Abstract
Spinal muscular atrophy (SMA) is a genetic neuromuscular disease characterized by progressive muscle weakness and atrophy. We report a case of a 36-year-old man with SMA type 3 who presented to our emergency department with epigastric pain and vomiting. He was found to have severe ketoacidosis on laboratory evaluation. The patient’s symptoms and ketoacidosis resolved after dextrose infusion and a relatively small amount of sodium bicarbonate infusion. Given the severity of the ketosis that seemed inconsistent with moderate starvation alone, we postulate that there must have been other contributing factors besides moderate starvation that might explain the severity of acidosis in this particular patient. These factors include low muscle mass, disturbed fatty acid metabolism, hormonal imbalances and defective glucose metabolism. Ketoacidosis is an under-recognized entity in patients with neuromuscular diseases and requires a high index of suspicion for prompt diagnosis and management.
Keywords: Ketoacidosis, Neuromuscular disease, Glucose metabolism, Spinal muscular atrophy
Introduction
Spinal muscular atrophy (SMA) is a genetic neuromuscular disease characterized by progressive muscle weakness and atrophy. It is inherited through an autosomal-recessive mutation of the survival motor neuron 1 (SMN1) gene on chromosome 5q13.2, leading to a deficiency of the SMN1 protein [1]. There are five different phenotypes of SMA (type 0–4) that seem to be related to an SMN2 gene adjacent to SMN1 gene on chromosome 5 [2]. Although SMA is a neuromuscular disease, several metabolic derangements have been described in those patients. We, hereby, present the case of a young man with SMA type 3 who developed severe ketoacidosis.
Case report
A 36-year-old man, wheel chair bound, secondary to genetically confirmed SMA type 3, presented to our emergency department with numerous episodes of vomiting (16 times) and epigastric pain of a 1-day duration. The patient reported decreased food intake in the preceding week in an attempt to lose weight. His diet consisted mainly of proteins and vegetables with minimal carbohydrates in the 48 h prior to his presentation. His last meal which was 16 h prior to the onset of his symptoms consisted of chicken, avocado and a salad. The patient denied any fever, chills, diarrhea, polyuria, polydipsia, or alcohol intake. He denied any sick contacts or recent travel history. He was not on any medications and denied any prior similar episodes. He denied any physical or psychological stressors. On physical examination, the patient’s vital signs showed a blood pressure of 132/84 mmHg, heart rate of 118 beats/min, temperature of 37 °C, and a respiratory rate of 29 breaths/min. His weight was 71 kg, his height 174 cm and his BMI 23 kg/m2. Apart from significant muscle atrophy and weakness in all four extremities, his physical examination was unremarkable. Specifically, his abdomen was soft, non-tender, and non-distended with no evidence of organomegaly.
On laboratory evaluation in the emergency department, the patient’s hemoglobin was 14.2 mg/dL and white blood cell count was 17,200/mm3 (upper limit of normal is 11,000/mm3). Serum creatinine was 0.2 mg/dL (baseline 0.1 mg/dL) and serum electrolytes were as follows: sodium was 139 mmol/L, potassium 4.1 mmol/L, chloride 95 mmol/L and bicarbonate 8 mmol/L. The patient was noted to have a high anion gap metabolic acidosis combined with metabolic alkalosis (Table 1). His serum albumin was normal at 3.9 g/L. His bicarbonate deficit was 1136 mEq. Serum ketone level (β hydroxy butyrate) was elevated to a value of 75.7 mg/dL (0.0–5.0 mg/dL). Serum lactate was normal at 0.96 mmol/L (0.55–2.20 mmol/L). Liver enzymes were also normal including: ALT 22 IU/L and g-GT 25 IU/L. Serum lipase was 20 U/L (13–60 U/L). Blood glucose level was 79 mg/dL and hemoglobin A1c was 4.6%. Further investigation showed a normal serum osmolar gap. Salicylate and acetaminophen levels were negligible at < 3 and < 5 mg/L, respectively. An enhanced computed tomography scan of the abdomen and pelvis revealed no acute abdominal process.
Table 1.
Laboratory values upon presentation and after treatment with dextrose water 50% and sodium bicarbonate
| Laboratory value | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Serum pH | 7.09 | 7.36 | 7.43 |
| pCo2 (mmHg) | 18.8 | 27 | 34.8 |
| Serum HCO3 (mmol/L) | 8 | 15 | 23.2 |
| Anion gap (mmol/L) | 36 | 28 | 15 |
| Delta anion gap/delta HCO3 | 1.5 | – | – |
| Serum lactate (mmol/L) | 0.96 | – | – |
| Serum ketone (mg/dL) | 75.7 | 43.2 | 2 |
| Serum osmolality (mOsm/kg) | 300 | – | 289 |
| Serum potassium (mmol/L) | 4.1 | 2.5 | 3.8 |
| Serum phosphorus (mmol/L) | 3.9 | 0.7 | 1.4 |
| Serum hemoglobin (g/dL) | 14.2 | 9.5 | 10.1 |
| Serum Creatinine (g/dL) | 0.2 | 0.2 | 0.2 |
(–), not applicable or not available
The patient was admitted to the hospital and received intravenous hydration for volume repletion. One liter of 0.9% saline per hour was given in the first 2 h then 1 L every 3 h for 6 h. For his acidosis, he was given 50% dextrose and sodium bicarbonate, a total of 240 mEq over 24 h with 160 mEq in the first 4 h. Proton pump inhibitors and anti-emetics were also administered for symptomatic management of his vomiting and epigastric abdominal pain. The patient felt much better and his laboratory values corrected appropriately as demonstrated in Table 1.
Initially, when the patient was first evaluated, we considered gastrointestinal etiologies such as gastroenteritis or pancreatitis as potential causes of his epigastric pain and vomiting. However, after the laboratory results revealed a high anion gap metabolic acidosis (HAGMA), the differential diagnosis broadened to include lactic acidosis, ketoacidosis from alcohol, diabetes mellitus or starvation, and toxic ingestions such as methanol, ethylene glycol, diethylene glycol, propylene glycol and aspirin. Other rare causes of HAGMA including d-lactic acidosis and pyroglutamic acid were also entertained.
First, lactic acidosis was ruled out when the patient’ serum lactate returned back as normal. Renal tubular acidosis (RTA) type II was considered because of low serum potassium and phosphorus but the high anion gap defies RTA. The patient denied any toxic ingestions and his serum osmolality was normal. High ketones further narrowed our differential diagnosis. Glycogen storage diseases secondary to inborn errors of metabolism were eliminated from our differential in view of the patient’s history of fasting without any prior complications (observing Ramadan for religious reasons). Euglycemic ketoacidosis has been reported with a normal sugar level in diabetics or in those receiving sodium–glucose cotransporter-2 (SGLT-2) inhibitors, both of which were not present in our patient. The combined metabolic alkalosis upon presentation was secondary to vomiting with loss of gastric secretions rich in hydrogen ions.
After hydration and volume expansion, the patient’s acidosis rapidly resolved with a relatively small amount of sodium bicarbonate, and his ketone levels slowly decreased. The patient’s abdominal pain and vomiting completely resolved the day after admission. Three days after admission, all his laboratory parameters normalized and the patient was discharged home. Upon discharge, he was advised to avoid fasting and to ingest a balanced diet. His laboratory values 2 weeks after his hospitalization were as follows: BUN 9 mg/dL, creatinine 0.1 mg/dL, sodium 143 mmol/L, potassium 4 mmol/L, chloride 104 mmol/L, HCO3 24 mmol/L and hemoglobin 11.6 g/dL.
Discussion
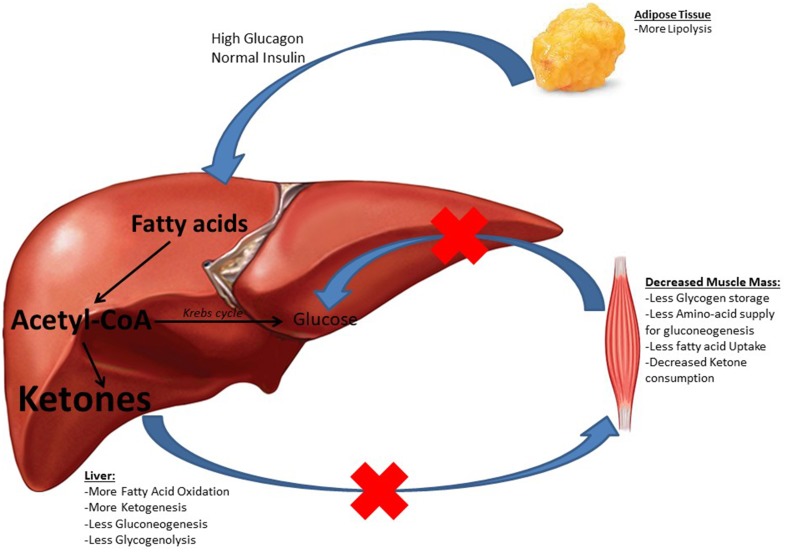
We presented a case of a 36-year-old male with SMA type 3 who presented with vomiting and was found to have severe ketoacidosis. After eliminating other major causes of acidosis and because the severity of the episode was inconsistent with that of moderate starvation alone, we postulate that there must have been other contributing factors besides moderate starvation that help explain the severity of acidosis in this particular patient. Ketosis seems to be more severe in myopathic patients than their normal counterparts even after equal duration of fasting [3]. Our review of the literature revealed only one case report of a patient with SMA type 2 who experienced recurrent stress-induced ketoacidosis [4]. No previous SMA type 3 patient has been reported to have this complication. Several elements might have contributed to ketoacidosis in our patient including a low muscle mass, disturbed fatty acid metabolism, hormonal imbalances and defective glucose metabolism (Fig. 1).
Fig. 1.
Proposed mechanisms for abnormal glucose and fatty acid metabolism in patients with SMA
Glucose is the main energy supplier for the body. In periods of glucose scarcity (fasting, starvation, intense exercise), the body turns towards ketone body production as the main source of energy [5]. Muscle fibers are the most to utilize ketones. In states of decreased muscle mass, such as in SMA, ketone consumption decreases resulting in ketosis. Nonetheless, the muscles are a major site for glycogen storage. They also serve as a pool of amino acids that are readily available for the liver to be converted to glucose upon demand. In patients with atrophic muscles, the supply diminishes and the body switches towards lipolysis and ketone body formation.
Few studies have shown an abnormal fatty acid metabolism in patients with SMA [6, 7]. Experimental studies suggested that denervated muscles have altered metabolism and response to hormones resulting in decreased uptake and utilization of fatty acids by these muscles [8]. Subsequently, this leads to a decrease in muscle fatty acid concentrations. Loss of neuronal influence on muscle β-oxidation leads to increased levels of acetyl-CoA in the liver which is precursors of ketone bodies.
Moreover, hormonal imbalances make SMA patients prone to ketoacidosis. A recent study by Bowerman et al. highlights an important function of the SMN gene in pancreatic islet development and glucose metabolism [9]. Bowerman et al. demonstrated that mice with SMA type 1 have predominantly glucagon-producing α cells in the pancreas with low-insulin-producing β cells. Similar results were noted in human subjects when autopsies of 6 babies with SMA type 1 were performed. Bowerman et al. also proved that mice with SMA type 1 had hyperglucagonemia with normal insulin levels. Extrapolating from the genetic similarity of SMA types 1 and 2 (as both have a deficiency in the SMN protein), patients with SMA type 3 may have the same abnormality, the extent of which is yet to be determined. Effectively, inappropriately high glucagon concentrations cause more acetyl-CoA, resulting from the beta-oxidation of fatty acids, to be converted into ketone bodies contributing to ketoacidosis in patients with SMA [3].
The onset of our patient’s symptoms at a later stage of life in spite of prior fasting (for 20 h sometimes) suggests either further slow decline in his muscle mass or a deterioration of his pancreatic function with time. The exaggerated ketoacidosis response to a period of relatively moderate starvation raised our suspicion for other inherent factors that might be playing a role in the pathogenesis of ketoacidosis in this particular set of patients. Neither glucagon nor insulin levels were measured in our patient so a further decline in his pancreatic function, which can be due to an increase in α cells and/or decrease in β cells, cannot be proven. Since SMA type 3 is less devastating than SMA type 1 and because it is associated with prolonged survival, it would be reasonable to produce mice models with this genetic mutation and take pancreatic samples at different ages, to compare the number of α and β cells and assess for any hormonal imbalances. Patients with SMA are prone to metabolic abnormalities; however, literature is scarce and hence arises the need for experimental models and human studies to highlight such gap.
Learning points
SMA patients are prone to metabolic derangements such as ketoacidosis.
Several factors might contribute to ketoacidosis in SMA patients. These include decreased muscle mass, defects in endocrine pancreatic function and abnormal fatty acid metabolism regardless of triggering factors.
Ketoacidosis in SMA can be easily managed with timely recognition from clinicians.
Conflict of interest
The authors report no conflict of interest.
Research involving human participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
An informed consent was obtained from the patient reported.
References
- 1.Kolb SJ, Kissel JT. Spinal muscular atrophy: a timely review. Arch Neurol. 2011;68(8):979–984. doi: 10.1001/archneurol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darras BT. Spinal muscular atrophies. Pediatr Clin N Am. 2015;62(3):743–766. doi: 10.1016/j.pcl.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Paulson DJ, et al. Ketogenic effects of carnitine in patients with muscular dystrophy and cytochrome oxidase deficiency. Biochem Med Metab Biol. 1988;39(1):40–47. doi: 10.1016/0885-4505(88)90056-4. [DOI] [PubMed] [Google Scholar]
- 4.Mulroy E, Gleeson S, Furlong MJ. Stress-induced ketoacidosis in spinal muscular atrophy: an under-recognized complication. J Neuromuscul Dis. 2016;3(3):419–423. doi: 10.3233/JND-160171. [DOI] [PubMed] [Google Scholar]
- 5.Paulson DJ, Hoganson GE, Traxler J, Sufit R, Peters H, Shug AL. Ketogenic effects of carnitine in patients with muscular dystrophy and cytochrome oxidase deficiency. Biochem Med Metab Biol. 1988;39(1):40–47. doi: 10.1016/0885-4505(88)90056-4. [DOI] [PubMed] [Google Scholar]
- 6.Tein I, Sloane AE, Donner EJ, Lehotay DC, Millington DS, Kelley RI. Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatr Neurol. 1995;12(1):21–30. doi: 10.1016/0887-8994(94)00100-G. [DOI] [PubMed] [Google Scholar]
- 7.Crawford TO, Sladky JT, Hurko O, Besner-Johnston A, Kelley RI. Abnormal fatty acid metabolism in childhood spinal muscular atrophy. Ann Neurol. 1999;45(3):337–343. doi: 10.1002/1531-8249(199903)45:3<337::AID-ANA9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Guth L. “Trophic” influences of nerve on muscle. Physiol Rev. 1968;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- 9.Bowerman M, Swoboda KJ, Michalski JP, Wang GS, Reeks C, Beauvais A, et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol. 2012;72(2):256–268. doi: 10.1002/ana.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]