograph of each patient was analyzed in a negatoscope for the definition of the pericoronal space. Measurements were performed by a single examiner with the aid of a millimeter ruler.
After extraction, the specimens were immediately fixed in a 10% formalin solution for 24 h. The material was then dehydrated with an increasing concentration of alcohol (70, 90 and 100%), cleared with xylol and embedded in paraffin. The paraffin blocks were sectioned with the aid of a microtome for the acquisition of slices measuring 5 µm in thickness. The slices were placed on glass slides and stained with hematoxylin and eosin. Glass cover slips were attached mounted over the slices with Permount resin (Fischer Scientific, Fair Lawn, NJ, USA).
The tissue samples were submitted to a morphological study under a light microscope. The analyses were performed by two independent experienced oral pathologists who had undergone a training and calibration exercise. The samples were classified into one of two groups: normal dental follicle without histopathological alterations and dental follicle with histopathological alterations. The following were the histopathological criteria for normal dental follicle: fragment comprised of dense or loose connective tissue, with or without islets or strings of odontogenic epithelium, amorphous basophilic mineralized material and/or areas of extravasation of red cells. When present, the epithelial lining was characterized by a thin epithelium formed by one to four layers of cuboidal cells and interpreted as reduced epithelium of the enamel organ. Samples that did not meet these criteria were interpreted as dental follicles with histopathological alterations when inflammatory infiltrate and/or squamous metaplasia was present in focal areas of the epithelium.
The Chi-squared test was used to determine associations between the variables. The Statistical Package for the Social Sciences (SPSS 15.0, SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A p value < 0.05 was considered indicative of statistical significance.
Results
Sixty-eight of the samples were from female patients and 41 were from male patients. Patient age ranged from 15 to 39 years (mean: 20.7 years). Seventy-two samples came from mandibles and 37 came from maxillas (Table 1).
Table 1.
Characteristics of sample
| Variables | N (%) |
|---|---|
| Age | 20.7 |
| Sex | |
| Female | 68 (62.4) |
| Male | 41 (37.6) |
| Anatomic location | |
| Maxilla | 9 (33.9) |
| Mandible | 72 (66.1) |
| Diagnosis | |
| Dental follicle with no histopathological alteration | 75 (68.8) |
| Dental follicle with inflammation alone | 18 (16.5) |
| Dental follicle with squamous metaplasia alone | 4 (3.7) |
| Dental follicle with both inflammation and squamous metaplasia | 11 (10.1) |
| Dentigerous cyst | 1 (0.9) |
| Total | 109 |
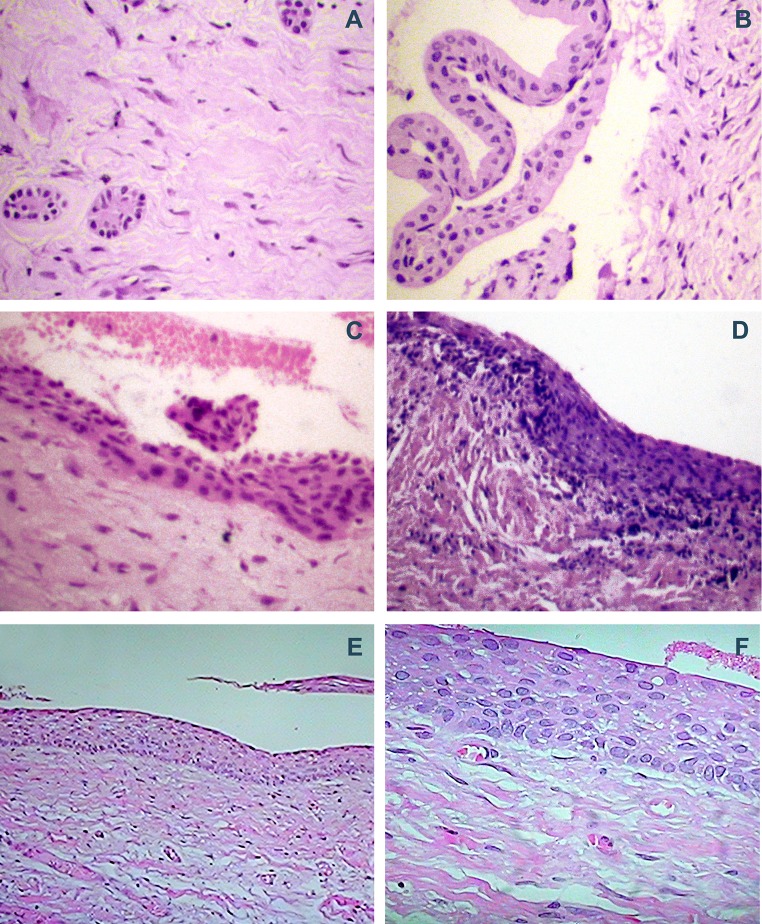
Histologically, one case (0.9%) was diagnosed as a dentigerous cyst and was excluded from the analysis. This sample exhibited stratified squamous epithelium with approximately five layers lining the capsule of dense connective tissue (Fig. 1a).
Fig. 1.
Histopathological features of dental follicle and dentigerous cyst (hematoxylin/eosin; magnification: 100×) a, b dental follicle with dense connective tissue displaying islands of odontogenic epithelium and epithelial lining composed of one to four layers of cuboidal cells; c dental follicle showing focal areas of squamous metaplasia; d squamous metaplasia associated with inflammation; e, f dentigerous cyst showing stratified squamous epithelium with approximately six layers
The remaining 108 cases were classified as dental follicles, 75 of which were normal (Fig. 1b) and 33 exhibited histopathological alterations: inflammatory infiltrate in the connective tissue (Fig. 1c) and/or focal areas of squamous metaplasia in the epithelium (Fig. 1d). Epithelial lining composed of one to four layers of cuboidal cells was found in 35 dental follicles and interpreted as reduced epithelium of the enamel organ. In many cases, the lining epithelium was absent, which may be explained by the fact that the epithelium participates in odontogenesis and is closely related to the dental organ. Thus, it likely was detached from the connective tissue during the collection of the sample or the mounting of the slide.
Histopathological alterations were found with greater frequency in patients older than 20 years of age. The frequency of inflammatory infiltrate in the dental follicle of patients older than 20 years of age was significantly higher than that of younger patients (p = 0.004), demonstrating an association between inflammation in the dental follicle and patient age (Table 2). The occurrence of squamous metaplasia was also greater in patients older than 20 years (p = 0.042), demonstrating that the prevalence of squamous metaplasia increases with age (Table 2). A significant association was also found between inflammation and squamous metaplasia (p < 0.001) (Table 3).
Table 2.
Association between clinical/histopathological variables and inflammatory infiltrate in dental follicle
| Variable | Dental follicle | Total | p | |||
|---|---|---|---|---|---|---|
| No inflammation | Inflammation | |||||
| n | % | n | % | |||
| Age (years) | ||||||
| ≤ 20 | 52 | 83.9 | 10 | 16.1 | 62 | 0.004 |
| > 20 | 27 | 58.7 | 19 | 41.3 | 46 | |
| Sex | ||||||
| Female | 53 | 79.1 | 14 | 20.9 | 67 | 0.074 |
| Male | 26 | 63.4 | 15 | 36.6 | 41 | |
| Location | ||||||
| Maxilla | 31 | 83.8 | 6 | 16.2 | 37 | 0.072 |
| Mandible | 48 | 67.6 | 23 | 32.4 | 71 | |
| Metaplasia | ||||||
| No | 75 | 80.6 | 18 | 19.4 | 93 | < 0.001 |
| Yes | 4 | 26.7 | 11 | 73.3 | 15 | |
| Total | 79 | 73.1 | 29 | 26.9 | 108 | |
Statistical difference is shown in bold
Result of Chi-squared test
Table 3.
Association between clinical/histopathological variables and metaplasia in dental follicle
| Variable | Metaplasia | Total | p | |||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | % | n | % | |||
| Age (years) | ||||||
| ≤ 20 | 57 | 91.9 | 5 | 8.1 | 62 | 0.042 |
| > 20 | 36 | 78.3 | 10 | 21.7 | 46 | |
| Sex | ||||||
| Female | 57 | 85.1 | 10 | 14.9 | 67 | 0.691 |
| Male | 36 | 87.8 | 5 | 12.2 | 41 | |
| Location | ||||||
| Maxilla | 32 | 86.5 | 5 | 13.5 | 37 | 0.935 |
| Mandible | 61 | 85.9 | 10 | 14.1 | 71 | |
| Total | 93 | 86.1 | 15 | 13.9 | 108 | |
Statistical difference is shown in bold
Result of Chi-squared test
Discussion
According to the literature, the prevalence of odontogenic cysts and tumors associated with impacted third molars is generally low [16–18] and cysts more frequent than odontogenic tumors. However, some authors describe a high prevalence rate of cystic and neoplastic changes associated with pericoronal tissue.
Among all cysts that can develop from the pericoronal follicle of impacted third molars, the dentigerous type is the most frequent, accounting for approximately 18–20% such developmental cysts [19]. In the present study, one case (0.9%) was diagnosed as a dentigerous cyst and had no clinical or radiographic evidence of pathological alteration, which is in agreement with data described by some authors [12]. Despite being the cyst most frequently associated with pericoronal tissue, the prevalence of the dentigerous type is low, ranging from 0.8 to 2.15% of samples, [17, 18] as confirmed in the present study. However, some authors report a high incidence (34–50%) of cysts associated with the pericoronal follicle of impacted mandibular third molars [1–3, 13].
The low incidence of cysts in the present study could be attributed to the sample size and mean age of the patients analyzed (20.7 years). Studies report that cystic alterations are more frequently found in patients older than 20 years of age [2, 4, 6, 12, 13, 16]. Another explanation could be the difficulty in histologically differentiating a normal dental follicle from a dentigerous cyst in the initial phase [15, 20]. The capsule of a dentigerous cyst is classically described in the histopathological exam as connective tissue lined with stratified squamous epithelium and the absence of inflammatory infiltrate. However, it is not clear in the literature whether this epithelium must line the entire sample or whether the occurrence of focal areas of squamous metaplasia is sufficient for the diagnosis of a dentigerous cyst, as some authors suggest [1, 2]. The correlation among histopathological, clinical/surgical and radiographic finings is necessary for an appropriate diagnosis, although clinical or radiographic evidence suggesting a disease process may not be observed in some cases [10].
According to some authors, reduced epithelium of the enamel organ delineating the connective tissue and focal areas of squamous metaplasia in the epithelial lining are frequent findings in dental follicles [15, 20]. Metaplasia is an adaptive replacement of one type of differentiated cell with another differentiated cell, generally in response to some form of cellular harm [21]. As the final stage of root development in third molars occurs between 18 and 25 years of age, [22] one may suggest that the physiological process of tooth eruption could cause areas of compression and ischemia in pericoronal tissues, which may undergo a degenerative and/or adaptive process, leading to the occurrence of areas of squamous metaplasia. This could explain the findings of the present study, in which a greater frequency of squamous metaplasia was found in patients older than 20 years of age.
Inflammation was the most frequent pathological alteration in the dental follicles analyzed, which is in agreement with results described in previous studies [8, 23] and was found in asymptomatic teeth. In such cases, inflammation may be explained by possible contamination of the periodontal space from an adjacent tooth, which could lead to contamination of the pericoronal space or could be the result of the physiological process of third molar eruption, as some authors suggest [15].
It has been described that an increase in patient age is associated with greater difficulty regarding the extraction of impacted third molars, which increases the risk of postoperative complications [24, 25]. Moreover, the removal of impacted third molars is associated with a better quality of life in the long term [26]. As pathological alterations in the dental follicle were associated with the increase in age and, in some cases, were found even in asymptomatic teeth with no clinical or radiographic evidence of disease, the prophylactic removal of asymptomatic impacted third molars in young patients could be justified by the fact that such surgery offers more benefits than harm. However, odontogenic cysts and tumors associated with non-erupted third molars are infrequent and, based on this criterion, the prophylactic extraction of asymptomatic teeth is not justified. Thus, each case should be evaluated individually, taking into consideration the risks and benefits of the procedure.
Acknowledgements
This study was supported by the Department of Pathology of the Federal University of Santa Maria.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study received approval from the ethics committee of the Federal University of Santa Maria (certificate number: 23081.017097/2009-89).
References
- 1.Adelsperger J, Campbell JH, Coates D, Summerlin DJ, Tomich CE. Early soft tissue pathosis associated with impacted third molars without pericoronal radiolucency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:402–406. doi: 10.1016/S1079-2104(00)70119-3. [DOI] [PubMed] [Google Scholar]
- 2.Baykul T, Saglam AA, Aydin U, Basak K. Incidence of cystic changes in radiographically normal impacted lower third molar follicles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:542–545. doi: 10.1016/j.tripleo.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Rakprasitkul S. Pathologic changes in the pericoronal tissues of unerupted third molars. Quintessence Int. 2001;32:633–638. [PubMed] [Google Scholar]
- 4.Yildirim G, Ataôlu H, Mihmanli A, Kizilôlu D, Avunduk MC. Pathologic changes in soft tissues associated with asymptomatic impacted third molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:14–18. doi: 10.1016/j.tripleo.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 5.NIH Consensus development conference for removal of third molars. J Oral Surg. 1980;38:235–236. [PubMed] [Google Scholar]
- 6.Steed MB. The indications for third-molar extractions. J Am Dent Assoc. 2014;145(6):570–573. doi: 10.14219/jada.2014.18. [DOI] [PubMed] [Google Scholar]
- 7.Adeyemo WL. Do pathologies associated with impacted lower third molars justify prophylactic removal? A critical review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:448–452. doi: 10.1016/j.tripleo.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Fuster Torres MA, Gargallo AJ, Berini AL, Gay EC. Evaluation of the indication for surgical extraction of third molars according to the oral surgeon and the primary care dentist. Med Oral Patol Oral Cir Bucal. 2008;13:499–504. [PubMed] [Google Scholar]
- 9.Ghaeminia H, Perry J, Nienhuijs ME, Toedtling V, Tummers M, Hoppenreijs TJ, Van der Sanden WJ, Mettes TG. Surgical removal versus retention for the management of asymptomatic disease-free impacted wisdom teeth. Cochrane Database Syst Rev. 2016;31(8):CD003879. doi: 10.1002/14651858.CD003879.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Tambuwala AA, Oswal RG, Desale RS, Oswal NP, Mall PE, Sayed AR, Pujari AT. An evaluation of pathologic changes in the follicle of impacted mandibular third molars. J Int Oral Health. 2015;7(4):58–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Akadiri OA, Okoje VN, Fasola AO, Olusanya AA, Aladelusi TO. Indications for the removal of impacted mandible third molars at Ibadan—any compliance with established guidelines? Afr J Med Med Sci. 2007;36:359–363. [PubMed] [Google Scholar]
- 12.Saravana GHL, Subhashraj K. Cystic changes in dental follicle associated with radiographically normal impacted mandibular third molar. Br J Oral Maxillofac Surg. 2008;46:552–553. doi: 10.1016/j.bjoms.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Glosser JW, Campbell JH. Pathologic change in soft tissues associated with radiographically “normal” third molar impactions. Br J Oral Maxillofac Surg. 1999;37:259–260. doi: 10.1054/bjom.1999.0061. [DOI] [PubMed] [Google Scholar]
- 14.Costa MG, Pazzini CA, Pantuzo MC, Jorge ML, Marques LS. Is there justification for prophylactic extraction of third molars? A systematic review. Braz Oral Res. 2013;27(2):183–188. doi: 10.1590/S1806-83242013000100024. [DOI] [PubMed] [Google Scholar]
- 15.Damante JH, Fleury RN. A contribution to the diagnosis of the small dentigerous cyst or the paradental cyst. Pesqui Odontol Bras. 2001;15:238–246. doi: 10.1590/S1517-74912001000300010. [DOI] [PubMed] [Google Scholar]
- 16.Shin SM, Choi EJ, Moon SY. Prevalence of pathologies related to impacted mandibular third molars. Springerplus. 2016;5(1):915. doi: 10.1186/s40064-016-2640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Katheeb TH, Bataineh AB. Pathology associated with impacted mandibular third molars in a group of jordanians. J Oral Maxillofac Surg. 2006;64:1598–1602. doi: 10.1016/j.joms.2005.11.102. [DOI] [PubMed] [Google Scholar]
- 18.Güven O, Keskin A, Akal UK. The incidence of cysts and tumors around impacted third molars. Int J Oral Maxillofac Surg. 2000;29:131–135. doi: 10.1016/S0901-5027(00)80011-9. [DOI] [PubMed] [Google Scholar]
- 19.Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med. 2006;35:500–507. doi: 10.1111/j.1600-0714.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Ellis GL. Dental follicular tissue: misinterpretation as odontogenic tumors. J Oral Maxillofac Surg. 1993;51:762–767. doi: 10.1016/S0278-2391(10)80417-3. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Abbas AK, Fausto N. Bases Patológicas das Doenças - Robbins & Cotran Patologia. São Paulo: Elsevier; 2004. p. 10. [Google Scholar]
- 22.Nolla CM. The development of the permanent teeth. J Dent Child. 1960;27:254–266. [Google Scholar]
- 23.Knutsson K, Brehmer B, Lysell L, Rohlin M. Pathoses associated with mandibular third molars subjected to removal. Oral Surg. 1996;82:10–17. doi: 10.1016/s1079-2104(96)80371-4. [DOI] [PubMed] [Google Scholar]
- 24.Park KL. Which factors are associated with difficult surgical extraction of impacted lower third molars? J Korean Assoc Oral Maxillofac Surg. 2016;42(5):251–258. doi: 10.5125/jkaoms.2016.42.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obimakinde O, Okoje V, Ijarogbe OA, Obimakinde A. Role of patients’ demographic characteristics and spatial orientation in predicting operative difficulty of impacted mandibular third molar. Ann Med Health Sci Res. 2013;3:81–84. doi: 10.4103/2141-9248.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath C, Comfort MB, Lo EC, Luo Y. Can third surgery improve quality of life? A 6-month cohort study. J Oral Maxillofac Surg. 2003;61:759–763. doi: 10.1016/S0278-2391(03)00150-2. [DOI] [PubMed] [Google Scholar]