Abstract
Purpose
The chemotherapeutics agent, 5-fluorouracil (5-FU), and curcumin (Cur), a natural antioxidant, has a wide pharmacological window to treat oral carcinoma; however, both drugs have limited bioavailability. This research study designs to develop a nanoemulsions (NEs) formulation by combining 5-FU and Cur to improve anticancer activity against oral cavity squamous cell carcinoma (OSCC) cells from the diversified origin for in vitro analysis, SCC090 (human tongue) and SCC152 (human hypo-pharynx).
Methodology
NEs formulated through homogenization, applying high-energy ultrasonication technique. The prepared 5-FUNE/Cur-NE/5-FU-Cur-NE were characterized and optimized by different in vitro assays to evaluate release system and treatment of OSCC cells to monitor cellular acceptability, such as in vitro anticancer activity by MTT assay, cell uptake studies and protein expression associated apoptotic study.
Results
5-FUNE/Cur-NE/5-FU-Cur-NE successfully formulated and show mean-value of the particle size (150–200 nm), surface charge (− 25.70 to − 37.91 mV), and PDI (0.194). In vitro release of 5-FUNE/Cur-NE/5-FU-Cur-NEs was monitored over a course of 04 days, where acidic pH shows higher release as compared to alkaline pH, along with acceptable stability data. Cytotoxicity study has shown higher-dose-dependent anticancer effect with a reduced IC50 value of NEs as compared to BLNE. Cellular uptake study of 5-FUNE/Cur-NE/5-FU-Cur-NEs upgraded many folds, comparatively BLNE and show potential cell arrest. Additionally, the cell protein (Blc2, Bax, P53, and P21) expression was revised and raised cell apoptosis.
Conclusion
The combinational loaded, 5-FU and Cur in nanoformulation system have proven their potency to deliver improved anticancer activity, against oral cancer.
Keywords: 5-Fluorouracil, Curcumin, Oral cancer, Nanoparticles, Drug Delivery
Introduction
The damaged cells build a mass of tissues may be converted into tumor or cancer [1]. In the year 2015–2016, there were 14.5 million cancer cases diagnosed, and the load of cancer cases was expected to increase up to 25 million by the year 2035, globally [2]. Oral cancer is highly rated solid tumor upon oral site, which affects approx 4.5 million populations and 2.15 million deaths every year, all around the world [3]. The report of tobacco survey of India in the year of 2010 that it would be projected that more than half population of the adults in India may be consumed any form of tobacco and by the year of 2035, it would be over 35% share in total cancer cases reported in India [4].
The incident of cancer within mouth and oropharynx mostly occurs due to the use of tobacco, alcohol and many chronic irritations, HPV and diet. Oral cancer mostly begins at squamous cells at the oral site and mask floor of mouth, lips, on the lateral and ventral surfaces of the tongue and hypo-pharynx, termed oral squamous cell carcinomas (OSCC) [5]. Most importantly, almost 38% of OSCC occur on the lower side of the lip, these are generally solar-related cancer on the external surface. The overexpression of OSCC may also be developed in the tissue of hypo-pharynx called hypopharyngeal cancer, which generated from the plane of the lining within the throat. It was reported that OSCC has 5-year survival rate and this was not improved significantly from many years [6]. Therefore, it is very necessary to identify the advanced treatment technology and drug delivery system with effective therapeutic efficacy against OSCC.
The most acceptable treatment against OSCC is surgery along with radiation and/or chemotherapy. Chemotherapy includes the use of anticancer drugs to counteract the growth of continuous isolation of cancer cells. It is the primitive treatment to eradicate the cancer cells and narrowing the tumor within the tumor site and recurrence of tumor growth. Anticancer drugs may damage cancer cell growth, involving through numerous cellular pathways within the cytosol, and formed apoptosis, called chemotherapy [7].
The chemotherapeutics drugs, which are using frequently for oral cancer treatment, are cisplatin, docetaxel, paclitaxel, carboplatin, and 5-fluorouracil (5-FU). Chemotherapy may also destruct healthy cell and produce obnoxious side effects, mostly fatigue, bleeding, shallow immunity, low blood count, diarrhea, mouth sores, hair loss, nausea, and vomiting [8, 9].
5-FU is pyrimidine analog, that irreversible inhibit of thymidylate synthase within the cell as suicidal inhibition, which mainly suppresses the DNA replication and forms thymineless cell death [10]. It was also noticed that the usages of the anticancer drug in combination with herbal drugs may strengthen up the therapeutic index and accomplish of sensitizing chemopreventive effects and chemotherapeutic effects [11].
Curcumin (Cur) is an ancient Indian aroma, with huge medicinal properties, typically anti-inflammatory, antioxidant, and anticancer effects. Cur is polyphenol isolated from the Curcuma longa herb, turmeric. Cur involved the diverse cellular pathways regulate oncogene expression, protein expression, tumorigenesis and coordinate the apoptosis within cancer cells [12].
Combinational delivery of 5-FU with Cur may improve the effectiveness because both drugs have proven their anticancer potency against cancer including OSCC. Besides of that chemo-activity, Cur has the low therapeutic effect because of poor solubility in aqueous media, extent hydrolysis and resultant low bioavailability. In addition, the effectiveness of 5-FU within nanoformulation was capable of minimizing, the 5-FU biological limitation such as short half-life, toxicity, and nonselective action against the cancer cell [13, 14]. The chemotherapy of 5-FU may be improved through nanoformulation-loaded drug delivery against OSCC as it combined with Cur.
Nanoparticles (NPs) are the drug delivery, which is being used to bypass numerous activity drawbacks through small particle size. They have the capability to engulf the drug in alone and/or combinational nanohybrid, chambered like structure. It has the efficiency in drug delivery system with increased half-life, bioavailability, less toxicity, improved therapeutic activity, and specific activity at tumor site. The nanotechnology drug deliveries may include polymer-based nanoparticles, liposomes, dendrimers, quantum dots, micelle, nanogel, and nanoemulsion (NE). These nanoparticulates system have the affinity to deliver combination of drugs in a single platform and control the release of the drug molecules, on the cancer site [15]. NE was liquid-phase system, where drug particle was distributed in liquid-phase system. It may be isotropic transparent or translucent system of oil, water, and mixer of surfactant and co-surfactant ranging the droplet size mostly from 50 to 350 nm. It is single-phase system, which has the capability to payload the hydrophilic and lipophilic drugs, because of the existence of several polarity domains within the single-phase drug delivery system for low soluble molecules to upgrade their bioavailability [16].
This study includes to develop a NEs formulations by combining a widely used anticancerous drug 5-FU and Cur, a natural antioxidant having wide pharmacological window and evaluation of its enhanced anticancer activity over the oral squamous cell carcinoma cell lines from diversified origin associated in vitro cell culture system, SCC090 (human tongue) and SCC152 (human hypo-pharynx). The prepared single and combination drugs, loaded within the nanoformulations formulation were evaluated, characterized, and examined through different in vitro assays, included anticancer activity, cell uptake study, protein expression associated apoptotic study in OSCC cells (SCC090 and SCC152). So the resultant formulation could be utilized as a better drug of choice for chemotherapeutic interventions with minimum or no side effects in oral cancer chemotherapy.
Material and Methods
Materials
5-FU, (99% pure) purchased from Himedia Chemical Ltd, Bangalore, India (Batch no. SV2905-5G); Cur (95% purity), purchased from Sigma Life Sciences, India (Lot No: BTUV745); tween 80 purchased from Merck Specialties Private Limited, India; MTT, from Sigma Aldrich Ltd, Bangalore, India; SCC090 (human tongue squamous cell carcinoma); SCC152 (human hypo-pharynx squamous cell carcinoma) cells were procured from NCCS, Pune, India. Remaining chemicals (analytical grade) obtained from Sigma–Aldrich, India.
Preparation of 5-FU and Cur Loaded NEs
5-FU and Cur were prepared by employing homogenization with high-energy ultrasonication technique. Firstly, 5-FU and Cur were weighed and formerly dissolved in ethanol to make drug solutions and afterword the drug solutions were dissolved in tween 80 (5%v/v) and soya oil (2%v/v). It was vigorously vortexed for 15 min and soya phosphatidylcholine (0.5% w/v) was poured in above solution and vortexed it again and further sonicated for 10 min at 20% amplitude to facilitate solubility and obtain a uniform mixture. This oil phase was then added gradually into 10 ml of aqueous-phase (water with 5%v/v glycerol) with continuous homogenization for 2 min at 5000 rpm followed by ultrasonication in pulse mode (6 s on and 3 off) at 20% amplitude for 18 min. The single-loaded, 5-FU nanoemulsion (5-FUNE) and Cur nanoemulsion (Cur-NE), and combined-loaded, 5-FU-Cur nanoemulsion (5-FU-Cur-NE), were collected and stored for further analysis.
Nanoparticles Size, Zeta Potential, and Polydispersity Index (PDI)
The average particle size of the globules, polydispersity index (PDI), and labeled charges of the NPs with payload of single and combination drugs, 5-FU and Cur, within single liquid phase of NEs were monitored through Malvern Zetasizer ver.7.12, (3000 HS, Malvern Instrument, UK) and optimized in triplet at 27 ± 03 °C.
Transmission Electron Microscopy (TEM)
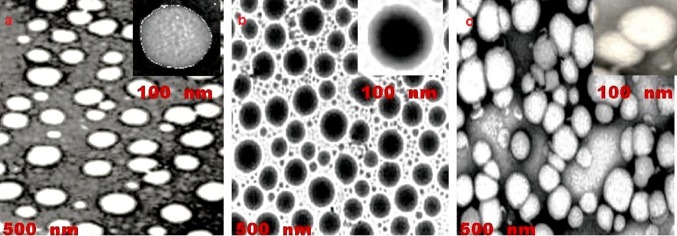
The morphological characteristic, specifical shape of the nanoformulation were monitored through TEM image (Tecnai TM G2 Sprit (FEI, Netherlands), the NEs samples were diluted and positioned on 400 mesh grid, coated with carbon (80 kV voltage) using negative stain with 2% phosphor-tungstic acid for 4 min and left it to dry for 12 h, the TEM image was taken at discrete magnifications.
Measurement of Drug Entrapment Efficiency (EE) and Drug Loading (DL)
The study data of EE and DL within single and combination drugs NEs, 5-FU and Cur were quantified through a plotted standard curve between the concentration (µg/ml) and absorbance value (nm) through the ultraviolet (UV) spectrophotometer, PerkinElmer, Lambda 25, USA, at absorption maxima, 267 and 430 nm for 5-FU and Cur, respectively. 5-FUNE/Cur-NE/5-FU-Cur-NE formulations were monitored the percentage of 5-FU and Cur entrapped, in respect to the absolute amount of drug (mg) loaded within the nanoformulations (mg/ml). The sample of NPs was centrifuged at 12,000 rpm for 35 min, and supernatant liquid was drawn from each samples and lyophilized, washed and weighed. The preserved pellets were scanned and recorded the absorbance value and standardized with the plotted standard curve for both the drugs. Each sample of NPs was quantified for three times. Following formulae were used for the quantification of the percentage of EE and DL value of the 5-FU and Cur in 5-FUNE/Cur-NE/5-FU-Cur-NE formulations. The weight of the lyophilized NPs, termed as the yield of the sample was calculated as total quantity of NPs retrieved to the total quantity of excipients and drugs used for the preparation of nanoformulation.
EE (%): entrapped drug concentration in NPs/Intake drug concentration × 100.
DL (%): entrapped drug concentration in NPs/weight of the lyophilized NPs (yield) × 100.
In Vitro Cumulative Drug Release Study of NEs
The release study of drugs, 5-FU and Cur from 5-FUNE/Cur-NE/5-FU-Cur-NE formulations was evaluated through the dialysis bag (Sigma Aldrich, India) method. The NPs sample, 2 ml (1 mg/ml) was dropped into treated dialysis bag, tied at ends and placed into a beaker, 25 ml of phosphate buffer solution (PBS) in two divergent pH ranges (7.4 and 4.5), incubated into a water bath shaker at room temperature. The release samples (1 ml) were drawn at a specific time duration (0, 6, 12, 24, 36, 48, 60, 72, 84, 96, 100 h) and fresh PBS was added into the medium to protect sink condition. The released samples were examined using UV spectrophotometer at the absorbance maxima 430 and 267 nm for the Cur and 5-FU, respectively, and quantify in respect to the plotted standard curve for both the drugs.
Drug Release (%): Release drug concentration at a respective time/entrapped drug concentration in NPs × 100.
Stability Study
The comparative stability study of nanoformulations, 5-FUNE/Cur-NE/5-FU-Cur-NE was optimized through measuring the physical appearance, phase separation, drug precipitation, entrapment efficiency, and a particle size within regular time interval up to 6 months. The stability study assessed the quality of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations under the effect of physical aspects like, light, temperature, aggregation, and humidity as per the stability guideline (ICH) [17]. The formulations were stored at 4 °C into glass vials (5 ml).
Cells and Culture Medium
OSCC cell lines, SCC090 and SCC152, from the different origin of the oral section were drawn for in vitro cell treatment. The cell lines cultured in DMEM/F12 medium and enriched with 10–12% concentration fetal bovine serum (inactivated) included 1% antibiotic (streptomycin and penicillin cocktail) (5% CO2, 95% air at 37 ± 0.5 °C, incubation condition).
MTT Cytotoxicity Assay
The in vitro anticancer activity of prepared 5-FUNE/Cur-NE/5-FU-Cur-NE formulations was evaluated through MTT cytotoxicity assay in SCC090 and SCC152 cells by in vitro cell treatment and optimized the percentage of cell viability. The cells suspension seeded (1 × 104 cells/well in 96 well plates) and leave to adhere for 1 day, and after that cells have incubated with the different concentrations of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations in regular time interval 24, 48, 72, 96 h.
Uptake Studies of OSCC Cells
The cellular uptake of the 5-FUNE/Cur-NE/5-FU-Cur-NEs formulation was estimated in OSCC cells by flow cytometry. Briefly, the cells were seeded (2 × 104 cells/well in 24 well plates) and left for 12 h. Subsequently, fluorescein isothiocyanate (FITC), labeled 5-FUNE/Cur-NE/5-FU-Cur-NEs formulation incubated with SCC090 and SCC152 cells for 4 h at 37 ± 0.5 °C at various concentrations. After incubation, the cells were gathered by trypsinization, washed and again poured in PBS (200 μl) washed with cold PBS, re-suspended in 200 μl PBS, flow cytometer was used to record the fluorescence intensity. About, 104 events per sample acquired at the excitation and emission wavelength of 495 and 521 nm, corresponding to FITC. Each analysis was performed in triplicate. Fluorescence intensity represented the amount of 5-FU and Cur, internalized within the cell, therefore the amount of 5-FU and Cur uptake by cells was estimated directly. FITC, fluorescence signal in cells was recorded and compared with BLNE treated cells as a control for autofluorescence.
Western Blotting
The altered expression of proteins marker of apoptosis (P53, P21, Bax, and Bcl2) was estimated. The NEs treated cells were blotted to quantify the changes in the expression of protein, briefly, the extraction, separation and probing. The protein mix within the cells was blotted through SDS-PAGE technique. The protein estimation was carried through Bradford’s reagent, and denatured protein was allowed to run on 10 SDS gel and blotted over polyvinlidene fluoride membrane under 250 mA current for 150 min by wet transfer technique. After that primary antibody was used to block specific protein (P53, P21, Bax, and Bcl2) and bind with that specific protein, it a detector of protein, after that secondary antibody was incubated for 120 min with secondary anti-primary immunoglobulin G (IgG)-conjugated with horseradish peroxidise, enzyme to produce product as color blots on membrane and target protein bands were indentified through Gel Docs (Alpha Inn, US).
Data Analysis
All experiments were redone thrice to validate the scientific data and asserted in the standard deviation and average mean and ANOVA analysis (one way) and least significance difference test tested for comparative analysis. GraphPad Prism 6.0, the software was used for the statistical analysis, P value, (p > 0.05).
Result
Formulation of 5-FUNE/Cur-NE/5-FU-Cur-NE
The ultrasonication method has been employed for the preparation of 5-FUNE/Cur-NE/5-FU-Cur-NE against SCC090 and SCC152 cells treatment and evaluation of in vitro analysis. The effect of ultrasonication time duration on NEs plays a major role in the contraction of nanoparticles size. The optimum sonication time was about 18 min at 20% amplitude. The oil phase was standardized and chambered within oil globules prepared through soya oil and tween 80 in different ratio with co-surfactant, soya phosphatidylcholine, along with series of the experiment were performed on formulation batches in different concentrations of oil and surfactant–co-surfactant to obtain stable nanosized oil droplets within o/w NEs.
Characterizations of 5-FUNE/Cur-NE/5-FU-Cur-NE
The particle size, zeta potential, and PDI values of prepared 5-FUNE/Cur-NE/5-FU-Cur-NE formulations were quantified through zetasizer. The mean sizes of the NPs in 5-FUNE/Cur-NE/5-FU-Cur-NE formulation were ranges from 150 to 200 nm. The average zeta potential of NEs was, − 25.70 to − 37.91 mV. The average PDI of the NEs was 0.194 and the pH of NEs was in the range of 5.41–6.72 (Table 1).
Table 1.
Particle size, zeta potential, and PDI values of NEs
| Formulation code | Nanoemulsion formulations | Particle size (nm) | Zeta potential (mV) | Polydispersity index (PDI) |
|---|---|---|---|---|
| BLNE | Blank nanoemulsion | 179 ± 6.85 | − 27.90 ± 4.12 | 0.35 |
| 5-FUNE | 5-Fluorouracil nanoemulsion | 183 ± 7.25 | − 18.85 ± 3.50 | 0.23 |
| Cur-NE | Curcumin nanoemulsion | 193 ± 5.85 | − 21.90 ± 4.93 | 0.31 |
| 5-FU-Cur-NE | 5-Fluorouracil curcumin nanoemulsion | 196 ± 6.35 | − 22.50 ± 4.87 | 0.29 |
The less difference between zeta potential value in single-loaded, 5-FUNE/Cur-NE and combined-loaded, 5-FU-Cur-NE in comparison with blank nanoemulsion (BLNE) optimized that the drugs payload in NEs may not adsorb at the interfacial layer to affect the charges, the negative charge of zeta potential may be existence of anionic type surfactant tween 80 and co-surfactant phosphatidylcholine in all NEs. The zetasizer image for 5-FUNE/Cur-NE/5-FU-Cur-NE formulations confirmed the size, surface charge, and PDI (Fig. 1a, b). The electron microscopy analysis confirmed that oil droplets were spherical in shape and uniformly distributed within the NEs (Fig. 2).
Fig. 1.
Zetasizer Image a peak for particle size and PDI, b peak for surface charge
Fig. 2.
TEM image, a 5-FUNE, b Cur-NE, c 5-FU-Cur-NE
Entrapment Efficiency and Drug Loading
The percentage of drug entrapment in 5-FUNE/Cur-NE/5-FU-Cur-NE formulations ranges from 71 to 73% for 5-FU and 76–83% for Cur. Drugs in combination (5-FU-Cur-NE) formulations expressed excellent entrapment efficiencies up to 73 and 83%, respectively, and Cur was quantified higher EE as related to 5-FU in 5-FU-Cur-NE. The immense EE confirmed that 5-FU highly distributed into the oil phase and excellent encapsulation with Cur. The DL of 5-FU was 21.58 and 13.25%, respectively, in 5-FUNE/5-FU-Cur-NE so as DL for Cur was 16.56 and 17.59%, respectively, in Cur-NE/5-FU-Cur-NE formulations (Table 2). The particle size of the drug is directly propositional to EE of the drug as the increased particle size may decrease the EE of the drug.
Table 2.
Entrapment efficiency and drug loading of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations
| Formulation code | EE (%) | DL (%) | ||
|---|---|---|---|---|
| 5-FU | Cur | 5-FU | Cur | |
| 5-FUNE | 73.29 ± 1.93 | – | 21.58 ± 2.13 | – |
| Cur-NE | – | 83.41 ± 2.78 | – | 16.56 ± 2.15 |
| 5-FU-Cur-NE | 71.59 ± 2.16 | 76.57 ± 2.63 | 13.25 ± 3.12 | 17.59 ± 3.46 |
In Vitro Release at Divergent pH Ranges
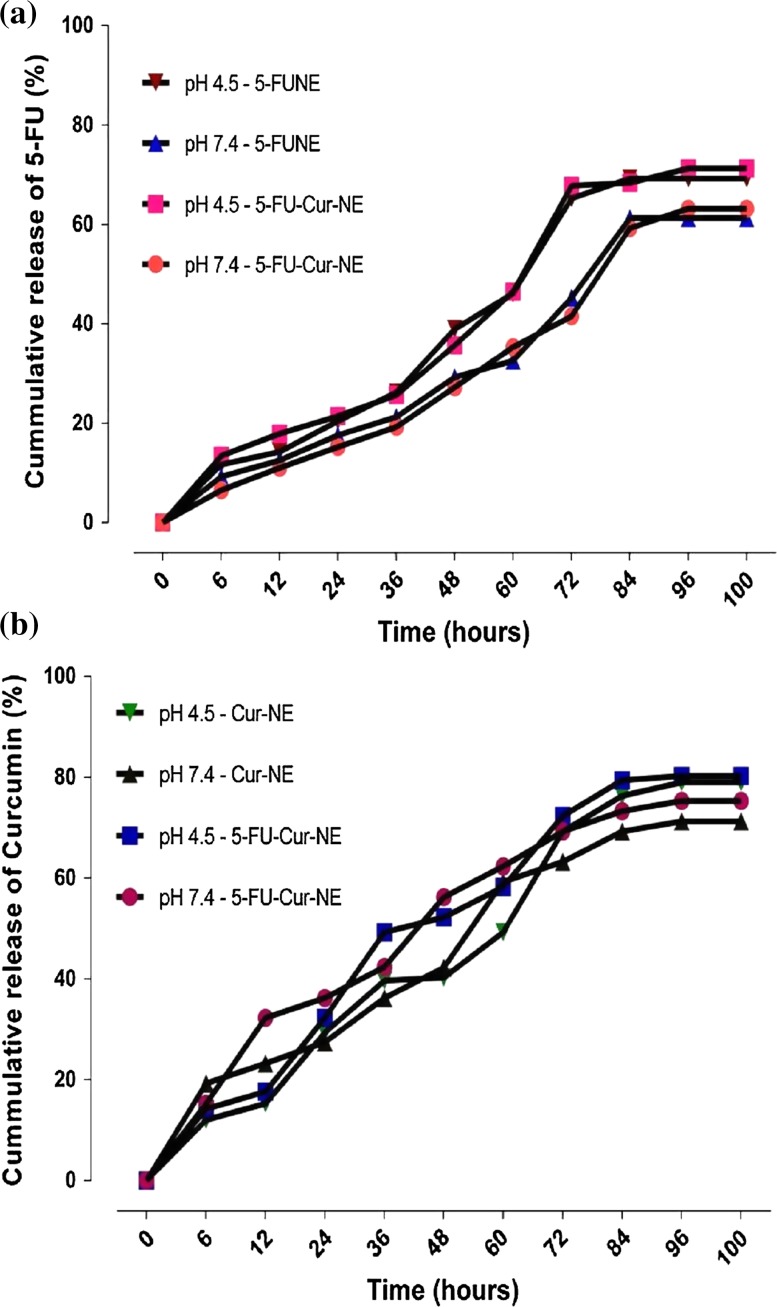
The in vitro drug release of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations was quantify in the diversified in vitro physiological condition such as temperature, pH and interfacial condition which show the impact on the release of drug in pH-restricted dissolution medium (pH 7.4 and 4.5). These diversified alkaline and acidic pH condition of the in vitro medium may be helpful to predict the in vivo condition during drug absorption. The release of drugs, 5-FU and Cur in different time (hours) interval from 6 to 100 h, (up to forth to fifth days). The results identified that acidic pH was much supportive to the release of 5-FU, as compared to alkaline pH, approximately 71 and 63%, respectively, in 5-FUNE/5-FU-Cur-NE formulations. The cumulative release of Cur confirmed high release more than 80% in Cur-NE/5-FU-Cur-NE formulations in acidic pH as compared to alkaline pH, approximately 71%. It was also noticed that bust release of Cur was found at 12–24 h and after that, it gradually releases up to 72 h. The combination releases of drugs were encapsulated in 5-FU-Cur-NE formulations were approximately 71 and 80%, respectively, up to the fourth day. The release of both the drugs, in respect to time, were gradually expanded, up to 72 h and at the time 84 h, it was found maximum release almost all the reported formulations and after 84 h and up to 100 h, steady-state release was reached. The different pH 7.4 and 4.5 ranges were confirmed the excellent release of drugs, 5-FU, and Cur from 5-FUNE/Cur-NE/5-FU-Cur-NE formulations and described the slop of release percentage (Fig. 3, Table 3).
Fig. 3.
In vitro drug release pattern a 5-FU loaded in 5-FUNE and 5-FU-Cur-NE, b Cur loaded in Cur-NE and 5-FU-Cur-NE
Table 3.
In vitro drug release of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations in divergent pH ranges (7.4 and 4.5)
| Time (h) | In vitro drug release at alkaline and acidic pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 5-FU (%) | Curcumin (%) | |||||||
| 5-FUNE | 5-FU-Cur-NE | Cur-NE | 5-FU-Cur-NE | |||||
| pH 4.5 | pH 7.4 | pH 4.5 | pH 7.4 | pH 4.5 | pH 7.4 | pH 4.5 | pH 7.4 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 11.69 ± 1.17 | 9.25 ± 0.51 | 13.45 ± 1.49 | 6.52 ± 1.37 | 14.23 ± 1.13 | 19.25 ± 2.87 | 14.23 ± 1.82 | 15.23 ± 4.15 |
| 12 | 14.27 ± 0.97 | 12.52 ± 1.98 | 17.89 ± 1.73 | 11.02 ± 2.98 | 17.65 ± 1.85 | 23.26 ± 1.79 | 17.65 ± 2.93 | 32.29 ± 1.43 |
| 24 | 20.41 ± 2.09 | 17.59 ± 2.02 | 21.41 ± 1.59 | 15.23 ± 1.53 | 32.26 ± 3.69 | 27.45 ± 2.59 | 32.26 ± 3.65 | 36.23 ± 2.75 |
| 36 | 26.23 ± 1.74 | 21.26 ± 2.94 | 25.68 ± 2.39 | 19.25 ± 1.55 | 49.23 ± 1.02 | 36.23 ± 1.89 | 49.23 ± 2.15 | 42.36 ± 2.29 |
| 48 | 38.96 ± 3.98 | 29.23 ± 1.58 | 35.65 ± 1.91 | 27.23 ± 2.93 | 52.23 ± 2.66 | 42.23 ± 1.91 | 52.23 ± 1.61 | 56.23 ± 2.15 |
| 60 | 46.17 ± 2.41 | 32.58 ± 3.17 | 46.46 ± 3.65 | 35.29 ± 3.55 | 58.23 ± 2.83 | 59.23 ± 2.59 | 58.23 ± 2.05 | 62.32 ± 1.59 |
| 72 | 65.23 ± 0.79 | 45.23 ± 3.74 | 67.76 ± 1.23 | 41.51 ± 3.59 | 72.32 ± 1.79 | 63.23 ± 1.85 | 72.32 ± 2.09 | 69.23 ± 1.13 |
| 84 | 69.23 ± 0.19 | 61.25 ± 0.12 | 68.36 ± 1.16 | 59.23 ± 1.38 | 79.36 ± 0.85 | 69.23 ± 0.98 | 79.36 ± 0.52 | 73.29 ± 0.55 |
| 96 | 69.24 ± 0.13 | 61.26 ± 0.09 | 71.25 ± 0.19 | 63.19 ± 0.35 | 80.23 ± 0.49 | 71.23 ± 0.57 | 80.23 ± 0.36 | 75.29 ± 0.21 |
| 100 | 69.24 ± 0.07 | 61.26 ± 0.07 | 71.25 ± 0.12 | 63.19 ± 0.16 | 80.23 ± 0.29 | 71.23 ± 0.41 | 80.23 ± 0.21 | 75.29 ± 0.21 |
Stability of 5-FUNE/Cur-NE/5-FU-Cur-NE
The follow-up accelerated stability study of 5-FUNE/Cur-NE/5-FU-Cur-NE was monitored up to 6 months at a regular time interval in respect to physical appearance, phase separation, drug precipitation, entrapment efficiency and particle size. There were nil physical changes, no phase separation, and drug precipitation seen in 5-FUNE/Cur-NE/5-FU-Cur-NE formulation. The nanoparticles size and EE of the 5-FUNE/Cur-NE/5-FU-Cur-NE formulations were also monitored on the regular time interval from the initial to 6 months (Table 4). First, the result data of storage stability for 5-FUNE/Cur-NE/5-FU-Cur-NE formulations shown that particle size of Cur in Cur-NE and 5-FU-Cur-NE were increased 47.66 and 42.42%, respectively; it was recorded that maximum 16.58% increased from first month storage to the second month and afterward approximately 8% increased in particle size was optimized on per month basis for Cur in Cur-NE and 5-FU-Cur-NE, this particle size increased was directly effects on the EE of the Cur, which reduced about 23%, from 83.41 to 64.19% and 76.57 to 59.55%, respectively. Second, 5-FU in 5-FUNE and 5-FU-Cur-NE shown 25.76% increased in particle size and third month to sixth month storage shown maximum increase in size of drug particles, briefly, 12.35% and an average of 3.5% increase per month with a reduction in entrapment efficiency about 16.84% for both 5-FUNE and 5-FU-Cur-NE formulations. The optimization of storage stability confirmed that 4 °C was found to be the excellent storage condition for 5-FUNE/Cur-NE/5-FU-Cur-NE.
Table 4.
Accelerated stability study of nanoformulations 5-FUNE/Cur-NE/5-FU-Cur-NE up to 6 months
| Nanoformulation | Days (s) | Physical description | Phase separation | Drug precipitation | EE (%) | Particle size (nm) | |
|---|---|---|---|---|---|---|---|
| 5-FU | Cur | ||||||
| 5-FUNE | Initial | White translucent solution | No | No | 73.29 | – | 183.98 |
| 30 | No change | Not seen | Not seen | 71.03 | – | 192.80 | |
| 60 | No change | Not seen | Not seen | 69.51 | – | 194.89 | |
| 90 | No change | Not seen | Not seen | 65.45 | – | 205.19 | |
| 180 | No change | Not seen | Not seen | 60.87 | – | 231.39 | |
| Cur-NE | Initial | Dark yellow translucent solution | No | No | – | 83.41 | 193.75 |
| 30 | No change | Not seen | Not seen | – | 76.23 | 225.61 | |
| 60 | No change | Not seen | Not seen | – | 68.25 | 239.37 | |
| 90 | No change | Not seen | Not seen | – | 65.47 | 259.34 | |
| 180 | No change | Not seen | Not seen | – | 64.19 | 285.73 | |
| 5-FU-Cur-NE | Initial | Light yellowish translucent solution | No | No | 71.59 | 76.57 | 196.27 |
| 30 | No change | Not seen | Not seen | 67.26 | 71.34 | 229.74 | |
| 60 | No change | Not seen | Not seen | 63.74 | 69.79 | 245.56 | |
| 90 | No change | Not seen | Not seen | 63.49 | 60.19 | 274.78 | |
| 180 | No change | Not seen | Not seen | 60.19 | 59.55 | 279.59 | |
In Vitro Anticancer Activity of 5-FUNE/Cur-NE/5-FU-Cur-NE
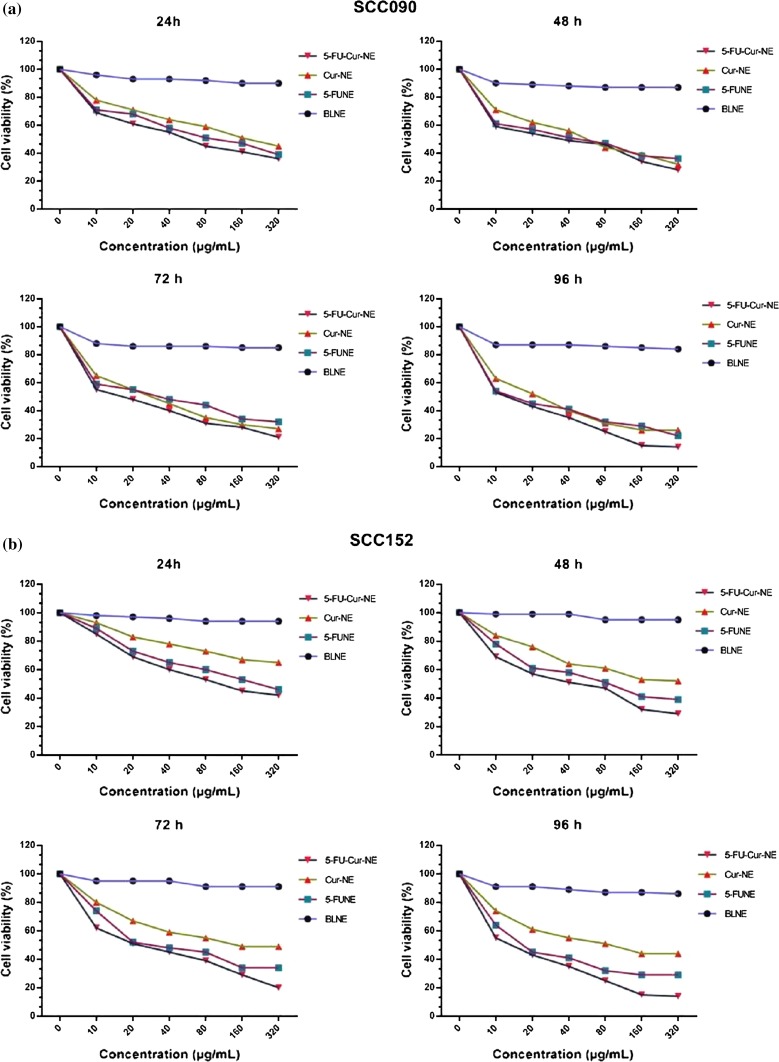
Cytotoxicity study of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations was evaluated through MTT assay, in respect to dose–response cell survival, on cell lines of OSCC from different origin, SCC090 and SCC152 at 24- to 96-h time interval. The percentage of cell viability versus concentration was ranging from lower to higher (10, 20, 40, 80, 160, 320 µg/ml), for the optimization growth inhibition within SCC090 and SCC152 cells at a different time interval. The cell viability of BLNE was ranging from 99 to 85% at given concentration range at 24–96 h, these data confirmed that BLNE was in-noxious in nature. 5-FUNE/Cur-NE/5-FU-Cur-NE formulations shown higher cell cytotoxicity, decreased the level of viability of cells, in respect to time–concentration-dependent (Fig. 4a, b). The SCC090, cells showing the lowest viability cell, 39, 45, and 36% at 24 h, 36, 32, and 28% at 48 h, 32, 27, and 21% at 72 h, and 22, 26, and 14% after 96 h of incubation for 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, respectively, the percentage of lowering in cell viability suggested that the 5-FUNE having more anticancer activity than Cur-NE, but when Cur treated with 5-FU, (5-FU-Cur-NE) shown maximum cytotoxicity, only 14% of cell viability. In SCC152, the result data suggested that BLNE was in-noxious and 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, shown less percentage of cell viability in dose and time-dependent manner, 5-FU-Cur-NE showing maximum lowering of the cell viability, 42, 29, 20, and 18% at (24, 48, 72, and 96 h, respectively) incubation at 320 µg/ml concentration and 5-FUNE showed effective cytotoxicity regularly decreased 8% of cell viability in dose and time response manner, and Cur-NE has shown less cytotoxicity alone but when combined with 5-FU as 5-FU-Cur-NE, there was increased cytotoxicity seen as high level of decreased cell viability. All the cytotoxicity results indicate that incubation time from 24 to 72 h having optimum decreased in cell viability which shows the controlled release of the drugs loaded within NPs and lastly maximum lower at 96 h and suggest the complete release of drug within the loaded nanoparticulate system. The decrease in cell viability inversely propositional to growth inhibition of cell against concentration suggested that the percentage of cell inhibition gradually increased with higher concentration and it may be more helpful to understand the rate of cytotoxicity of the 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, which was increased with the rate of incubation up to 96 h. The data with respect to all observation concentration were suggested that the emphasis of the improved anticancer effect of co-delivery of 5-FU and Cur, loaded with NE system. The cytotoxicity results were optimized in triplicate. In SCC090 and SCC152 cells, IC50 of 5-FU in 5-FUNE was found higher, 51.79 and 62.04 µg/ml in SCC090 and SCC152, respectively, as compared to 5-FU-Cur-NE when combined with Cur, 31.05 and 37.46 µg/ml, further endorse the upgraded cell cytotoxicity. Cur shows higher IC50 about 79.16 and 84.18 µg/ml against SCC090 and SCC152 cells, respectively, in comparison with other NEs, suggested Cur in single-loaded drug system performed infirm inhibition, despite that cur proven to be effective chemo-sensitizer or chemopreventive that could overcome the synergistic effects along with another chemotherapeutic drug (Table 5). Hence, the uppermost decreased in cell viability coordinately elevated the inhibition and lowering the IC50 value in 5-FU-Cur-NE suggest the optimal ratio of the drug and effectively against SCC090 and SCC152.
Fig. 4.
Cells viability in concentration–time-dependent manner a SCC090, b SCC152
Table 5.
IC50 value of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations
| Nanoformulations | IC50 value (μg/ml) | |
|---|---|---|
| SCC090 | SCC152 | |
| 5-FUNE | 51.79 | 62.04 |
| Cur-NE | 79.16 | 84.18 |
| 5-FU-Cur-NE | 31.05 | 37.46 |
Intracellular Uptake Studies in OSCC
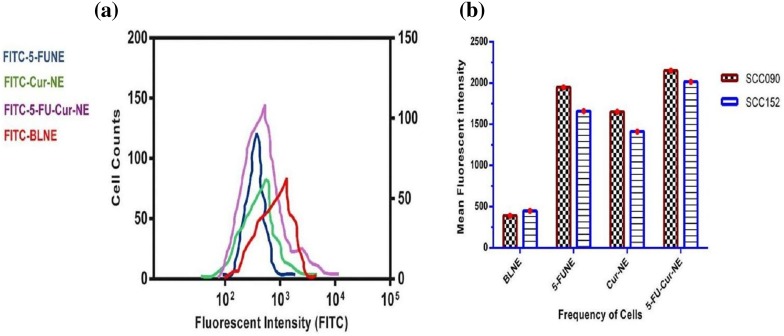
The cellular uptake of SCC090 and SCC152, treated with 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, the FITC was tagged with the NPs molecules and intercellular drug emanation was monitored through flow cytometry analysis, to record the quantification of fluorescent signals intensity. The SCC090 and SCC152 uptakes were found extremely upgraded abundantly treated with 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, in compression to free 5-FU and Cur. The uptake intensity was apparently depicted that 5-FUNE/Cur-NE/5-FU-Cur-NE formulations may be adequately accumulated in OSCC, and comparatively shown negligible accretion when treated with control cell lines up to 1 day. The fluorescent intensity was elevated sixfold and fourfold for in FITC-5-FU-Cur-NE and fourfold and threefold for FITC-5-FUNE/FITC-Cur-NE in SCC090 and SCC152, respectively, that of the free drug, manifesting the upgraded receptor-mediated-endocytosis cellular uptake of nanoformulations into SCC090 and SCC152. The FITC tagged to 5-FU-Cur-NE shown high intensity in compression to 5-FUNE and Cur-NE. The internalization competency of 5-FUNE/Cur-NE/5-FU-Cur-NE formulations was recorded in time–dose-dependent mannerism, the total incubated period was 4 h with different concentrations of nanoformulations and the recorded fluorescent intensity suggested that by 1.20 h there was vast number of NPs engrossed within extracellular membrane of the cells and by 4 h, ultimate sum of NPs accumulated within OSCC cells (Fig. 5). The initialization mechanism involved the fluorophore-tagged nanoformulation, FITC-5-FUNE/FITC-Cur-NE/FITC-5-FU-Cur-NE, receptor-mediated-endocytosis, pinocytosis, or phagocytosis, and the cell surface has a different domain of cation which attracted the negatively charged NPs [18]. The cellular uptake study evidently supported the nanoformulation concentration with increased incubation time, efficiently elevated the intracellular accumulation–concentration of the drug for the advancement of therapeutic effect to treat OSCC.
Fig. 5.
Cellular uptake a flow cytometry, b frequency of cells versus mean fluorescent intensity in 5-FUNE/Cur-NE/5-FU-Cur-NE
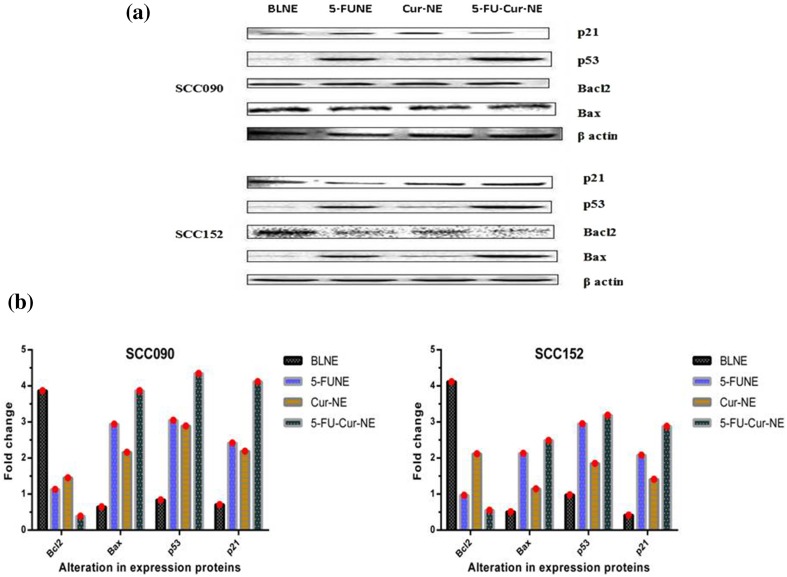
Expression of Proteins for Cell Apoptosis
The western blotting analysis was performed to quantify the alteration in expression proteins affiliated with apoptosis in SCC090 and SCC152 cells treated with 5-FUNE/Cur-NE/5-FU-Cur-NE formulations. 5-FUNE/Cur-NE/5-FU-Cur-NE formulations have effectively decreased the expression of the Bcl2 protein in SCC090 cells in compression with SCC152, the degree of expression of the protein was decreased with the increased concentration (20, 50, and 100 µg/ml) in a time-dependent manner. Additionally, both cancer cells have shown the much decreased level of Bcl2 protein expression, when exposed to 5-FUNE. The results, related Bcl2 protein, attached with mitochondria cell death pathways as anti-apoptosis protein marker, suggested the down-regulation for combinational exposure of drugs, 5-FU-Cur-NE formulations, were most significantly alerted the expression of the Bcl2 protein as compared to the single-loaded drug and confirmed the upgraded apoptosis in SCC090 and SCC152. The cancer cells show high expression of the Bcl2 protein and act to antagonize the Bax protein expression, ultimately obstructed the mitochondrial membrane devastation [19]. The results confirmed that expression of the Bcl2 protein was curtailed acute level in SCC090 and SCC152 cells when treated with combinational 5-FU-Cur-NE formulations and simultaneously the expression of Bax protein was increased in both OSCC cells with time–dose-dependent manner up to 48 h (Fig. 6a, b). The intensity of p53 and p21 band in protein expression to differentiate the apoptosis cell death was increased appreciably in dose-dependent aspect (20, 50 and 100 µg/ml), and confirmed the cell death caused through 5-FUNE/Cur-NE/5-FU-Cur-NE formulations. The p53 tumor suppressor gene acts as guardian in cellular apoptosis, p53 can invest cell death when DNA damages un-repairable and 5-FU molecule strives their anticancer action through RNA and DNA damage within the cancer cells [20]. The untreated SCC090 and SCC152 cells confirmed the down-regulation and further mutation in expression marker protein, p53, and p21, which was responsible for tumor suppression, the 5-FUNE/Cur-NE/5-FU-Cur-NE formulations treated OSCC cells have shown up-regulation in expression protein, p53, and p21, and activated the apoptosis in OSCC cells.
Fig. 6.
Alteration in expression of proteins, Blc2, Bax, P53, and P21 in SCC090 and SCC152, treated, 5-FUNE/Cur-NE/5-FU-Cur-NE and untreated, blank nanoemulsion (100 µg/ml concentration for 48 h), a Western blot protein expression, b fold change
Discussion
In India, including south Asian countries, OSCC is becoming very common type of cancer which covers 35% cases in all type of cancer [3]. Firstly, the prime treatment for OSCC is performed surgically by removing a tumor from oral site. Secondly, radiotherapy and chemotherapy may also be exhorted to rectify the remaining OSCC cells. The chemotherapy with the help of numerous anticancer drugs may be used to block the chance of recurrence of growth of malignant cells on tumor site [9].
In this study, firstly, the intention behind to prepare nanoformulation-loaded combinational drugs was, 5-FU with herbal agent Cur, to overcome the drawback of conventional formulations. Secondly, to identify the potency of combinational drugs over OSCC cells lines by comparing the in vitro activity of the single-loaded drug and combinational loaded drug in a single-phase NEs. The nano-system has been prepared by applying ultrasonication method and standardized through several aspects, such as excipients ratio and ultrasonication duration time to optimize stable formulations and many more. The ideal combination of NE system was shown in the particles size of NEs which ranges from 175 to 200 nm. The particle size of BLNE, marginally shrink, in comparison with 5-FUNE/Cur-NE/5-FU-Cur-NE formulations, may be due to the existence of 5-FU and Cur molecules on the surface of NPs. The spherical shape and uniform distribution of the NPs globules were formally denoted that the drugs particles have equally distributed throughout NE system. The NPs with the negative surface charge were reported more anticancer activity, due to high eligibility to navigate blood–brain barrier. The surfactant, tween 80, has 15.0 values in HLB scale (hydrophilic–lipophilic balance). Which may comparatively reduce high degree of interfacial tension within the NEs, and minimum distressed in GI environments such as pH and ionic bond changes [21]. The combinational drugs loaded in NE show an advanced degree of EE, 70 and 77% and DL, 10 and 20% for 5-FU and Cur, respectively, the encapsulation percentage shows that 5-FU and Cur optimally entrapped into NPs system.
The in vitro release pattern of 5-FU/Cur-NE/5-FU-Cur-NE formulation confirmed the controlled release of the drug molecules from NPs droplet. The release pattern was pH sensitive and complemented in acidic pH that ultimately enhanced the intracellular uptake within the cancer cell which lies from 6.5 to 7.6 pH. The earlier report on NEs was stated that NE formulations have shown aggregation within drug particle, and so it reflected over the release pattern, and ultimately down-regulation of antitumor activity was reported due to large particle size [22]. 5-FU/Cur-NE/5-FU-Cur-NE formulations stability data were also collected up to 6 months, to fallow the time-dependent alteration in physical appearance, phase separation, drug precipitation, entrapment efficiency and particle size, and collected data have shown satisfactory results.
The combinational drug system of 5-FU and Cur has potentially cropped the synergistic antagonist effects on both diversified origin, OSCC cells (SCC090 and SCC152). The BLNE formulation was comparatively used to record the survival rate of the OSCC. The 5-FU in combination with Cur reduced IC50 value approximately 28.05% and confirmed the advantage of co-delivery. The other important point was to be noticed that Cur reduced IC50 value almost 39.04% when it was combined with 5-FU, as compared to a single Cur delivery, confirmed Cur improved chemotherapy in combinational drug delivery. Cytotoxicity of 5-FU within the NE formulation was resulted to enhance the DNA damage, and as in combination with Cur, it may advance the engulfment of drug molecules, specifically over cancer cells and suppress the cytosol pathways and caused cytotoxicity of the cells, with concentration–time ratio, and ultimately reduce the cell survival rate. The prolongation of antitumor activity was possessed through adsorption of NPs loaded with drugs over cell surface, which eventually made a continuous flow, and maintain concentration gradient within the cell. These situational changes were led to make endocytosis and cell death [23].
Cellular uptake of the drug concentration may be analyzed through sorting the treated cell frequency with the intensity of tagged fluorescent [24]. The quantitative scrutiny of cell uptake has monitored the affinity of NE-loaded drug molecules, in single and combination delivery, into the intracellular cytoplasm of OSCC cells. This phenomenon was to quantify the mean fluorescent intensity, comparatively standardized with the concentration of 5-FU/Cur-NE/5-FU-Cur-NEs formulations. The drug samples were allowed to incubate for 4 h with NEs and found maximum intracellular uptake through endocytosis in time–concentration-dependent manner.
The transition of healthy or normal cells into malignant cells is a diversified alteration mechanism within the cellular genome [25]. The drug molecules may be intercellulite and interact with multifarious cellular pathways and change the expression of macromolecules. Bcl2 protein inhibits the cell to form mitochondrial pores and antagonize the discharge of apoptogenic factors know as antagonist to apoptosis [19]. The untreated SCC090 and SCC152 cells were shown a high level of Bcl2 protein, as compared to 5-FU/Cur-NE/5-FU-Cur-NEs treated cells, which further confirmed the inhibition of overexpressed Bcl2 protein, and ultimately provoke the drug-induced apoptosis. This mechanism may directly increase the expression of protein Bax, in the cytosol, which breaches the surface crust of mitochondria and construct mitochondrial pores for apoptogenic factors permeability ultimately favors apoptosis. P53, supposed to be the curator of the genome, which activated if any single of abnormality occurred within the cellular pathways. Firstly, p53 may try to repair the damage to normalize the cell pathways, if not, secondly, it induced apoptosis via programmed cell death [20]. 5-FU/Cur-NE/5-FU-Cur-NEs treated tumor cells, SCC090 and SCC152 have shown increased expression of the protein, p53, and p21, and activate the apoptosis in time–dose-dependent. Comparatively, the BLNE incubated as non-treated OSCC cells, shown limited expression of the protein, p53, and p21, and shown reduced apoptosis.
Overall, this study was evidently established that co-delivery of 5-FU and Cur (5-FU-Cur-NE) have the capability to increase anticancer activity against, SCC090 and SCC152 cells, from the lower-dose to higher-dose concentration with time span incubation. The in vitro drug experiments have successfully confirmed the supportive information about control release, combined effect, increased bioavailability, sufficient cell arrest, and high induction of apoptosis.
Conclusion
Overall, this study concluded that co-delivery of 5-FU and Cur in a single-phase NE drug release system across in vitro oral cancer cells culture system. This drug–herb combined formulation was successfully invaded over OSCC cells and capable to produce many possibilities of combination drug delivery against all types of cancer. 5-FU and Cur, both drugs were highly unstable and for that, they have a limited therapeutic window, and this nano-platform provides vast bioavailability and control release system. 5-FU/Cur-NE/5-FU-Cur-NEs were non-toxic and created maximum intracellular uptake and limit cell survival with reduced IC50 value in dose–time-dependent manner. The changes in expression of proteins in OSCC cells, SCC090 and SCC152, specifically Blc2, Bax, P53, and P21 have clearly established the high induction of apoptosis. The overall results have proven the advancement in drug delivery by multichannel chemotherapy against OSCC.
Funding
The author, Saurabh Srivastava, is very grateful to Indian Council of Medical Research (ICMR), New Delhi, India (Project id-45/21/2013-NAN-BMS), for providing the fellowship grant to conduct his Ph.D. work.
Compliance with Ethical Standards
Conflict of interest
All the authors declared no conflicts of interest in this research work for the publication.
Contributor Information
Saurabh Srivastava, Email: saurabhsrivastava.kgmu@gmail.com.
Shadab Mohammad, Email: shadab31aug@yahoo.com.
References
- 1.Aktipis CA, Boddy AM, Jansen G, Hibner U, Hochberg ME, Maley CC, Wilkinson GS. Cancer across the tree of life: cooperation and cheating in multicellularity. Philos Trans R Soc Lond B Biol Sci. 2015;370(1673):1–21. doi: 10.1098/rstb.2014.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Karnov KKS, Gronhoj C, Jensen DH, Wessel I, Charabi BW, Specht L, Kjaer A, von Buchwald C. Increasing incidence and survival in oral cancer: a nationwide Danish study from 1980 to 2014. Acta Oncol. 2017;56:1204–1209. doi: 10.1080/0284186X.2017.1307516. [DOI] [PubMed] [Google Scholar]
- 4.Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010–2020) by cancer groups. Asian Pac J Cancer Prev. 2010;11(4):1045–1049. [PubMed] [Google Scholar]
- 5.Tapia JL, Goldberg LJ. The challenges of defining oral cancer: analysis of an ontological approach. Head Neck Pathol. 2011;5(4):376–384. doi: 10.1007/s12105-011-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015;33(29):3269–3276. doi: 10.1200/JCO.2015.61.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundela S, Sharma A, Bisen PS. Potential compounds for oral cancer treatment: resveratrol, nimbolide, lovastatin, bortezomib, vorinostat, berberine, pterostilbene, deguelin, andrographolide, and colchicine. PLoS ONE. 2015;10(11):1–31. doi: 10.1371/journal.pone.0141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratajczak MZ, Jadczyk T, Schneider G, Kakar SS, Kucia M. Induction of a tumor-metastasis-receptive microenvironment as an unwanted and underestimated side effect of treatment by chemotherapy or radiotherapy. J Ovarian Res. 2013;6(95):2–9. doi: 10.1186/1757-2215-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Wei Q, Zhou Y, Wang J, Liu Q, Xu H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst Biol. 2017;11(87):27–43. doi: 10.1186/s12918-017-0464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias JL. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13(10):2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4(1):421–427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavan AR, Silva GD, Jornada DH, Chiba DE, Fernandes GF, Man Chin C, Dos Santos JL. Unraveling the anticancer effect of curcumin and resveratrol. Nutrients. 2016;8(11):628–678. doi: 10.3390/nu8110628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan S, Sun Y, Qi X, Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012;13(1):159–166. doi: 10.1208/s12249-011-9732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Z, Li Z, Yan J, Wang P. Irinotecan and 5-fluorouracil-co-loaded, hyaluronic acid-modified layer-by-layer nanoparticles for targeted gastric carcinoma therapy. Drug Des Devel Ther. 2017;11:2595–2604. doi: 10.2147/DDDT.S140797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy - Strategies and perspectives. J Control Release. 2016;240:489–503. doi: 10.1016/j.jconrel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pangeni R, Choi SW, Jeon OC, Byun Y, Park JW. Multiple nanoemulsion system for an oral combinational delivery of oxaliplatin and 5-fluorouracil: preparation and in vivo evaluation. Int J Nanomedicine. 2016;11:6379–6399. doi: 10.2147/IJN.S121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part II: stability assessment, in vivo pharmacodynamic evaluations and toxicological studies. Int J Pharm. 2012;431(1–2):149–160. doi: 10.1016/j.ijpharm.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Wang ZQ, Liu K, Huo ZJ, Li XC, Wang M, Liu P, Pang B, Wang SJ. A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy. J Nanobiotechnol. 2015;13(63):1–10. doi: 10.1186/s12951-015-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indran IR, Tufo G, Pervaiz S. Brenner C (2011) Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 1807;6:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Russo A, Maiolino S, Pagliara V, Ungaro F, Tatangelo F, Leone A, Scalia G, Budillon A, Quaglia F, Russo G. Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles. Oncotarget. 2016;7(48):79670–79687. doi: 10.18632/oncotarget.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuomela A, Hirvonen J, Peltonen L. Stabilizing Agents for Drug Nanocrystals: effect on Bioavailability. Pharmaceutics. 2016;8(2):1–18. doi: 10.3390/pharmaceutics8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro LM, Lione VF, do Carmo FA, do Amaral LH, da Silva JH, Nasciutti LE, Rodrigues CR, Castro HC, de Sousa VP, Cabral LM. Development and characterization of a new oral dapsone nanoemulsion system: permeability and in silico bioavailability studies. Int J Nanomedicine. 2012;7:5175–5182. doi: 10.2147/ijn.s36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TH, Lee GJ, Kang JH, Kim HJ, Kim TI, Oh JM. Anticancer drug-incorporated layered double hydroxide nanohybrids and their enhanced anticancer therapeutic efficacy in combination cancer treatment. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/193401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unciti-Broceta JD, Cano-Cortes V, Altea-Manzano P, Pernagallo S, Diaz-Mochon JJ, Sanchez-Martin RM. Number of nanoparticles per cell through a spectrophotometric method: a key parameter to assess nanoparticle-based cellular assays. Sci Rep. 2015;5:10091. doi: 10.1038/srep10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vranic S, Boggetto N, Contremoulins V, Mornet S, Reinhardt N, Marano F, Baeza-Squiban A, Boland S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part Fibre Toxicol. 2013;10(2):1–16. doi: 10.1186/1743-8977-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]