Abstract
Patients with an indwelling tunneled dialysis catheter (TDC) for hemodialysis access are at a high risk of developing methicillin-resistant Staphylococcus aureus (MRSA) infection. MRSA bacteremia complications rarely include infected aneurysm. Here, we report the first case of an infected thoracic aneurysm associated with TDC-related MRSA bacteremia. An 86-year-old Japanese male with a TDC for hemodialysis access developed TDC-related MRSA bacteremia. Intravenous vancomycin was initiated, and the TDC was removed on day 3. Despite removal of the catheter and initiation of vancomycin treatment, MRSA bacteremia persisted. Chest computed tomography (CT) showed no aneurysm; however, calcification of the thoracic aorta was detected on admission. The patient subsequently developed hemosputum. CT revealed a thoracic aneurysm, which turned out to be caused by MRSA bacteremia. The patient eventually died because of the rupture of the infected aneurysm, as confirmed by autopsy. This report demonstrates TDC management in a patient with TDC-related MRSA bacteremia and the importance of investigating a metastatic infection to a calcified artery if bacteremia persists.
Keywords: Infected aneurysm, Hemodialysis, Tunneled dialysis catheter, MRSA bacteremia
Introduction
Patients with an indwelling tunneled dialysis catheter (TDC) for hemodialysis access are at a high risk of developing methicillin-resistant Staphylococcus aureus (MRSA) infection [1]. Although MRSA bacteremia rarely leads to an infected aneurysm, we report an exceptionally rare case of a pathologically confirmed infected aneurysm in the thoracic aorta caused by TDC-related MRSA bacteremia.
Case report
An 86-year-old Japanese male who was undergoing maintenance hemodialysis was admitted to our hospital with complaints of fever and purulent discharge from a TDC site. Four months prior to admission, he presented at our emergency department with dyspnea due to diabetic nephropathy, and hemodialysis was initiated. Due to dementia, he had difficulty in enduring a puncture during hemodialysis. Thus, although the creation of an arteriovenous fistula was possible, a TDC was inserted in the right internal jugular vein for vascular access. He reported that he had smoked 40 cigarettes per day for 62 years but did not consume alcohol. No allergies were reported. His medical history comprised type 2 diabetes, hypertension, dyslipidemia, and coronary artery bypass grafting at the age of 65 years. Although a tumor was found in his left lung at the age of 86 years, further enquiry related to the tumor was not conducted because of the tumor’s low criticality and the patient’s deteriorating dementia condition. His level of activities of daily living was 50 as per the Barthel Index.
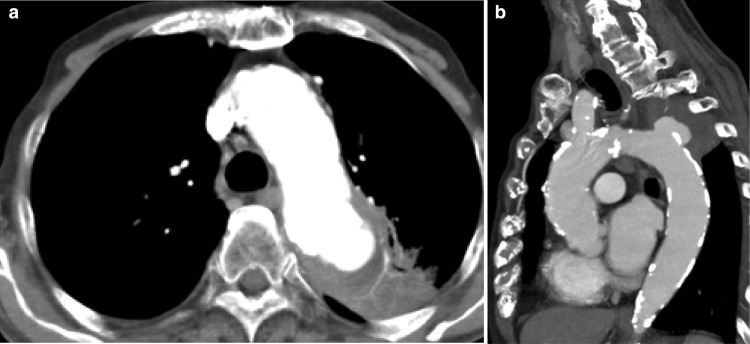
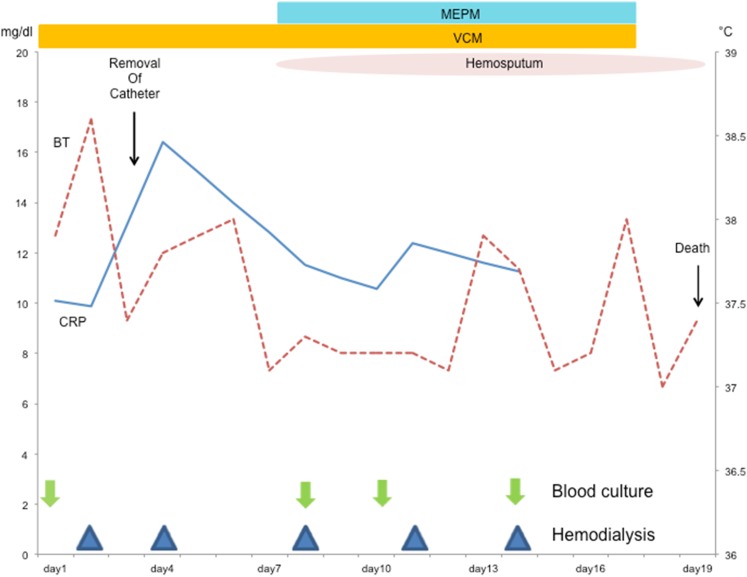
On admission, his body temperature was 38 °C, blood pressure was 137/67 mmHg, heart rate was 60 beats/min, and oxygen saturation was 98% in room air. Physical examination results were normal except for the presence of purulent discharge from the exit site of the TDC and redness of the skin extending up to 5 cm from the exit site along the subcutaneous tunnel. The results of laboratory investigations are summarized in Table 1. Although the white blood cell count was within the normal range, the C-reactive protein concentration was elevated. The serum corrected calcium level and phosphate level was appropriate for a dialysis patient. His hypoalbuminemia was believed to be caused by decreased consumption of food. Computed tomography (CT) revealed no fever focus and aneurysm (Fig. 1). Antibiotic treatment with intravenous vancomycin (22 mg/kg of body weight) was initiated [2]. The TDC was not immediately removed because the patient’s dementia forced us to avoid puncture during hemodialysis. Blood culture was positive for MRSA, which was also detected in a bacterial culture of the catheter tip, thereby confirming TDC-related MRSA bacteremia. The TDC was removed on day 3. Antibiotic treatment with intravenous vancomycin was continued. The minimum inhibitory concentration of vancomycin was 1 µg/mL. The blood concentrations of vancomycin trough levels were monitored, which varied from 12 to 17 mg/L. Subsequent blood culture specimens were obtained on days 8, 10, and 14, which were positive for MRSA and sensitive to vancomycin. Despite catheter removal and vancomycin treatment, the patient’s fever and MRSA bacteremia persisted. He developed hemosputum on day 8. Chest contrast-enhanced CT revealed an aneurysm (45 mm in diameter) of the thoracic aorta that had ruptured and was sealed within the left lung (Fig. 2). Considering inflammation of the left lung, meropenem treatment was initiated. The aneurysm was not evident at the time of admission. Rapid expansion of the aneurysm and persistent MRSA bacteremia suggested an infected aneurysm. Surgical intervention was not considered a treatment option because of the patient’s dementia; thus, conservative treatment was continued. Hemodialysis was discontinued because of the risk of exacerbating the ruptured aneurysm by anticoagulation. The patient suffered massive hemoptysis and subsequently died on day 18. The clinical course is depicted in Fig. 3.
Table 1.
Laboratory data on admission
| Complete blood cell count | Blood chemistry | ||||
|---|---|---|---|---|---|
| WBC | 4400/µL | LDH | 200 U/L | TP | 6.0 g/dL |
| Neutro | 81.6% | AST | 16 U/L | Alb | 3.1 g/dL |
| Eos | 1.2% | ALT | 4 U/L | CRP | 10.0 mg/dL |
| Ba | 0.4% | UA | 4.0 mg/dL | BNP | 1638 mg/dL |
| Mo | 5.6% | BUN | 42 mg/dL | CEA | 2.3 ng/mL |
| Ly | 8.5% | Cre | 4.43 mg/dL | CYFRA | 5.2 ng/mL |
| RBC | 315 × 104/µL | Ca | 8.4 mg/dL | Pro GRP | 118.5 pg/mL |
| Hb | 9.9 g/dL | P | 4.4 mg/dL | HbA1c | 5.2% |
| Ht | 30.8% | Na | 136 mEq/L | ||
| MCV | 97.7% | K | 3.7 mEq/L | ||
| Plt | 11.4 × 104/µL | Cl | 100 mEq/L | ||
Alb albumin, ALT alanine aminotransferase, AST aspartate aminotransferase, Ba basophil, BNP brain natriuretic peptide, BUN blood urea nitrogen, CEA carcinoembryonic antigen, Cre creatinine, CRP C-reactive protein, CYFRA cytokeratin subunit 19 fragment, Eos eosinophil, Hb hemoglobin, Ht hematocrit, LDH lactate dehydrogenase, Ly lymphcyte, MCV mean corpuscular volume, Mo monocyte, Neuro neutrophil, Plt platelet, Pro GRP pro-gastrin-releasing peptide, RBC red blood cell, TP total protein, UA uric acid, WBC white blood cell
Fig. 1.
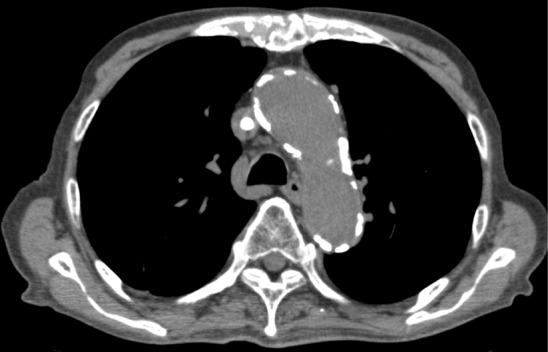
A calcified thoracic artery on admission
Fig. 2.
A ruptured thoracic aneurysm sealed within the left lung; a transverse plane, b sagittal plane
Fig. 3.
Clinical courses of body temperature and C-reactive protein levels. BT body temperature, CRP C-reactive protein, VCM vancomycin, MEPM meropenem
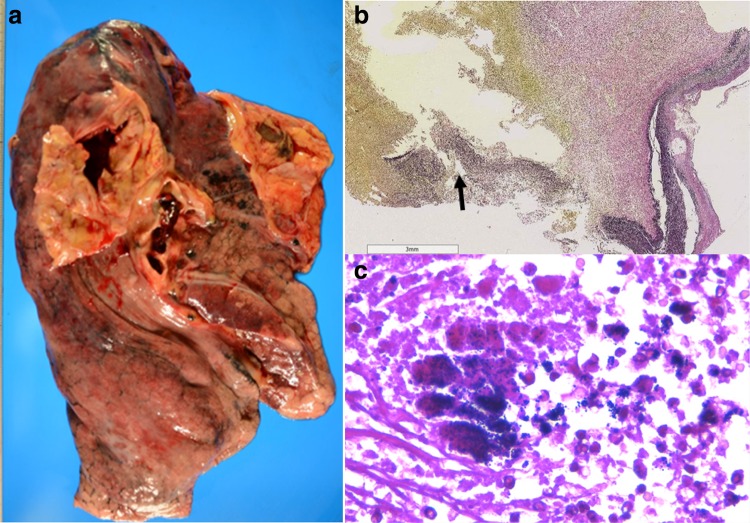
Autopsy revealed invasion by neutrophils and gram-positive cocci of the lamina elastica of the ruptured aneurysm (Fig. 4). Infective endocarditis was not observed. The tumor in the patient’s left lung was confirmed as small-cell carcinoma with 4-mm-diameter metastasis in the left lobe of the liver. There were no malignant cells in the lamina elastica of the ruptured aneurysm, indicating that the tumor was not related to the aneurysm. A definitive diagnosis of an infected thoracic aneurysm associated with TDC-related MRSA bacteremia was made.
Fig. 4.
a An autopsy revealed a ruptured atherosclerotic aneurysm of the arch aorta. b Elastica van Gieson staining showing necrosis of the lamina elastic fibers of the aorta (magnification: ×20) (arrow). c Gram-positive cocci are shown at the indicated area in b with an arrow (magnification: ×400)
Discussion
MRSA bacteremia rarely leads to an infected aneurysm [3]. There are few published cases involving infected aneurysms caused by TDC-related MRSA bacteremia. Generally, bacteremia in hemodialysis patients is caused by infection at the site of vascular access. The risk of access-related infection associated with the use of a TDC is higher than that associated with the use of arteriovenous (AV) fistulas and AV grafts. Infection with coagulase-negative staphylococci and S. aureus account for approximately 80% of the cases in most studies [4]. MRSA has also become an increasingly important pathogen in hemodialysis patients [5].
With TDC-induced bacteremia, the optimal timing for TDC removal has been the subject of discussion. It is a concern because TDC is both the source of the infection and the vascular access required for the ongoing hemodialysis. Catheter salvage, defined as the continuous use of the same catheter throughout an episode of catheter-related bloodstream infection (CRBSI), may be considered in hemodialysis patients with limited vascular access such as in our patient with dementia in whom the TDC was not immediately removed to avoid puncture during hemodialysis. However, catheter salvage should not be used in case of S. aureus, Pseudomonas, and fungal infections; unresolved infection symptoms after 48–72 h of initiation of antibiotics; metastatic complications; and concomitant tunnel infection [6]. Although the TDC was removed on day 3 in our case, we should have removed the TDC earlier. A previous study reported that a delay of > 3 days in the removal of the infection focus is associated with an increased risk of persistent S. aureus bacteremia [odds ratio (OR) 2.18; 95% confidence interval (CI) 1.05–4.55] [7]. Delay in the removal of the TDC may have influenced the outcome. Briefly, when a patient has CRBSI with tunnel infection, the catheter should be immediately removed irrespective of the type of bacterial infection.
Metastatic infection must be considered when bacteremia is persistent after the removal of the catheter and initiation of effective antibacterial agents. An infected aneurysm was the reason for persistent MRSA bacteremia in our patient. S. aureus bacteremia complications in hemodialysis patients usually include infective endocarditis, osteomyelitis, septic arthritis, metastatic abscess, pulmonary emboli, pleural empyema, and meningitis, whereas infected aneurysm is infrequent [8]. There are only three reports in the literature on infected aneurysms caused by TDC-related MRSA bacteremia (Table 2) [3, 9, 10]. In all three cases, the aneurysms were located in the abdominal aorta. An infected aneurysm of the thoracic aorta is rare [11]; this is the first case of an infected thoracic aortic aneurysm associated with TDC-related MRSA bacteremia to be reported.
Table 2.
Previous reports of infected aneurysms associated with TDC-related MRSA bacteremia
| Type of VA | Location | Bacteria | Antibacterial | Location of infected aneurysm | Outcome | |
|---|---|---|---|---|---|---|
| Lee [3] | TDC and forearm AVF | Right IJV | MRSA | Vancomycin and rifampicin | Abdominal aorta | Death |
| Matsui [9] | TDC | Right IJV | MRSA | Cefazolin changed to vancomycin | Abdominal aorta | Death |
| Yoon [10] | TDC | Right IJV | MRSA | Vancomycin | Abdominal aorta | Resolved by extra-anatomic reconstruction |
AVF arteriovenous fistula, IJV internal jugular vein, MRSA methicillin-resistant Staphylococcus aureus, TDC tunneled dialysis catheter, VA vascular access
As evidenced by the CT findings, our patient had a highly calcified arch aorta at the site of the infected aneurysm. In addition, an atherosclerotic aneurysm (20 mm in diameter) was observed in the left iliac artery. The location of the infected aneurysm demonstrated a 10-mm-thick substantial atherosclerosis that was the most prominent among all arteries, with additional minute sporadic atherosclerosis in the aorta. Although the aortic intima is generally highly resistant to infection, atherosclerosis-induced disruption of the intima reduces its resistance to infection. S. aureus has the ability to produce a polysaccharide dextran that allows it to adhere to damaged intima [12]. Although the precise mechanism of bacterial adherence is poorly understood, previously injured sites, such as calcification sites, are more likely to be colonized by S. aureus [13]. Lee [3] and Matsui [9] also proposed that highly atherosclerotic arteries in immunodeficient hemodialysis patients are vulnerable to S. aureus infection. Taken together with these findings, we consider the following mechanism for the formation of infected thoracic aortic aneurysm in our patient: The atherosclerotic plaque may have permitted bacterial passage into the deeper layers of the arterial wall that eventually led to arterial wall infection.
Infected aneurysm is a serious clinical condition that is associated with significant mortality. The major determinants of mortality of infected aneurysms are reportedly advanced age, non-salmonella infection, no surgery, severe aortic infection, aneurysm rupture, suprarenal location, and shock [14, 15]. Mortality among patients who are managed without surgical repair of an infected thoracic aneurysm is reportedly > 85% [16]. Although the 1-year mortality rate among patients who underwent extra-anatomic reconstruction of a thoracic aneurysm was 50%, a systematic review reported that the 2-year survival rate after endovascular stent grafting is approximately 82% [17]. However, an optimal treatment regimen for repair of an infected aneurysm remains controversial.
In conclusion, this is the first report of an infected aneurysm of a calcified thoracic aorta associated with TDC-related MRSA bacteremia in a hemodialysis patient. It is important to investigate metastatic infection if bacteremia persists, particularly in patients with calcified arteries because it can be a site of infected aneurysm.
Acknowledgements
This work was supported by JSPS KAKENHI Grant numbers 16L08698 to KN.
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from the participant included in this article.
References
- 1.Centers for Disease Control and Prevention Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients-United States, 2005. Morb Mortal Wkly Rep. 2007;56:197–199. [PubMed] [Google Scholar]
- 2.Launay-Vacher V, Izzedine H, Baumelou A, Deray G. FHD: an index to evaluate drug elimination by hemodialysis. Am J Nephrol. 2005;25:342–351. doi: 10.1159/000086591. [DOI] [PubMed] [Google Scholar]
- 3.Lee CC, Ng YY, Chou YH, et al. Mycotic aneurysm of the abdominal aorta in a patient undergoing hemodialysis: an unusual complication of Staphylococcus aureus bacteremia. Clin Infect Dis. 2000;30:823–824. doi: 10.1086/313769. [DOI] [PubMed] [Google Scholar]
- 4.Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004;44(5):779–791. doi: 10.1016/S0272-6386(04)01078-9. [DOI] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention Invasive methicillin-resistant Staphylococcus aureus infection among dialysis patients-United States, 2005. Morb Mortal Wkly Rep. 2007;56(9):197–199. [PubMed] [Google Scholar]
- 6.Miller LM, Clark E, Dipchand C, et al. Hemodialysis Tunneled Catheter-Related Infections. Can J Kidney Health Dis. 2016;3:1–11. doi: 10.1177/2054358116669129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong YP, Park SJ, Kim HS, et al. Persistent Staphylococcus aureus bacteremia. A prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine. 2013;92:98–108. doi: 10.1097/MD.0b013e318289ff1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marr KA, Kong L, Fowler KVGA, et al. Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int. 1998;54:1684–1689. doi: 10.1046/j.1523-1755.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsui S, Hatta T. Mycotic abdominal aortic aneurysm in a dialysis patient with catheter-related methicillin-resistant Staphylococcus aureus bacteremia. Ther Apher Dial. 2011;15:113–114. doi: 10.1111/j.1744-9987.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoon WJ, Conley A, Herrera S, et al. Ruptured mycotic abdominal aortic pseudoaneurysm in a patient on hemodialysis complicated with oxacillin-resistant Staphylococcus aureus bacteremia. Ann Vasc Surg. 2016;35:204.e1–204.e4. doi: 10.1016/j.avsg.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Lopes RJ, Almeida J, Dias PJ, et al. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32:488–490. doi: 10.1002/clc.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal A, Singh KP, Jain P. Medical significance and management of staphylococcal biofilm. FEMS Immunol Med Microbiol. 2010;58:147–160. doi: 10.1111/j.1574-695X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuypers JM, Proctor RA. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu RB, Chen RJ, Wang SS, Chu SH. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004;40:30–35. doi: 10.1016/j.jvs.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T, Hirota M, Kondo S, et al. Post-thoracic endovascular aortic repair complicated with mycotic aneurysm rupture, repaired by redo thoracic endovascular aortic repair. Ann Vasc Surg. 2016;33:228.e1–228.e4. doi: 10.1016/j.avsg.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Weis-Muller BT, Rascanu C, Sagban A, et al. Single-center experience with open surgical treatment of 36 infected aneurysms of the thoracic, thoracoabdominal, and abdominal aorta. Ann Vasc Surg. 2011;25:1020–1025. doi: 10.1016/j.avsg.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46:906–912. doi: 10.1016/j.jvs.2007.07.025. [DOI] [PubMed] [Google Scholar]