Abstract
We encountered a case of gemcitabine (GEM)-induced secondary thrombotic microangiopathy (TMA) with nephrotic syndrome. Advanced pancreatic cancer with liver metastasis had originally been diagnosed. Renal biopsy showed focal reduplication of the glomerular basement membrane, endothelial cell swelling, and narrowed capillary lumens with fragmented erythrocytes and fibrin deposition, compatible with TMA. Regular monitoring of renal function during GEM treatment and discontinuation of treatment if acute kidney injury (AKI) might occur is crucial, because AKI combined with TMA is life-threatening.
Keywords: Thrombotic microangiopathy, Gemcitabine, AKI, Nephrotic syndrome
Introduction
Thrombotic microangiopathy (TMA) is a rare but serious disease with pathologic features including widening of the subendothelial space with detachment of endothelial cells from the glomerular basement membrane, intraluminal thrombi, glomerular mesangiolysis, thickening of the vessel wall, and microvascular obstruction. Clinically, TMA includes thrombotic thrombocytopenic purpura (TTP), Shiga toxin-producing Escherichia coli (STEC)–hemolytic uremic syndrome (HUS), atypical HUS (aHUS), and secondary TMA.
Gemcitabine (GEM) is a nucleoside analog used against various cancers, including pancreatic cancer, breast cancer, ovarian cancer, non-small cell lung cancer, and bladder cancer. The major side effects of GEM are myelosuppression, mild liver dysfunction, respiratory failure, gastroenterological symptoms, and elevation of blood pressure. In addition, cases of GEM-associated secondary TMA have been reported [1].
Here we report a case of GEM-induced acute kidney injury (AKI) accompanied by nephrotic syndrome, hemolytic anemia and thrombocytopenia. Since few reports have described the nephrotic range of proteinuria in GEM-induced secondary TMA, renal biopsy was performed to clarify pathological changes such as severe endothelial cell injury in the present case.
Case report
A 46-year-old woman was hospitalized with severe cough, systemic edema, hypoventilation (SpO2 93%) and high blood pressure (175/108 mmHg). Four days after admission, she was transferred from the Department of Surgery to the Department of Nephrology for the treatment of AKI. Advanced pancreatic cancer with liver metastasis had originally been diagnosed before this admission, and administration of GEM was started at another local clinic. The dose of GEM was started at 400 mg/day and increased to 600 mg/day. She received a total 11,000 mg of GEM over the course of 8 months. She had no medical history of renal disease before GEM therapy was started, at which point she had normal kidney function (serum creatinine 0.53 mg/dL) and no proteinuria.
The major laboratory findings on admission are shown in (Table 1). Electrocardiography showed no abnormalities, with a heart rate of 86 beats/min. Chest X-ray disclosed a cardiothoracic ratio of 46% and mild bilateral pleural effusion.
Table 1.
Laboratory findings on admission
| TP | 5.0 | g/dl | Ca | 8.1 | mg/dl | Blood gas analysis (shunt) | ||
| Alb | 2.9 | g/dl | P | 5.2 | mg/dl | pH | 7.362 | |
| T-bil | 1.7 | mg/dl | CRP | 10.42 | mg/dl | HCO 3 | 20.9 | mmol/L |
| AST | 47 | U/L | WBC | 6420 | /µl | BE | −3.5 | mmol/L |
| LDH | 1172 | U/L | Hb | 6.5 | g/dl | HPT | 10.8 | mg/dl |
| CK | 101 | U/L | Ht | 20.7 | % | Coombs test | Neg | |
| BUN | 24.9 | mg/dl | MCV | 101.0 | Pg | Fe | 57 | µg/dl |
| Cr | 1.54 | mg/dl | Plt | 6.1 | × 104/µl | TSAT | 30.6 | % |
| UA | 2.7 | mg/dl | PT | 11.7 | s | FER | 217.1 | ng/ml |
| Na | 142 | mEq/L | APTT | 24.0 | s | ret RBC | 1.6 | % |
| K | 4.2 | mEq/L | Fib | 237.6 | mg/dl | Fecal oc bld | Neg | |
| Cl | 109 | mEq/L | ATIII | 93.1 | % | Eosino | Neg | |
| HbA1C | 3.6 | % | IgG | 771.8 | mg/dl | U blood | 3+ | |
| P,C-ANCA | Neg | IgA | 157.1 | mg/dl | Urinary sediment | |||
| CA19-9 | 25.6 | U/ml | IgM | 74.1 | mg/dl | RBC 50–99/HPF, WBC 50–99/HPF, Granular cast (+) | ||
| DUPAN | 96 | U/ml | T-IgE | 226.0 | IU/ml | U Pro | 7.83 | g/day |
| U β2MG | 662 | µg/L | ||||||
Bold indicates a value higher than the normal reference range. Italic indicates a value lower than the normal reference range
Taken together, AKI accompanied by nephrotic syndrome (NS), hemolytic anemia and thrombocytopenia were confirmed on admission. Neither fever nor neurological symptoms were observed. A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADTAMTS13) activity was 63.1% (normal reference > 70%) and ADAMATS13 inhibitor screening yielded negative results. GEM-induced TMA was considered the most probable diagnosis, and GEM was therefore, discontinued. Drug lymphocyte stimulation testing for GEM yielded an indeterminate (+/−) result. The patient was treated with rest and protein restriction to conservatively improve AKI, resulting in amelioration of thrombocytopenia and kidney dysfunction (Fig. 1). Because GEM-induced TMA seldom presents with NS [2, 3], renal biopsy was performed after red blood cell transfusion.
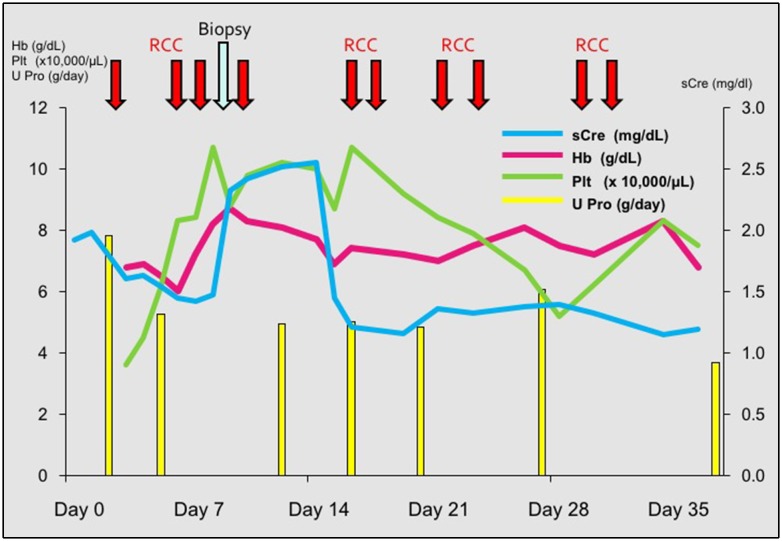
Fig. 1.
Clinical course. The patient initially presented with proteinuria (about 8 g/day), which gradually decreased. As serum creatinine (sCre) increased unexpectedly on day 7, renal biopsy was performed to assess the cause of AKI. Hb hemoglobin, Plt platelet count, U Pro urinary protein, RCC red cell concentrate transfused
Biopsy specimens included 31 glomeruli and 2 of them showed global sclerosis in light microscopy (Fig. 2). No segmental sclerosis was found in any glomerulus. Glomeruli showed diffuse global changes of endocapillary proliferation and endothelial cell swelling. Moreover, segmental double-layered basement membranes were diffusely identified. Mesangial proliferation (lobulation), crescent formation and epithelial proliferation were not found. No deposition was identified in PAM-Masson stain. Several renal tubules were atrophic, and lymphoplasmacytes and a few eosinophils focally and mildly infiltrated the interstitium. Immunofluorescence staining showed IgG(−), IgA(−), IgM(periphery+), C1q(−), C3(periphery+), and C4(−). In electron microscopy, glomeruli showed endocapillary cell proliferation and endothelial swelling with mild increase of mesangial matrix. In subendothelial and paramesangial areas, there were lots of heterogeneous dense materials, probably exudation as well as filamentous materials, namely fibrin deposition. Capillary lumens were narrowed and hardly identified. Foot processes were preserved and slight swelling of epithelial cells were identified. These renal biopsy findings were compatible with TMA. Proteinuria gradually ameliorated with supportive therapy. The patient was discharged 36 days after admission, and kidney function eventually normalized (estimated glomerular filtration rate > 70 ml/min/1.73 m2) by the 3-month follow-up. Urinary protein gradually decreased after discharge. It was 3.64 g/day just after discharge, and 0.87 g/day 1 month later. Urinary protein finally decreased to 0.54 g/day at 3 months follow-up.
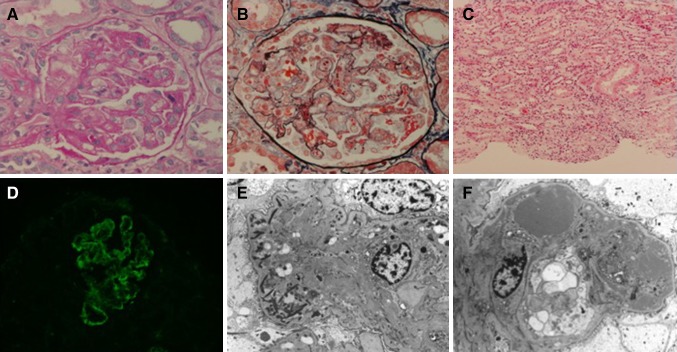
Fig. 2.
Renal biopsy findings. a Periodic acid–Schiff staining. The glomerulus shows thickening of the glomerular capillary walls. b Periodic acid methenamine silver (PAM)/Masson staining. Focal reduplication of the glomerular basement membranes with fragmented erythrocytes is seen. c Periodic acid–Schiff staining. Focal lymphocyte infiltration is clearly apparent in the interstitial tissue. d Immunofluorescence microscopy. Non-specific positive IgM are seen in the subendothelial and mesangial areas, corresponding to deposits. e Electron micrograph of a glomerular basement membrane. Fibrins are clearly recognizable in the capillary loop. f Endothelial cell swollen with electron-dense deposition at the subendothelial area, indicating severe endothelial cell injury
Discussion
TMA is a pathophysiological disease with: (1) microangiopathic hemolytic anemia (MAHA); (2) consumptive thrombocytopenia; and (3) organ injury with platelet-induced microvascular occlusion. The concept of TMA generally includes TTP, STEC-HUS, aHUS, and secondary TMA, although no international consensus has yet been determined [4, 5].
HUS is a thrombotic disease characterized by the triad of: (1) microangiopathic hemolytic anemia; (2) thrombocytopenia; and (3) AKI. The classic form of HUS, also known as diarrhea-positive (D+) HUS, is a disease of infants and children caused by STEC. This form represents 90% of all HUS. Stool culture to detect STEC helps diagnosis. TTP is generally diagnosed with significant reduction of ADAMTS13 activity (< 10%) [6]. However, 5–12% of HUS is aHUS, formerly known as diarrhea-negative (D−) HUS; this form is related to complement dysregulation. Not only complement factor H, but also C3, factor B, complement factor I, CD46, and others have been reported as dysregulated in aHUS [7].
Secondary TMA has been associated with a variety of triggers, including bacterial and viral infections, drugs, malignancy, maternity, and systemic disease. Drug-induced secondary TMA is caused by chemotherapeutic agents such as mitomycin C, cisplatin, and GEM. The present patient had a history of malignancy treated with GEM, compatible with secondary TMA.
Among TMA, STEC-HUS and aHUS are known as the major causes of kidney injury. Malignancy-related secondary TMA has generally been reported with gastric, lung, ovarian, breast, and prostate cancer [8]. This patient presented with proteinuria (7.8 g/day; nephrotic range) and urinary protein selectivity was low (> 0.2). GEM-induced TMA appears quite rare (0.25–0.4% among patients using GEM) [9]. Phase 2 trials of GEM reported World Health Organization grade 1–2 proteinuria in 58% of patients [1, 10]. Only a small number of reports have described the nephrotic range of proteinuria in GEM-induced TMA according to the literature [1–3]. To the best of our knowledge, only one report has described NS during GEM treatment, although renal biopsy showed membranous nephropathy rather than TMA [11]. Therefore, we cannot deny that TMA in the present case might have been complicated or triggered by some other glomerular disease which often causes NS, such as membranous nephropathy and focal segmental glomerulosclerosis though no typical findings of these diseases were disclosed by renal biopsy. A recent report detected excretion of podocytes in urine among 2 cases with TMA and advanced cancer that rapidly developed AKI and NS [12]. This indicates that severe endothelial cell dysfunction during the acute phase of glomerular TMA can lead to podocyte loss. In the present case, endothelial cells were swollen and electron-dense deposits were seen in the subendothelial area, indicating severe endothelial cell injury that might have led to some podocyte injury (Fig. 2f).
Another reason to perform renal biopsy was that in the present case, AKI was found with rapid elevation of serum creatinine levels from the normal range. AKI might rapidly progress to irreversible end-stage renal disease and fatal multiple-organ failure in several days. We therefore, considered renal biopsy as necessary in this patient after treating anemia and thrombocytopenia with blood transfusion. Findings from renal biopsy specimen were compatible with TMA, mildly complicated by interstitial nephritis (Fig. 2c).
GEM can result in direct injury to the endothelium and cause TMA [13]. The detailed mechanisms remain unclear, but prostacyclin has been suggested to decrease the secretion of von Willebrand factor, subsequent platelet aggregation and endothelial cell injury [9]. Because AKI is life-threatening and expected to increase in aging societies with the use of anti-cancer agents [14, 15], the risk of TMA with GEM has been noted in the United States’ Food and Drug Administration (FDA)-approved information to evaluate renal function. Onset of TMA has been reported to occur after 5–8 months of treatment [9]. Newly onset hypertension after GEM treatment have been reported as common clinical symptom of GEM–TMA. The present case also showed elevated blood pressure. Discontinuation of GEM, anti-hypertensive therapy, plasma exchange, and dialysis have been suggested as treatments for GEM-induced TMA [1].
Conclusion
Here we have reported a very rare case of biopsy-confirmed GEM-induced TMA with NS. Monitoring blood pressure and renal function is crucial when administering GEM, because TMA combined with AKI obviously represents an emergency.
Acknowledgements
We wish to thank Dr. Makoto Tokuhara from the Department of Surgery at the National Center for Global Health and Medicine, Tokyo, Japan, for referring the patient, and Dr. Makoto Mochizuki from the Department of Pathology at Teikyo University Hospital, Tokyo, Japan for his advice regarding the pathology. This study was partly supported by overseas research fellowships (to D.K) from Uehara Memorial Foundation and 46th KANAE grants (to D.K) from the KANAE Foundation for the Promotion of Medical Science.
Conflict of interest
Authors have declared that no conflict of interest exists for this work.
Ethical statement
All of the treatment and the examination followed the guideline laid down in the Declaration of Helsinki.
Informed consent
Informed consent for the treatment and the renal biopsy was obtained from the patient.
References
- 1.Humphreys BD, Sharman JP, Henderson JM, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004;100:2664–2670. doi: 10.1002/cncr.20290. [DOI] [PubMed] [Google Scholar]
- 2.Lee HW, Chung MJ, Kang H, et al. Gemcitabine-induced hemolytic uremic syndrome in pancreatic cancer: a case report and review of the literature. Gut Liver. 2014;8:109–112. doi: 10.5009/gnl.2014.8.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada Y, Suzuki K, Nobata H, et al. Gemcitabine-induced hemolytic uremic syndrome mimicking scleroderma renal crisis presenting with Raynaud’s phenomenon, positive antinuclear antibodies and hypertensive emergency. Intern Med. 2014;53:445–448. doi: 10.2169/internalmedicine.53.1160. [DOI] [PubMed] [Google Scholar]
- 4.Scully M, Goodship T. How I treat thrombotic thrombocytopenic purpura and atypical haemolytic uraemic syndrome. Br J Haematol. 2014;164:759–766. doi: 10.1111/bjh.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokiranta TS. HUS and atypical HUS. Blood. 2017;129:2847–2856. doi: 10.1182/blood-2016-11-709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah N, Rutherford C, Matevosyan K, et al. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP) Br J Haematol. 2013;163:514–519. doi: 10.1111/bjh.12569. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Nangaku M, Hataya H, et al. Clinical guides for atypical hemolytic uremic syndrome in Japan. Clin Exp Nephrol. 2016;20:536–543. doi: 10.1007/s10157-016-1276-6. [DOI] [PubMed] [Google Scholar]
- 8.Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore) 2012;91:195–205. doi: 10.1097/MD.0b013e3182603598. [DOI] [PubMed] [Google Scholar]
- 9.Blake-Haskins JA, Lechleider RJ, Kreitman RJ. Thrombotic microangiopathy with targeted cancer agents. Clin Cancer Res. 2011;17:5858–5866. doi: 10.1158/1078-0432.CCR-11-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green MR. Gemcitabine safety overview. Semin Oncol. 1996;23:32–35. [PubMed] [Google Scholar]
- 11.Ata A, Gurses I, Kiykim A, et al. Nephrotic syndrome associated with gemcitabine use in a patient with ovarian cancer. Am J Case Rep. 2012;13:268–270. doi: 10.12659/AJCR.883583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usui J, Yokoyama C, Hagiwara M, et al. The detection of urinary podocytes from drug-induced glomerular thrombotic microangiopathy in advanced cancer patients. Clin Lab. 2016;62:2413–2417. doi: 10.7754/Clin.Lab.2016.160525. [DOI] [PubMed] [Google Scholar]
- 13.Brocklebank V, Wood K, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol 2017; in press. [DOI] [PMC free article] [PubMed]
- 14.Katagiri D, Hamasaki Y, Doi K, et al. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J Am Soc Nephrol. 2013;24:2034–2043. doi: 10.1681/ASN.2013020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katagiri D, Hamasaki Y, Doi K, et al. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int. 2015;89:374–385. doi: 10.1038/ki.2015.327. [DOI] [PubMed] [Google Scholar]