Abstract
Renal vein thrombosis in a transplanted kidney is an uncommon but critical complication that can result in graft loss if management is delayed. A 31-year-old male with known atresia of the inferior vena cava who received a deceased donor renal transplant 7 years previously presented to hospital with severe graft site pain and a week of nausea, vomiting, and chills. Serum creatinine was markedly elevated from baseline. Sonographic examination revealed external iliac vein thrombosis with extension of the thrombus into the transplant renal vein. Urgent angiographic administration of tissue plasminogen activator and suction thrombectomy was performed, then followed by heparin and clopidogrel post procedure. Within 24 h, his serum creatinine improved, and within 2 weeks returned to his baseline. He was started on lifelong warfarin anti-coagulation to reduce the risk of rethrombosis secondary to his uncorrectable aberrant venous anatomy. Due to the turbulent and sometimes reversed flow in the major veins, lifelong anticoagulation should be strongly considered for such transplant patients with recipient aberrancy of the large veins.
Keywords: Kidney transplant, Veins, Interventional radiology, Thrombosis, Anticoagulation
Introduction
Renal vein thrombosis (RVT) complicates 1–4% of allograft transplants [1]. Consequences of unrecognized RVT are severe, often resulting in transplant loss [2]. However, early recognition and treatment can permit graft salvage. Interventional procedures and thrombolytic agents have been increasingly used in the management of RVT. While the risk of potentially life-threatening hemorrhage with thrombolytic therapy is high in the immediate postoperative period [3], this is much less of a concern in a patient who received their graft more than a few months previously. These patients may benefit from interventional and medical, rather than operative, management.
Case
The patient is a 31-year-old male with a history of diabetes mellitus, hypertension, dyslipidemia, and significantly abnormal venous anatomy who underwent uncomplicated deceased donor renal transplant in 2009 for end-stage renal disease secondary to vesicoureteral reflux. Prior to transplant, he was dependent on hemodialysis thrice weekly. A CT angiogram of the abdominal and pelvic arteries and veins done prior to transplant revealed atresia of the intrahepatic IVC with significant venous drainage to the lumbar plexus through the azygous and hemiazygous veins (Fig. 1). The preoperative CT angiogram was beyond the usual vascular investigations prior to transplant and was ordered due to the patient’s identical twin being found to have congenitally absent IVC and iliac veins while undergoing assessment for live donor suitability. Since the recipient’s iliac veins had normal anatomy suitable for iliac vein implantation, with well-formed collateral venous circulation development, the patient had been deemed suitable for transplant and was listed for deceased donor kidney transplantation.
Fig. 1.
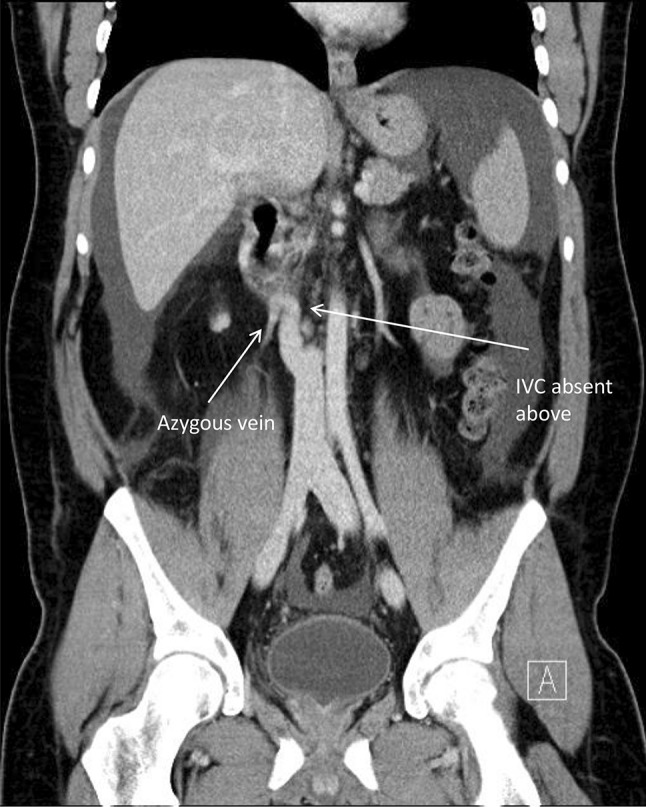
CT angiogram of the recipient prior to transplant, showing absent vena cava above the level of the renal veins, prominent azygous vein compensating, and no intrahepatic vena cava
After undergoing deceased donor kidney transplantation, the patient’s transplanted kidney functioned well and he did not suffer any acute complications. He established a baseline creatinine of 120 µmol/L and was maintained on an immunosuppressive regimen of tacrolimus, mycophenolate, and prednisone.
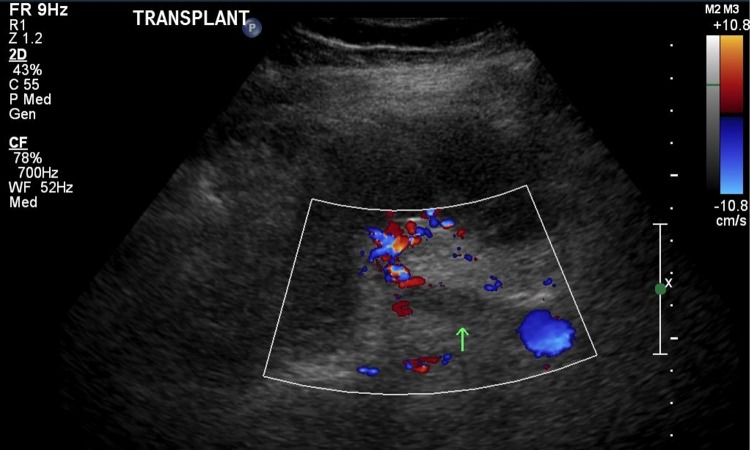
The patient presented to hospital 7 years after his transplant with pain over his graft site after suffering from a week of nausea, vomiting, chills, and progressive lower back pain, leaving him bedbound. He was found to have a significantly elevated serum creatinine of 502 µmol/L, markedly elevated from his usual baseline of 120 µmol/L.His INR and aPTT were normal. Ultrasound evaluation revealed an occlusive thrombus in the right external iliac vein adjacent to the pelvic fossa renal transplant, with extension of the clot into the transplant renal vein (Fig. 2). No compressive lesions were identified upon imaging investigations that may have contributed to thrombus development.
Fig. 2.
Doppler ultrasound taken at the time of presentation to the emergency department with elevated creatinine. Figure shows external iliac vein thrombus that extends into the transplant renal vein (note the clips around where the transplant renal vein would normally show flow on Doppler)
The patient was taken to the angiography suite for attempted dissolution of the thrombus. The right posterior tibial vein was cannulated, and contrast injection revealed that the right external iliac vein contained a substantial thrombus and was dilated to 3.5 cm (Fig. 3). The venous flow was reversed such that flow in the external iliac vein was primarily in a caudal direction. The catheter could not be advanced further and was therefore withdrawn. An Angiojet device was then inserted, and 10 mg of tissue plasminogen activator (TPA) was instilled into the thrombus. Suction thrombectomy was successful at removing some of the thrombus, but was unsuccessful in re-establishing flow, given the quantity of clot. Attempts at cannulation of the transplant renal vein were also unsuccessful, and the procedure was therefore concluded.
Fig. 3.
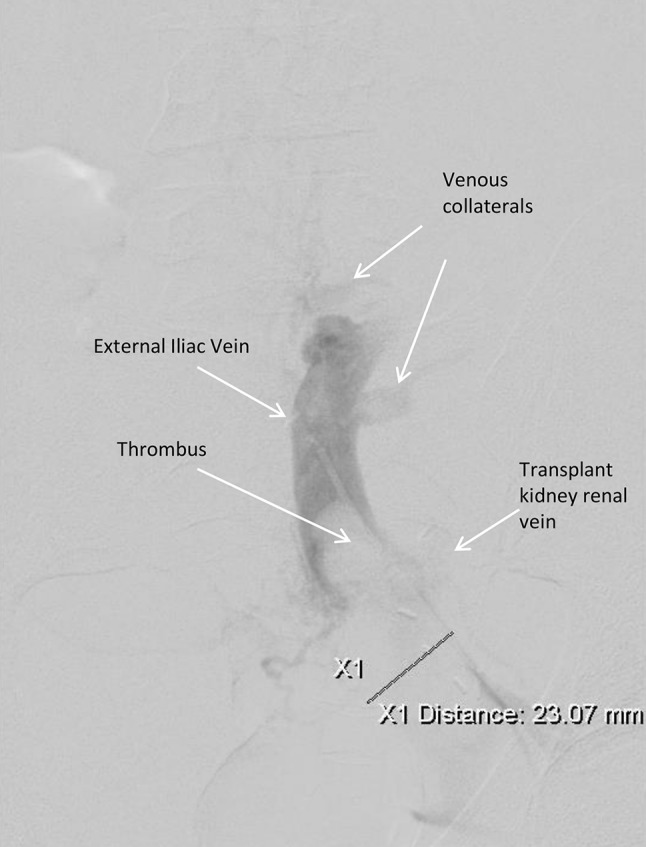
Angiogram showing the large thrombus in the external iliac vein and extending into the transplant renal vein. Also note the extensive collaterals and complete occlusion cephalad to the external iliac vein. In real time, the flow of contrast was seen to be reversed, ie, flowing caudally towards the lower extremity
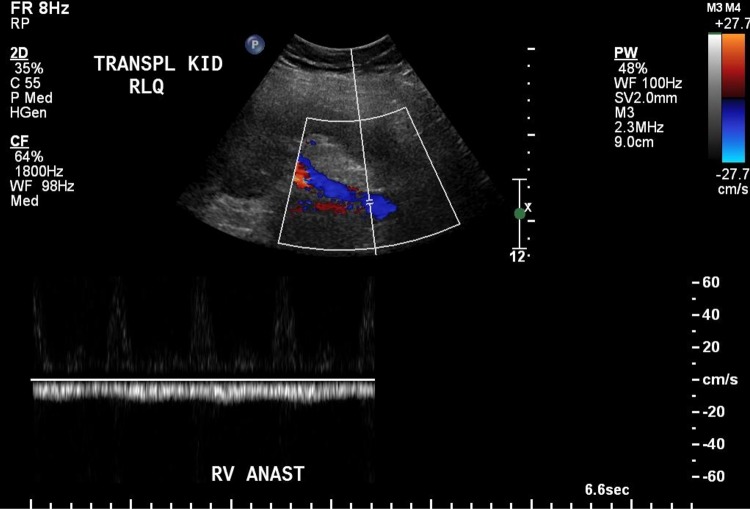
After the interventional procedure, the patient was started on heparin and clopidogrel. Within hours, his urine output improved and his serum creatinine decreased. He was maintained on high levels of anticoagulation and observed for several days in hospital, and by the time of discharge, his serum creatinine had improved to 147 µmol/L. He was discharged on clopidogrel or 1 month and warfarin, which was suggested to be continued indefinitely. An ultrasound 3 months later (Fig. 4) revealed resolution of the renal thrombus. Creatinine had returned to his baseline of 120 µmol/L.
Fig. 4.
Doppler ultrasound taken 3 months after the venous thrombosis was treated with TPA and anticoagulants, showing the thrombus completely resolved and flow restored to that segment of external iliac vein, albeit reversed
Outpatient investigations by hematology for additional risk factors predisposing to hypercoagulability were negative, and it was concluded that the development of the thrombus was most likely the result of venous stasis and epithelial damage secondary to aberrant IVC anatomy, compounded by acute inflammatory mediators and immobilization due to the preceding flu-like illness.
Discussion
RVT occurs most commonly within 2 weeks of transplantation [4] and is often attributable to surgical complications, including kinking of the renal vein or renal vein compression, but also to thrombophlebitis, hypovolemia, or acute rejection [5]. Our case is unique in that RVT developed years after transplantation. Late-onset RVT has been observed in relation to immune-complex glomerulonephritis, immunosuppressive treatment, elevated hematocrit, acute rejection, and extension of lower extremity thrombosis [2].
Our patient had significant lower extremity thrombosis that led to RVT. We believe that our patient’s history of a week of flu-like illness preceding development of his lower extremity deep venous thrombosis, combined with his previously known aberrant venous anatomy, contributed significantly to the development of his RVT, as previously described [6].
Virchow’s triad, interestingly named after, but not described by Dr. Rudolph Virchow over a century ago [7], continues to be applicable in the analysis of cases of venous thrombosis. Virchow’s triad describes how vascular epithelial damage, circulatory stasis, and hypercoagulable states are predisposing factors for coagulation. In this particular case, the patient’s viral illness likely increased production of inflammatory mediators, and this along with relative dehydration and immobilization, contributed to a hypercoagulable state. Furthermore, our patient’s anomalous venous anatomy significantly increased his risk of coagulation. His altered IVC anatomy likely contributed to venous stasis, with delayed cardiac return. Additionally, turbulent blood flow may have produced epithelial damage. Immunosuppressants have also been identified as a risk factor in late-onset RVT [2].
The diagnosis of late RVT is difficult given that the presenting symptoms are often non-specific. Patients may present with pain over the graft site, oliguria or anuria, or unilateral lower extremity swelling [2, 4, 8]. Laboratory investigations are often remarkable for an elevated serum creatinine, proteinuria, and hematuria [2, 8]. Our patient presented with similar findings of graft site and lower back pain, as well as an acutely elevated creatinine above baseline.
Once graft dysfunction was recognized, our patient underwent ultrasonography that identified thrombosis as the cause of the acute kidney injury. Doppler ultrasound, venography, and renogram are imaging modalities that have been used in the identification of RVT [2, 4]. While venography is considered the gold standard, it must be used with caution in a patient with a renal transplant given the nephrotoxic nature of contrast agents [4]. Sonographic evaluation is being increasingly used in the evaluation of graft failure as it allows for examination of vascular abnormalities, parenchymal damage, and urological complications without risk of adverse effects [9].
Early diagnosis and intervention are essential in the management of transplant RVT and preservation of graft function. The goal of treatment is the preservation of graft function. Increasing sensitivity of diagnostic imaging and interventional procedures have contributed to the success of graft salvage through interventional and medical, as opposed to surgical, means [4, 10]. Catheter-directed infusion of thrombolytic agents such as TPA, streptokinase, and urokinase in patients with renal transplantation with stable grafts and no other contraindications to systemic thrombolytics [11, 12] can be graft-saving. After thrombolysis, ongoing anticoagulation, usually with Heparin, is indicated, with the duration of anticoagulation dependent on identification of reversible or irreversible risk factors [11]. Patients with reversible risk factors may receive 3–6 months of anticoagulation, while patients with irreversible risk factors should be anticoagulated indefinitely, as in this case.
Conclusion
This case report highlights the importance of the recognition of known risk factors for thrombosis, particularly aberrant venous anatomy and consideration of such transplant patients for life-long anticoagulation.
Conflict of interest
The authors have declared that no conflict of interest exists.
Informed consent
Informed consent was obtained from the individual included in the study.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee of the University of Saskatchewan and the Saskatoon Health Region (Bio REB#12-44).
References
- 1.DELBEKE DOMINIQUE, SACKS GLYNIS A., SANDLER MARTIN P. Diagnosis of Allograft Renal Vein Thrombosis. Clinical Nuclear Medicine. 1989;14(6):415–420. doi: 10.1097/00003072-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Schweiger J, Reiss R, Cohen JL, et al. Acute renal allograft dysfunction in the setting of deep venous thrombosis: a case of successful urokinase thrombolysis and a review of the literature. Am J Kidney Dis. 1993;22(2):345–350. doi: 10.1016/S0272-6386(12)70330-X. [DOI] [PubMed] [Google Scholar]
- 3.Duckett T, Bretan PN, Cochran ST, et al. Non-invasive radiological diagnosis of renal vein thrombosis in renal transplantation. J Urol. 1991;146:403–406. doi: 10.1016/S0022-5347(17)37806-0. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaswamy SK, Rajan M, Prabahar A. Successful thrombolysis of renal allograft venous thrombosis – a case report. Indian J Transpl. 2014;8:57–59. doi: 10.1016/j.ijt.2014.05.003. [DOI] [Google Scholar]
- 5.Ehrlich RM, Smith RB. Surgical complications of renal transplantation. Urology. 1977;10:43–39. [PubMed] [Google Scholar]
- 6.Muscianese L, Seese RR, Graham W, Williams JH. Congenital atresia of the inferior vena cava and antithrombin III deficiency in a young adult: compounding risk factors for deep vein thrombosis. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-205729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malone PC, Agutter PS. The aetiology of deep venous thrombosis. QJM Int J Med. 2006;99(9):581–593. doi: 10.1093/qjmed/hcl070. [DOI] [PubMed] [Google Scholar]
- 8.Fathi T, Samhan M, Gawish A, et al. Renal allograft venous thrombosis is salvageable. Transpl Proc. 2007;39(4):1120–1121. doi: 10.1016/j.transproceed.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Muradahli D, Chawla T. Organ transplantation. In: Rumack CM, editor. Diagnostic ultrasound. 4. Philadelphia: Mosby; 2011. pp. 639–706. [Google Scholar]
- 10.Freitas C, Fructuso M, Rocha MJ, Almeida M, Pedroso S, Martins LS, Dias L, Castro Henriques A, Almeida R, Cabrita A. Late venous thrombosis of renal allograft: two cases with different treatment and outcome. Nefrologia. 2011;31(1):115–117. doi: 10.3265/Nefrologia.pre2010.Sep.10641. [DOI] [PubMed] [Google Scholar]
- 11.Witz M, Korzets Z. Renal vein occlusion: diagnosis and treatment. Isr Med Assoc J. 2007;9(5):402–405. [PubMed] [Google Scholar]
- 12.Hogan JL, Rosenthal SJ, Yarlagadda SG, Jones JA, Schmitt TM, Kumer SC, Kaplan B, Deas SL, Nawabi AM. Late-onset renal vein thrombosis: a case report and review of the literature. Int J Surg Case Rep. 2015;6C:73–76. doi: 10.1016/j.ijscr.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]