Abstract
A 55-year-old man with Marfan syndrome taking warfarin for anticoagulant therapy after aortic valve replacement developed acute kidney injury (serum creatinine level of 9.01 mg/dL) and gross macrohematuria. Renal biopsy showed red cell casts in the renal tubules, glomerular crescent formation in the glomeruli with immunoglobulin A deposition, and global sclerosis. Based on these findings, the patient was diagnosed with warfarin-related nephropathy with acute kidney injury characterized by immunoglobulin A nephropathy with crescents. The warfarin was withdrawn, and his hematuria and renal function improved without immunosuppressive agents.
Keywords: Acute kidney injury, Warfarin-related nephropathy, Immunoglobulin A nephropathy, Crescents
Introduction
Warfarin is widely used as anticoagulant therapy to prevent primary and secondary thromboembolic events. Several adverse effects of warfarin therapy on the kidney have been reported; these adverse effects are collectively called warfarin-related nephropathy, which is characterized by hematuria and acute kidney injury [1–4]. This condition is more common in patients with than without renal diseases, including immunoglobulin A (IgA) nephropathy, nephrosclerosis, focal segmental glomerulosclerosis, and lupus nephritis [5]. In most cases of warfarin-related nephropathy, red blood cell casts in the renal tubules obstruct urine flow, resulting in acute kidney injury; however, the glomeruli are not usually involved [5]. We herein report a case of warfarin-related nephropathy characterized by crescents in the glomeruli in addition to red blood cell casts in the renal tubules in a patient with IgA nephropathy.
Case report
A 55-year-old man underwent aortic valve replacement with a mechanical valve at the age of 42 years for the treatment of aortic regurgitation due to Marfan syndrome. He began taking warfarin as an anticoagulant agent after the operation. Most recently, he had been taking warfarin at 3 mg/day, aspirin at 100 mg/day, lansoprazole at 15 mg/day, and ticlopidine at 200 mg/day. His last checkup (7 months before admission) had revealed mild microscopic hematuria without proteinuria, normal renal function (serum creatinine level of 0.76 mg/dL), and a prothrombin time-international normalized ratio (PT-INR) of 1.14. He noticed gross macrohematuria 8 days before admission. He underwent a work-up for the gross macrohematuria 2 days before admission, and severe renal impairment was found. He was then referred to our nephrology center for diagnosis and treatment of his renal impairment.
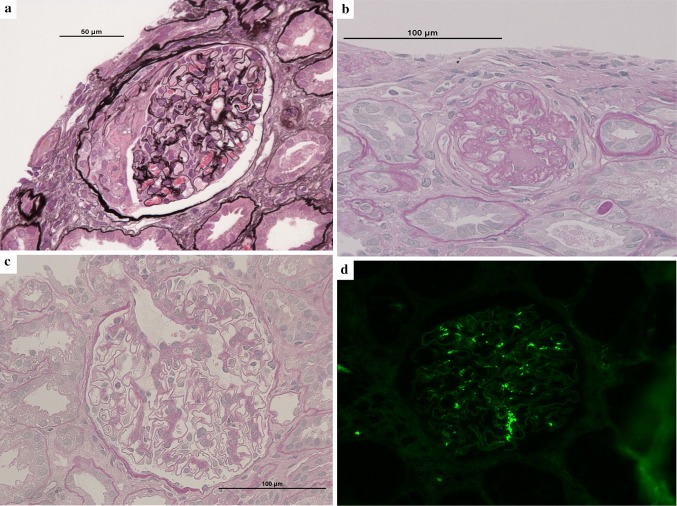
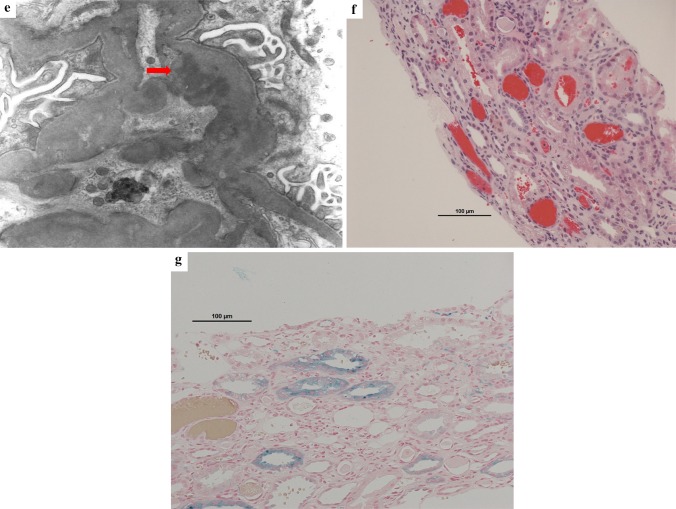
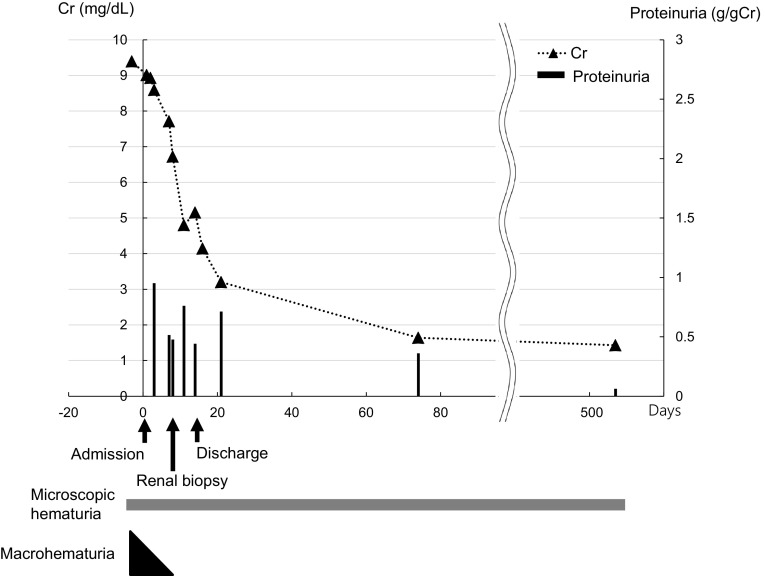
Upon admission, mild leg edema was detected, but no extrarenal bleeding was observed. His systolic/diastolic blood pressure was 168/94 mmHg. His serum creatinine level was elevated at 9.01 mg/dL, and his PT-INR was prolonged to 3.75 beyond the treatment range. Urinalysis showed macrohematuria and numerous microscopic foci of hematuria with many dysmorphic red blood cells and moderate proteinuria at 0.95 g/gCr. The patient was negative for antineutrophil cytoplasmic antibody, antiglomerular basement membrane antibody, antinuclear antibody, and antistreptolysin O. The concentrations of serum complement, such as C3 and C4, were within the normal range. The other laboratory data obtained at admission are shown in Table 1. Ultrasonography and computed tomography showed that the size of the bilateral kidneys was normal with no urinary tract obstruction. Warfarin was suspended, because the PT-INR was prolonged beyond the treatment range and gross hematuria was present. After the PT-INR had normalized, renal biopsy was performed with administration of heparin on the 9th hospital day. The histological analysis revealed that 13% (7/53) of the glomeruli showed crescents (11% cellular crescents and 2% fibrocellular crescents) (Fig. 2a) and that 38% (20/53) of the glomeruli showed global sclerosis (Fig. 2b). The mesangial matrix showed mildly increased cellularity and IgA and C3 deposition (Fig. 2c, d). Electron microscopy showed the presence of electron-dense deposits in mesangial areas (Fig. 2e). No subepithelial or endothelial deposits were observed. Several red blood cell casts were observed in the renal tubules (Fig. 2f). Perls’ Prussian blue stain revealed hemosiderin deposition in 20% of the proximal tubular epithelial cells (Fig. 2g). Based on these histological findings, the patient was diagnosed with acute kidney injury with warfarin-related nephropathy in the presence of IgA nephropathy. He was treated by supportive care including infusion of extracellular fluid solution without any immunosuppressive agents. On the 11th hospital day, his serum creatinine level decreased to 4.8 mg/dL. The gross macrohematuria and proteinuria also decreased. The warfarin was then carefully resumed at a dose of 1 mg/day. His renal function and macrohematuria and proteinuria gradually improved, and he was discharged from our center on the 15th hospital day. He has since received regular outpatient treatment in our department. At 18 months after discharge, his serum creatinine level had decreased to 1.43 mg/dL, his proteinuria had decreased to the normal range (0.06 g/gCr), and his urine continued to show microscopic hematuria (50–100 red blood cells per high-power field) (Fig. 1).
Table 1.
Laboratory results on admission
| Examination | Value | Reference range |
|---|---|---|
| Blood test | ||
| White blood cells (/µL) | 18,960 | 3,900–9,800 |
| Red blood cells (/µL) | 365 × 104 | 427–570 × 104 |
| Hemoglobin (g/dL) | 12.0 | 12.0–17.6 |
| Hematocrit (%) | 35.4 | 39.8–51.8 |
| Mean corpuscular volume | 97.0 | 83–101 |
| Platelets (× 103/µL) | 389 | 130–369 |
| Sodium (mEq/L) | 136 | 138–145 |
| Potassium (mEq/L) | 5.0 | 3.6–4.8 |
| Chloride (mEq/L) | 101 | 100–110 |
| Calcium (mg/dL) | 7.9 | 8.6–10.1 |
| Phosphate (mg/dL) | 4.6 | 2.7–4.6 |
| Blood urea nitrogen (mg/dL) | 75 | 8–20 |
| Creatinine (mg/dL) | 9.01 | 0.65–1.07 |
| Blood glucose (mg/dL) | 157 | 70–100 |
| HbA1c (%) | 5.1 | 4.6–6.2 |
| IgG (mg/dL) | 1,922 | 870–1,700 |
| IgA (mg/dL) | 392 | 110–410 |
| IgM (mg/dL) | 48 | 33–190 |
| Antistreptolysin O (U/mL) | 91 | < 250 |
| C3 (mg/dL) | 116 | 65–135 |
| C4 (mg/dL) | 24 | 13–35 |
| CH50 (U/mL) | 48.4 | 30.0–45.0 |
| Antinuclear antibody | 40 | < 40 |
| PR3-ANCA (IU/mL) | < 1.0 | < 1.0 |
| MPO-ANCA (IU/mL) | < 1.0 | < 1.0 |
| Antiglomerular basement membrane antibody (IU/mL) | < 1.0 | < 3.5 |
| Urinary test | ||
| Red blood cells (/HPF) | Numerous (dysmorphic) | < 5 |
| Proteinuria (g/gCr) | 0.95 | < 0.15 |
| Urinary β2-microglobulin (µg/L) | 11,521 | < 230 |
ANCA antineutrophil cytoplasmic antibody, HbA1c hemoglobin A1c, IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, C3 complement component 3, C4 complement component 4, CH50 50% complement activity, MPO myeloperoxidase, PR-3 proteinase-3, HPF high-power field
Fig. 2.
Renal biopsy findings. a Glomerulus with cellular crescentic formation (periodic acid-Schiff stain; magnification, × 400). b Glomerulus with global sclerosis (periodic acid–Schiff stain; magnification, × 400). c Glomerulus with increased mesangial cellularity (periodic acid-Schiff stain; magnification, × 400). d Granular mesangial IgA deposits (immunofluorescence; magnification, × 200). IgA immunoglobulin A. e Scattered small electron-dense deposits in the paramesangial area (uranyl acetate lead citrate stain; magnification, × 3000). f Intratubular red blood cell casts (hematoxylin–eosin stain; magnification, × 200). g Hemosiderin deposits in proximal tubular epithelial cells (Perls’ Prussian blue stain; magnification, × 200)
Fig. 1.
Patient’s clinical course. The horizontal axis shows the number of days from admission. A renal biopsy was performed on the 9th hospital day. Cr, creatinine
Discussion
We have herein described a case of warfarin-related nephropathy with crescents leading to acute kidney injury in a patient with IgA nephropathy. Warfarin-related nephropathy has been reported to induce acute kidney injury [4]. The mechanism of acute kidney injury by warfarin is considered to involve obstruction of tubules by red blood cell casts and hemoglobin-induced nephrotoxicity. These pathological changes are induced by glomerular bleeding caused by the over-anticoagulation effect of warfarin [6, 7]. A previous study showed that over-anticoagulation due to a warfarin overdose and a PT-INR that exceeds 3.0 may be considered to cause glomerular bleeding [8]. In the present case, the PT-INR was prolonged to 3.75, and this over-anticoagulation by warfarin may have triggered the warfarin-related nephropathy. The cause of the prolonged PT-INR could not be identified. The PT-INR often shows considerable intra-patient variability. Various factors such as patients’ adherence, drug interactions, comorbidities, and acute illnesses are known to influence the PT-INR. No factors that may affect the PT-INR were identified in the present case. Another study revealed that the histological findings of warfarin-related nephropathy are characterized by the obstruction of a low percentage of tubules (2–20%) with red blood cell casts and no active glomerular lesions [9]. In a different study, accumulation of hemosiderin (iron-based degraded hemoglobin from red blood cells) was detected in the renal tubulointerstitial space [10]. The histological changes of the kidney in the present case are consistent with those in previous reports. Notably, most patients with warfarin-related nephropathy reportedly have underlying kidney diseases including IgA nephropathy (Table 2) [5, 11–14]. In addition, cellular and fibrocellular crescents were observed in 13% of the glomeruli of our patient. To our knowledge, this is the first study to show crescentic changes in the glomeruli in association with warfarin-related nephropathy. The patient may have had IgA nephropathy as an underlying renal disease, because the renal biopsy showed mildly increased mesangial matrix cellularity and IgA and C3 deposition on immunofluorescent staining. The microscopic hematuria before and after admission also suggests this possibility. Furthermore, the elevations of serum IgA level and IgA/C3 ratio, which can predict diagnosis of IgA nephropathy, were observed in this patient [15]. Macrohematuria-induced acute kidney injury has been reported in several cases of IgA nephropathy. However, we consider that the cause of acute kidney injury in this case was warfarin-related nephropathy rather than IgA nephropathy. First, there was no evidence of prior infection. Prior infection was reportedly observed in many cases of IgA nephropathy with macrohematuria. Second, mesangial proliferation was mild. In one study, diffuse mesangial proliferation was observed in all patients with AKI and concurrent IgA nephropathy [16]. Third, deposition of IgA with immunofluorescence staining in the kidney was mild. Immunofluorescence staining showed strong IgA staining in patients with AKI with IgA nephropathy, but not in patients with warfarin nephropathy [5]. Conversely, a relationship among Marfan syndrome, IgA nephropathy, and warfarin nephropathy has not been reported. Further studies are required to investigate the mechanism of glomerular crescent formation in patients with warfarin-related nephropathy.
Table 2.
Clinicopathological findings in warfarin-related nephropathy
| Patient [references] | Age/sex | Underlying renal disease | PT-INR (IU) | Basal sCr (mg/dL) | sCr at biopsy | Crescents (mg/dL) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 [1] | 27/F | Lupus nephritis | 8 | 0.6 | 3.1 | (−) | Complete recovery |
| 2 [1] | 73/M | Moderate interstitial fibrosis | 3 | 2.6 | 6.5 | (−) | Dialysis |
| 3 [1] | 61/M | Nephrosclerosis | 2 | 3 | 4.8 | (−) | Dialysis |
| 4 [1] | 76/F | FSGS | 7 | 0.9 | 3.4 | (−) | Dialysis |
| 5 [1] | 38/F | IgA nephropathy | 3.9 | 0.7 | 0.9 | (−) | Complete recovery |
| 6 [1] | 82/F | IgA nephropathy | 2.8 | 1.1 | 4.5 | (−) | Complete recovery |
| 7 [1] | 80/M | DN and IgA nephropathy | 5.2 | 1.1 | 3.9 | (−) | Dialysis |
| 8 [1] | 63/M | Nephrosclerosis | 3.7 | 1.0 | 2.5 | (−) | Complete recovery |
| 9 [1] | 55/M | IgG nephropathy | 3.8 | 0.8 | 9.1 | (−) | Complete recovery |
| 10 [11] | 59/M | Thin basement membrane disease | 3.6 | 1.0 | 8.4 | (−) | Incomplete recovery |
| 11 [12] | 74/M | CKD (unknown cause) | 4.62 | 1.8 | 3.5 | (−) | Dialysis |
| 12 [13] | 56/M | FSGS | 6.08 | 1.4 | 11.5 | (−) | Incomplete recovery |
| 13 [14] | 56/F | Nephrosclerosis | 4.95 | 0.8 | 3.5 | (−) | Incomplete recovery |
| 14[our case] | 55/M | IgA nephropathy | 3.75 | 0.76 | 6.7 | (−) | Incomplete recovery |
DN diabetic nephropathy, CKD chronic kidney disease, FSGS focal segmental glomerulosclerosis, IgA immunoglobulin A
In conclusion, we have described a case of AKI with warfarin-related nephropathy with crescents in a patient with IgA nephropathy. Regular blood and urine examinations including measurement of the PT-INR should be regularly performed in patients who are taking warfarin.
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient in this case report.
References
- 1.Volpi A, Ferrario GM, Giordano F, Antiga G, Battini G, Fabbri C, et al. Acute renal failure due to hypersensitivity interstitial nephritis induced by warfarin sodium. Nephron. 1989;52(2):196. doi: 10.1159/000185636. [DOI] [PubMed] [Google Scholar]
- 2.Dajani YF. Hematuria in patients on anticoagulants. N Engl J Med. 1977;297(4):222. doi: 10.1056/nejm197707282970419. [DOI] [PubMed] [Google Scholar]
- 3.Klinger ME, Tanenbaum B, Elguezabal A. Pseudo-tumors of the kidney secondary to anticoagulant therapy. J Urol. 1971;106(4):507–511. doi: 10.1016/S0022-5347(17)61328-4. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):182–90. doi: 10.1038/ki.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54(6):1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Kincaid-Smith P, Bennett WM, Dowling JP, Ryan GB. Acute renal failure and tubular necrosis associated with hematuria due to glomerulonephritis. Clin Nephrol. 1983;19(4):206–210. [PubMed] [Google Scholar]
- 7.Heyman SN, Brezis M. Acute renal failure in glomerular bleeding: a puzzling phenomenon. Nephrol Dial Transplant. 1995;10(5):591–593. [PubMed] [Google Scholar]
- 8.Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, et al. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115(2):c142-6. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodsky SV. Anticoagulants and acute kidney injury: clinical and pathology considerations. Kidney Res Clin Pract. 2014;33(4):174–80. doi: 10.1016/j.krcp.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin Cleary C, Moreno JA, Fernandez B, Ortiz A, Parra EG, Gracia C, et al. Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol Dial Transplant. 2010;25(12):4103–4106. doi: 10.1093/ndt/gfq493. [DOI] [PubMed] [Google Scholar]
- 11.Abt AB, Carroll LE, Mohler JH. Thin basement membrane disease and acute renal failure secondary to gross hematuria and tubular necrosis. Am J Kidney Dis. 2000;35(3):533–536. doi: 10.1016/S0272-6386(00)70209-5. [DOI] [PubMed] [Google Scholar]
- 12.Santos C, Gomes AM, Ventura A, Almeida C, Seabra J. An unusual cause of glomerular hematuria and acute kidney injury in a chronic kidney disease patient during warfarin therapy. Nefrologia. 2013;33(3):400–403. doi: 10.3265/Nefrologia.pre2012.Oct.11617. [DOI] [PubMed] [Google Scholar]
- 13.Larpparisuth N, Cheunsuchon B, Chawanasuntorapoj R, Vasuvattakul S, Vareesangthip K. Warfarin related nephropathy: the first case report in Thailand. J Med Assoc Thai. 2015;98(2):212–216. [PubMed] [Google Scholar]
- 14.Ng CY, Tan CS, Chin CT, Lim SL, Zhu L, Woo KT, et al. Warfarin related nephropathy: a case report and review of the literature. BMC Nephrol. 2016;17:15. doi: 10.1186/s12882-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda A, Gohda T, Funabiki K, Horikoshi S, Shirato I, Tomino Y. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J Clin Lab Anal. 2003;17(3):73–6. doi: 10.1002/jcla.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett WM, Kincaidsmith P. Macroscopic hematuria in mesangial IgA nephropathy: correlation with glomerular crescents and renal dysfunction. Kidney Int. 1983;23(2):393–400. doi: 10.1038/ki.1983.32. [DOI] [PubMed] [Google Scholar]