Abstract
Mood disorders are associated with significant psychosocial and occupational disability. It is estimated that major depressive disorder (MDD) will become the second leading cause of disability worldwide by 2020. Existing pharmacological and psychological treatments are limited for targeting cognitive dysfunctions in mood disorders. However, growing evidence from human and animal studies has shown that treatment with erythropoietin (EPO) can improve cognitive function. A recent study involving EPO-treated patients with mood disorders showed that the neural basis for their cognitive improvements appeared to involve an increase in hippocampal volume. Molecular mechanisms underlying hippocampal changes have been proposed, including the activation of anti-apoptotic, antioxidant, pro-survival and anti-inflammatory signalling pathways. The aim of this review is to describe the potential importance of glycogen synthase kinase 3-beta (GSK3β) as a multi-potent molecular mechanism of EPO-induced hippocampal volume change in mood disorder patients. We first examine published associations between EPO administration, mood disorders, cognition and hippocampal volume. We then highlight evidence suggesting that GSK3β influences hippocampal volume in MDD patients, and how this could assist with targeting more precise treatments particularly for cognitive deficits in patients with mood disorders. We conclude by suggesting how this developing area of research can be further advanced, such as using pharmacogenetic studies of EPO treatment in patients with mood disorders.
Mood disorders and cognitive deficits
Mood disorders affect ∼20% of the general population1 and for individuals suffering from a mood disorder, there is a 5–6% lifetime risk of completed suicide2. Major depressive disorder (MDD) is ranked as the third most prevalent condition associated with disability3 and is estimated to be the second leading cause of disability worldwide by 20204. Bipolar disorder (BD) is also on the top ten list of most debilitating mental illnesses3 and is associated with significant psychosocial and occupational disability5. Both mood disorders, MDD and BD, are debilitating and chronic psychiatric disorders that cause significant suffering and burden in individuals with these illnesses and their families and friends, as well as reducing their quality of life6–8.
Treatment of MDD and BD has focused on reducing mood symptoms;9 however, cognitive deficits are a core symptom domain of mood disorders10 that prolongs illness duration and reduces the likelihood of recovery11,12. Cognitive dysfunction also contributes to socio-occupational impairment13,14, which represents the largest economic cost of mood disorders for society15,16. Patients with MDD have consistently displayed difficulties in attention (e.g., in effortful attention, as well as automatic processing), declarative memory (e.g., verbal learning and memory, visuospatial learning and memory and episodic memory), and executive function (e.g., response inhibition, problem solving and planning, verbal fluency, decision-making and mental flexibility)17. These deficits are particularly pronounced in response to information that is emotionally or socially relevant. Similar but more severe deficits, specifically in verbal learning, spatial working memory, set-shifting and sustained attention, have been reported in patients with BD18,19. While neurobiological mechanisms of cognitive impairments in mood disorders are unclear, converging preclinical, human neuroimaging and post-mortem evidence suggest that they may arise from disrupted neuroplasticity and associated structural changes in hippocampal volume20–22. This highlights the potential of novel treatments with direct and lasting effects on neuroplasticity changes to induce enduring structural alterations and effectively alleviate cognitive deficits.
Pharmacological treatments for mood disorders have limited effects on cognitive dysfunction23,24 and are, in some cases, associated with adverse effects on cognition due to anticholinergic, sedative, extrapyramidal and/or blunting effects25, which may exacerbate patients’ persistent cognitive impairments during periods of remission (i.e., when patients are relatively symptom-free)26. Existing cognitive enhancing drugs (i.e., medications aiming to improve cognitive functions) have shown limited pro-cognitive effects in depressed patients27. Among the most promising cognition treatments are vortioxetine, which has shown replicated effects on psychomotor speed in symptomatic MDD28, modafinil that improved some aspects of cognition in a study of remitted MDD29, transcranial direct current stimulation that improved working memory in symptomatic MDD30,31, lurasidone that improved a global measure of cognition in remitted BD32 and erythropoietin (EPO) that improved several cognitive domains in symptomatic MDD and remitted BD33,34. However, despite these promising findings, there are no clinically available effective treatments for cognitive impairment in mood disorders to date35,36. Indeed, many studies have examined the efficacy of existing and novel interventions to reduce cognitive dysfunction in patients with mood disorder;35,36 however, cognition trials in this area have faced some important methodological challenges that may negate the interpretations and significance of findings36,37. Although preliminary evidence showed promising effects of psychological interventions for cognitive dysfunction, such as cognitive remediation in patients with MDD33,38, we recently demonstrated a lack of beneficial effects of this intervention for BD patients in a randomized, controlled clinical trial39. Notably, this trial was limited by a small sample size (n = 44), short follow-up times (12 weeks) and lack of enrichment for the primary outcome (objectively-assessed verbal memory dysfunction). Indeed, emerging evidence indicates that cognitive remediation programs may be useful in BD and there are several ongoing cognitive remediation trials in BD.
Recent randomized, placebo-controlled trials demonstrated that 8 weekly doses of erythropoietin (EPO) reduced cognitive dysfunction in patients with treatment-resistant depression (TRD)33 and in patients with BD in partial remission34. Treatment-resistant depression was defined as lack of remission after ≥ 2 adequate antidepressant treatments with 2 different classes of antidepressant drugs in previous or current depressive episodes33. The improvement of verbal memory after EPO vs. saline treatment across TRD patients and BD patients was of a moderate effect size (change in RAVLT total score, mean [SD]: EPO: 6.4 [8.8]; saline: 2.1 [8.0]; d = 0.54). Structural magnetic resonance imaging (MRI) assessments of patients from these two trials revealed that memory improvement was associated with normalization of volume loss in a subfield of the left hippocampus corresponding to the cornu ammonis 1–3 (CA1–3) and subicilum40. Post hoc exploratory assessments of the mean surface displacement values revealed that the subfield hippocampal volume change was of a large effect size (hippocampal surface displacement, mean [SD]: EPO: 0.04 [0.08]; saline: −0.05 [1.0]; d = 0.90). However, the biological mechanisms linking EPO to increased hippocampal volume in mood disorders remain unknown.
EPO biology
EPO is a glycoprotein hormone cytokine that plays important roles in regulating red blood cell synthesis (i.e., hematopoiesis)41, trafficking of immune cells, anti-apoptotic actions, neurodevelopment42, neuroprotection and cognitive function43,44. EPO and its receptor are expressed in multiple organ systems and have been shown to interact closely with the nervous, vascular, immune and reproductive systems45–47. EPO is produced and secreted predominantly in the kidney, but it is also expressed in brain regions including the hippocampus, amygdala, temporal cortex, prefrontal cortex, internal capsule and midbrain45,48,49 as well as the liver and the uterus47. Expression of EPO and its receptor have also been found in neurons, glial cells, endothelial cells and adult neural progenitor cells. Expression levels are high during human embryonic brain development, but remain present in adulthood45. EPO functions in a hypoxia-sensitive manner meaning that stimuli such as hypoxia and stress (i.e., cellular changes such as hypoglycaemia, electrolyte imbalance, anaemia, infections and loss of endogenous anti-oxidants, etc.) can affect EPO and its receptor45–47, which can have pleiotropic effects in the modulation of apoptotic and immune activities50 as well as neurotrophic and neuroprotection effects46. Specifically, hypoxia-inducible factor (HIF) rapidly upregulates the expression of the EPO receptor, EPO-R, in cells of the Central Nervous System (CNS) and of EPO synthesis by neurons and astrocytes45. Extracellular EPO then binds to EPO-R on the cell membrane, which triggers the intracellular JAK2 (janus kinase 2) signalling. This results in the activation of several signal transduction pathways including STAT5 (signal transducer and activator of transcription 5), PI3K (phosphatidylinositol 3-kinase)/Akt (protein kinase B), NFκB (nuclear factor-κB) and MAPK (mitogen-activated protein kinase). These pathways switch on signalling cascades that lead to long-lasting biological protective and reparative responses, which may be important for future treatment of cognitive impairments in neuropsychiatric disorders including depression46. Specifically, relevant down-steam effects of these signalling cascades include activation of anti-apoptotic, antioxidant and anti-inflammatory signalling in neurons, glial and cerebrovascular endothelial cells, and promotion of dendritic sprouting, neurogenesis, hippocampal brain-derived neurotrophic factor (BDNF) and long-term potentiation51–53. Erythropoietin was also shown to exert neuroprotective effects by inhibiting the activity of the enzyme glycogen synthase kinase 3-beta (GSK3β)54,55, as will be discussed in greater detail later in this review. This may be particularly relevant in relation to mood disorders since GSK3β is a key activator of cell death and other functions involved in mood disorders, hippocampal volume, glucocorticoid regulation and neuroplasticity56–58.
It was a conceptual break-through that systemic administration of high-dose (> 500 International Units [IU]/kg) EPO was shown to cross the blood-brain barrier (BBB)49 and facilitate neuroprotection and neuroplasticity in animal models of neurodegenerative and neuropsychiatric conditions59 in addition to after acute neural injury60–62. While it is unclear whether EPO crosses the BBB via an active transport mechanism or in an unspecific manner, it is evident that systemically administered high-dose EPO enters the brain to an extent that is sufficient for neuroprotection (ibid.). Accordingly, administration of such high doses of EPO to humans (through injections of 40,000–48,000 IU/ml)33,34,63–65 improved brain function and cognition after short-term (1 week) and longer-term (8–12 weeks) treatment. In contrast, short-term administration (3 days) administration of lower-dose EPO (30,000 IU to men of 74 ± 7 kg [mean ± SD]; corresponding to < 500 IU/kg) produced no cognitive benefits in healthy men66 and 12 weeks low-dose EPO treatment (8000 IU/ml) produced no neural or cognitive benefits with schizophrenia63,67. Although no more precise pharmacokinetic or pharmacodynamic studies have been performed, this evidence indicates that high doses of EPO are required for neuroprotection and cognitive enhancement.
EPO has also been used to treat anaemia, ischaemia and reperfusion injuries (i.e., stroke, heart attack)68, neurological disorders (i.e., seizures69, spinal cord ischaemia, Alzheimer’s disease, Parkinson’s disease and demyelinating disease47), and retinal disease47 and neuropsychiatric disorders33,34,46. Thus, knowledge of the underlying mechanisms of EPO may provide important insights for future therapeutic strategies for the treatment of neuropsychiatric, neurodegenerative, inflammatory and autoimmune-related disorders.
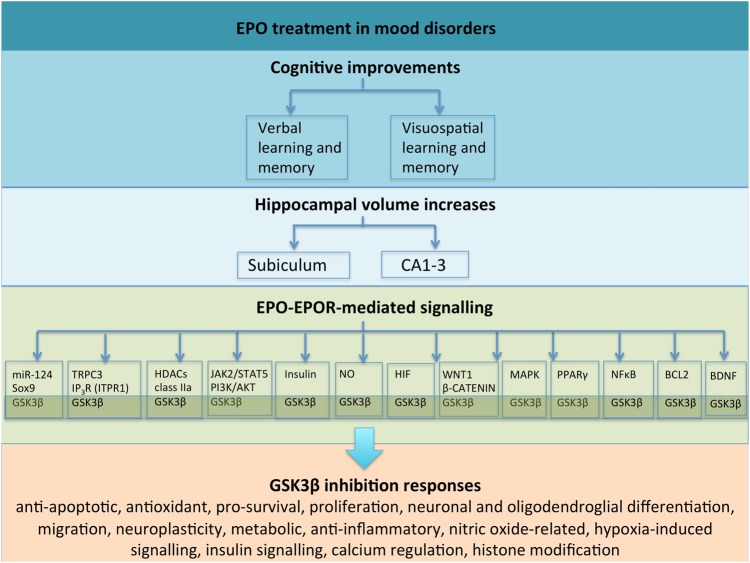
In this review, we highlight evidence collectively suggesting that inhibition of GSK3β acts as a multi-potent molecular mechanism that may mediate multi-potent effects of EPO on hippocampal volume changes in depression (Fig. 1). Understanding the complex relationship between EPO and GSK3β (and its pleiotropic regulatory role across its large genetic network) on cognitive functioning in depressed patients may help reveal new drug targets (both upstream and downstream), aid precision medicine, and ultimately reduce disability and mortality for mood disorders.
Fig. 1. This overview schematic summarizes the complex, interrelated relationships between EPO treatment in mood disorders, cognitive deficits, hippocampal changes and EPO’s potential mechanisms of action through the GSK3β inhibition.
. Notably, complex relationships exist across signalling pathways and molecules, which have not been illustrated
Narrative review search methodology
The following search terms were included in this review: cognition, cognitive functions or dysfunction or impairment or deficits, cognitive enhancers or enhancement, mood disorders, depression, bipolar disorder (BD), major depressive disorder (MDD), treatment-resistant depression (TRD), erythropoiten (EPO), glycogen synthase kinase-3 beta (GSK3β), hippocampus, hippocampal volume or structure, molecular pathway and biology or biological. Several search engines were used, including PubMed and Medline. This review has mainly focused on unipolar and bipolar depression and therefore, only the most recent reviews on other disorders such as neurological and cardiac diseases have been included for references. Two factors led us to choosing a narrative style for the review paper: firstly, to our knowledge, this is the first review paper to bridge these complex interrelated topics in the literature and, secondly, it was not our intention to perform an extensive systematic search for each of the topics independently as this would be an enormous undertaking beyond the scope of our narrative approach.
EPO treatment and cognitive function
Studies in patients with schizophrenia, and multiple sclerosis, have shown that 8–12 weeks of high-dose (40,000–48,000 IU) EPO treatment improves cognitive functioning that lasts for up to 6 months after treatment completion, long beyond red blood cell normalization63,64. This indicates that the pro-cognitive effects of EPO are not directly related to changes in the vascular system. Indeed, the effects of EPO on neurocognitive function in humans seem to be mediated through neurobiological actions rather than indirect increases in red blood cells65,70. In particular, these studies demonstrated that a single high dose of EPO (40,000 IU) versus saline improves neural and cognitive measures of memory and executive functioning in healthy volunteers without affecting red blood cells (ibid.). Based on this evidence, Miskowiak et al.33,34 conducted a randomised, placebo-controlled clinical trial examining the effects of 8 weekly infusions of EPO (40,000 IU) on mood symptoms and cognitive dysfunction in patients with TRD and patients with BD in partial remission. EPO treatment improved verbal memory in TRD patients and speed of complex cognitive processing across attention, memory and executive function in BD patients relative to placebo treatment. These cognitive changes were independent of changes in mood symptoms and were maintained several weeks after red blood cell normalisation at a 6-week follow-up at which time EPO-treated patients displayed structural increase in the left hippocampus40 and changes in task-related neural activity within a fronto-parietal network71,72. Importantly, post hoc analyses showed that the structural hippocampal increase and task-related neural activity change correlated with the observed improvements in EPO-treated patients’ cognitive functions, whereas no influence was found of changes in red blood cells, mood symptoms, diagnosis, age or gender40,71,72.
Effects of EPO have also been demonstrated on neural and cognitive responses to facial expressions in healthy volunteers70,73 and were subsequently replicated in a sample of patients with acute depression74. Long-term EPO treatment did not improve the primary measure of depression severity in an 8-week trial (Hamilton Depression Rating Scale [HDRS] score), but this may be a result of suboptimal statistical power75 and the use of HDRS, which might underestimate other less relevant depressive symptom domain and burden of illness that correlate poorly with depression severity;76 however, improvement in several other depression-relevant outcomes including self-rated depression and quality of life were observed, suggesting that further investigations of the antidepressant efficacy of EPO in larger-scale trials are warranted33. Given this evidence demonstrating the potential impact of EPO on cognitive function and mood symptoms, it is important to elucidate the biological mechanisms underlying alterations of neural processing.
GSK3β: biological mechanism of mood disorders
GSK3β is a highly active proline-directed serine-threonine protein kinase. It contributes to diverse cellular functions including gene expression, neurogenesis, neuroplasticity, cell survival, differentiation, migration, stress responses, cell structure, cell death, the immune system, neurotransmitter systems, metabolism and other functions77–80. GSK3β inhibitors increase proliferation, migration and differentiation of neural stem cells in the adult hippocampal dentate gyrus81. GSK3β is ubiquitously expressed throughout the brain, most prominently in the cerebral cortex and hippocampus (Allen Brain Atlas). GSK3β is a particularly unique protein kinase82 that can be inactivated through the action of various kinases, such as Akt/protein kinase B, protein kinase A and protein kinase C on the ninth position of serine (Ser9)83.
Several neurogenetics studies have investigated associations between GSK3β and mood disorders. The GSK3β gene (GSK3β; OMIM 605004) was mapped to chromosome 3q13.384. Functional single nucleotide polymorphisms (SNPs) have been identified in GSK3β; for example, a promoter T to C polymorphism at position −50 (rs334558) with the T allele having a higher in vitro transcriptional activity and an intron 5T to C polymorphism at position −157 (rs6438552) with the T allele lacking exons 9 and 11 and has been associated with an increased level of GSK3β85. Several studies have investigated genetic variants in GSK3β as risk factors for MDD86,87 and BD88. Other studies have focused on anxiety symptoms in MDD and P300 waveform89, psychotic symptoms in MDD and BD90, age of onset in MDD91 and BD92, suicidal behaviour in MDD93 and combined cases of MDD and schizophrenia patients94. Furthermore, GSK3β polymorphisms have been examined as a predictor of antidepressant response95 and lithium response96,97.
Neuroimaging genetic studies of mood disorders have reported associations between GSK3β variation and hippocampal volume. A genetic association study of numerous GSK3β SNPs and brain-wide grey matter volume using MRI-based voxel-based morphometry was conducted in a sample of 134 patients with recurrent MDD and 144 healthy controls56. Disease modulated associations were reported between grey matter volume in the right hippocampus and bilateral temporal cortex and a functional intronic GSK3β polymorphism, rs6438552. The same direction of association was observed in a larger, independent sample of healthy volunteers between the same GSK3β polymorphism and hippocampal volume using different neuroimaging methods98. This polymorphism has also been associated with altered resting state networks in MDD patients99. Based on in vitro work, this polymorphism alters the splice acceptor site leading to exclusion of exons 9 and 11, which alters the protein’s function to then hyperphosphorylate the substrate, microtubule-associated protein tau85. Further in vivo and in vitro work is required to understand how this modified GSK3β protein regulates other substrates. Additional associations between hippocampal volume and genetic variation involving GSK3β-related pathways and other directly interacting proteins have also been reported57,58.
Identifying putative connections between GSK3β, erythropoietin, hippocampus, cognition and mood disorders
The hippocampus is an important brain region implicated in mood disorders. Specifically, changes in the neural circuitry of the hippocampus have been implicated in cognitive deficits in patients with mood disorders100–102, which may arise in part from the disruption of neuroplasticity67. Disturbance in hippocampal neuroplasticity has been hypothesised to play an aetiological role in mood disorders and may result from chronic inflammatory processes and over-activation of stress responses103–105. This is consistent with evidence showing that stress-induced glucocorticoid production is associated with reduced hippocampal neurogenesis, hippocampal memory deficits and depression-like behaviour in animals106–109. Moreover, a recent meta-analysis110 supported an overall significant hippocampal volume reduction in patients with MDD relative to controls and several additional studies reported hippocampal subiculum shape abnormalities in patients with depression111–113.
The involvement of GSK3β in EPO-mediated neuroprotection via PI3K/AKT is well documented in the literature (e.g. see refs 45,114–116). In the context of primary hippocampal neurons, EPO treatment triggers pro-survival mechanisms by activation of PI3K/AKT45, which suppresses downstream target GSK3β (i.e., by increasing phosphorylation of Ser9 in GSK3β)117. In contrast, PI3K/AKT pathway inactivation results in GSK3β pro-apoptotic functions. In a recent study, Ma and colleagues116 administered exogenous EPO to rats for 4 weeks using an animal model of vascular dementia. Their results indicated improvements in memory impairment, promotion of hippocampal dendritic spine growth as well as deactivation of GSK3β via an EPO-R/JAK2/STAT5/PI3K/Akt/GSK3β pathway116 (Fig. 1).
Another mechanism of action of EPO treatment that could be linked with GSK3β function is through the central role that GSK3β plays in neuronal and oligodendroglial differentiation. A recent study by Hassouna and colleagues118 examined the effects of EPO in young, healthy mice administered EPO for 3 weeks. The authors reported an approximately 20% increase in hippocampal CA1/CA3 neurons and oligodendrocytes, and they detected a significant enhancement of neuronal and oligodendroglial differentiation rather than proliferation118. Using neural stem cells and hippocampal cultures, the authors found that EPO administration decreased the transcription factor Sry-box 9 (Sox9) and increased micro RNA 124 (miR-124). miR-124 is known to regulate Sox9 function and drive neuronal differentiation118. We highlight evidence showing an interconnected relationship between GSK3β, Sox9 and miR-124. Sox9 interacts with GSK3β via its targets in the Wnt signalling pathway119,120. For example, Sox9 inhibits the GSK3β-dependent Wnt/beta-catenin signaling pathway in chondrocyte differentiation by promoting beta-catenin phosphorylation in the nucleus. This finding is in keeping Hassouna and colleagues118 in that EPO inhibits Sox9 although it should be noted that different tissues and models were used and so further investigations are warranted for mood disorders. Other Sox-related genes should also be explored given that, for example, Sox17 regulates the Wnt/β-catenin signaling pathway via GSK3β in oligodendrocyte progenitor cells121. Furthermore, miR-124 co-regulates neuronal differentiation and dendritic architecture via the AKT/GSK3β-dependent pathway122. Its regulation of GSK3β hippocampal expression may have implications for chronic stress and mood disorder pathophysiology123,124. Further evidence has shown that miR-124 regulates HDAC4 and GSK3β expression in the hippocampus, which may have important implications for chronic stress and depression124 and another study identified associations between HDAC4 genetic variation and reduced hippocampal volume in two independent MDD cohorts58. Notably, another class IIa histone deacetylases (HDAC5) has been implicated in the therapeutic action of EPO whereby researchers found that EPO regulates phosphorylation at two different sites stimulating nuclear export of HDAC5 in rat hippocampal neurons125. Collectively and indirectly, these diverse studies provide a plausible link between EPO treatment and its downstream effects on GSK3β function (Fig. 1), which require much greater examination in order to delineate specific and selective effects.
Research has shown that EPO stimulates calcium influx. In terms of biological mechanisms, one study demonstrated that interactions between inositol 1,4,5-trisphosphate (ITPR1; alias, IP3R) and transient receptor potential cation channel subfamily C member 3 (TRPC3) is required for epo-modulated Ca2+ influx, which was reduced under conditions of mutated or deleted IP3R binding sites on TRPC3126. ITPR1 (alias, IP3R) genetic variation was recently associated with reduced hippocampal volume in two independent MDD cohorts, which lead the authors to speculate that mood disorders, and specifically cognitive changes, may involve mechanisms related to ITPR, endoplasmic reticulum (ER) stress, the unfolded protein response (UPR) system and GSK3β signalling56.
Another possible way in which EPO treatment could be linked with GSK3β function is through anti-apoptotic mechanisms. Several biological models have implicated GSK3β as a key activator of cell death79 and so inactivation of GSK3β may therefore promote cell viability. For example, evidence has demonstrated a molecular relationship between EPO, GSK3β and the mitochondrial cell death pathway; EPO suppresses 6-hydroxydopamine (6-OHDA)-induced apoptosis by increasing phosphorylation of Ser9 in GSK3β (i.e., increasing GSK3β inhibition)54. Neuroprotective effects against apoptosis were observed for both EPO and the GSK3B inhibitor 4-benzyl-2-methyl-1, 2,4-thiadiazolidine-3, 5-dione (TDZD8). In contrast, 6-OHDA decreased phosphorylation of Ser9 in GSK3β (i.e., increased GSK3β activity). In this study, decreases in mitochondrial expression of the anti-apoptotic gene B-cell lymphoma 2 (Bcl-2) were also observed (Fig. 1). Other related work has also described a relationship between EPO treatment, increased phosphorylation of Ser9 in GSK3β, and oxidant stress-induced apoptosis127,128. Further investigation is crucial to understand how EPO treatment interacts with GSK3β function in different brain tissue types, cellular environments and diseases.
An additional relationship between EPO and GSK3β involves the downstream increase in hippocampal brain-derived neurotrophic factor (BDNF) expression, neurite growth and spine density53,129 (Fig. 1). BDNF is highly involved in neuroplasticity, cell survival, differentiation and cell death130,131 as well as learning and memory132–134. Evidence has shown that GSK3β interacts with BDNF at the protein level; GSK3β overexpression inhibits BDNF-induced cAMP response element-binding (CREB) phosphorylation135,136. GSK3β and BDNF genotype combinations have been associated with MDD86.
A complex relationship between EPO, GSK3β, the hippocampus and depression may exist, in part, through nitric oxide (NO)-related pathways. In brief, increased GSK3β mRNA expression was found in post-mortem hippocampal samples from MDD patients, which is consistent with previous animal studies of depression. GSK3β mRNA expression was also significantly correlated with nitric oxide synthase 1 (NOS1) in these same patients137, which is in keeping with previous evidence suggesting that nitric oxide activates GSK-3β. EPO can influence oxygen delivery through stimulation of NO production45, which may contribute to its neuroprotective role; however, this relationship is complex and very much dependant on the cell and tissue type, and different dose-time exposure conditions (i.e., short-term versus long-term exposure, hypoxia versus normoxia conditions etc.). Possible relationships between EPO, GSK-3β, NOS and hypoxia may exist, although specific mechanisms remain unclear. Hypoxia modulates NOS mRNA and protein levels under specific conditions138, GSK-3β overexpression is associated with reduced hypoxia-inducible transcription factor 1α (HIF-1α)139, while EPO-R expression is rapidly upregulated by HIF45. More detailed work in this area is needed to understand the isoform-specific interactions however (e.g., the role of HIF-1α versus HIF-2α etc.).
GSK3β acts centrally in the canonical Wnt signalling pathway, which is essential for regulating neurodevelopment as well as synaptic maintenance and plasticity in the adult brain140. Independent evidence has implicated the Wnt pathway in mood disorders57,141. Biological interactions between EPO and the canonical Wnt signalling pathway have been observed in elevated D-glucose models of diabetes142. These authors142 found that EPO triggered anti-apoptotic responses via the modulation of Wnt1 protein expression that subsequently promoted beta-catenin translocation. The authors142 also reported that Wnt1 gene silencing and Wnt1 antagonist administration prevented the protective EPO treatment. Notably, biological interactions between EPO and Wnt signalling have also been a proposed mechanism of action for neurodegenerative diseases47.
Peroxisome proliferator-activated receptor-gamma co-factor 1A (PPARGC1A) is involved in the PPAR-γ system, which interacts with numerous pathways including the Wnt signalling pathway57. Evidence has shown that PPARGC1A genetic variation is associated with altered brain volume in MDD patients57. Activation of the PPAR-γ system has been shown to improve depressive-like behaviours105. It has been proposed that PPAR-γ plays a protective role against ER stress105 and that PPAR-γ pro-survival activity is inhibited by HDAC4 activation143. Furthermore, the PPAR-γ system has been linked to EPO function144. For example, a study examining the therapeutic implications of EPO in type 2 diabetes and insulin resistance found that EPO regulates the PI3K/AKT signalling pathway via PPARγ-dependent activation144 (Fig. 1).
Insulin signalling pathways also share complex relationships with both EPO and GSK3β. Evidence has shown that insulin-like growth factor leads to increased EPO and EPOR expression in neuronal cells46 and that GSK3β is inhibited by insulin-mediated mechanisms145. It has been proposed that impaired insulin receptor-mediated regulation of GSK3β activity is involved with the cognition and depression146.
EPO, GSK3β and pharmacological treatments
Evidence from animal studies suggests that inhibition of GSK3β is a potential mechanism contributing to the antidepressant-like effects of lithium, ketamine147 and valproate148. Lithium is considered to be the gold standard pharmacological treatment for BD and has pleiotropic effects on multiple cellular systems and pathways149. Additionally, lithium treatment results in significant inhibition of GSK3 activity150,151, which has been shown to mediate neuroprotective, anti-oxidative and neurotransmission mechanisms. The effect of lithium-induced GSK3 inhibition has also previously been shown to reduce tauopathy and neurodegeneration152, and another study demonstrated that lithium (Li+) inhibits GSK3 by competition for magnesium (Mg2+)153. With regard to ketamine, while the literature is inconclusive, there is an indication that ketamine may be effective at treating depression154, in particular severe depression, TRD and acute suicidality. With its fast-acting properties155, ketamine has been shown to interact with EPO156,157. The combination of EPO and ketamine may offer new areas of investigation for mood disorder treatments. The antidepressant actions of ketamine involve GSK3β inhibition147. Lithium and other selective GSK3β inhibitors enhance the effects of low doses of ketamine158 and the authors suggested that GSK3β activation is an underlying mechanism related to ketamine-induced apoptosis. Low-dose interactions may be of particular interest for reducing the risk of side effects and possible misuse given prior evidence implicating ketamine with misuse and addiction159. Ketamine has been shown to modulate inflammatory responses160. Acute or chronic use of ketamine has been found to induce cognitive impairments with hyperphosphorylation of tau and apoptosis161, and transient behavioural changes similar to schizophrenia (i.e., motor and social behavioural disturbances)162,163. However, studies have previously shown that ketamine has anti-inflammatory effects under inflammatory conditions and has been used in surgical procedures in patients with sepsis164,165, chronic stress-induced depression166, mood disorders in general167 and severe TRD168. The effect of ketamine in reducing depressive symptoms has been shown to be fast-onset but short-lived and requires continual or maintenance treatment; however, the safety of long-term ketamine use has not yet been examined169. Evidence has suggested the involvement of the serotonergic and dopaminergic systems in addition to the glutamate N-methyl-D-aspartate (NMDA) receptor and BDNF169. Studies have postulated that excessive or ill-timed NMDA antagonism by ketamine may induce glutamate excitotoxicity, which further complicates the role of ketamine in neuroprotection or neurotoxicity, and its clinical utility169. Future clinical trials that examine the EPO-ketamine combination treatment would be of interest, especially in patients who have molecular measures of GSK3β given its interactions with EPO and ketamine. GSK3β cellular signalling is extremely fast acting and responsive to cellular changes. Animal studies have also shown that the monoamine reuptake inhibitor antidepressants, fluoxetine and imipramine, increase the inhibitory control of phosphorylation of Ser9 in GSK3170,171. Furthermore, valproate directly inhibits GSK3β and was shown to protect cells from ER stress and apoptosis148. Inhibition of GSK3β is therefore a possible mechanism of action shared by several classes of antidepressant medication and other emerging medications (i.e., ketamine) for the treatment of depression. Whether the pharmacological effects of these antidepressants on GSK3β contribute to the reduction of depressive symptoms is yet to be established.
Future directions and challenges
Here we have reviewed literature that examines the relationship between EPO, mood disorders, cognition and the hippocampus. We then speculated that EPO inhibits GSK3β activity and subsequently might alter complex signalling cascades to improve cognition via hippocampal brain changes. A key limitation of this review is that the selective molecular effects of the treatment with EPO remain unclear. Also, other antidepressant therapies have strong overlap with these cellular pathways presented in this review indicating that much more work is required to unravel directly relevant versus secondary molecular events in the context of cognitive and hippocampal in mood disorders. One key area that needs prioritising is to explore cellular differentiation molecular mechanisms. Future studies investigating the effects of EPO on different cellular networks mediated by GSK3β are highly warranted to identify its common and specific roles in the treatment of mood disorders and other neuropsychiatric illnesses. Given the similarities and known differences between MDD and BD, further exploration of the underlying mechanism that differentiates unipolar and bipolar depression is necessary for novel treatment of these debilitating and chronic mood disorders. Nevertheless, the beneficial effects of EPO on cognition and hippocampal volume have been observed across several neuropsychiatric diseases including MDD, BD40 and schizophrenia67, suggesting that EPO modulates common signalling pathways involved in neuroplasticity and cognition across these disorders. Preliminary evidence suggests that GSK3β inhibition may play a role in improving a range of cognitive deficits172,173. We therefore recommend that further studies directly test for associations between hippocampal-related cognitive measures in mood disorders and GSK3β-related genetic networks (e.g., ITPR1) as well as considering co-treatment designs with EPO, such as ER stress inhibitors58. This may lead to future potential treatment options more targeted for illness-related cognitive impairments173.
Additional research is required to elucidate the role of the EPO gene (OMIM: 133170) and its related genetic variation; surprisingly, this has not been studied in mood disorders (or psychiatric disorders more generally). To our knowledge, only one study to date has investigated genetic variants across EPO and EPOR in schizophrenia, which showed initial promising results in cognitive modulation43. Given the caveats for genetic association studies and recruitment challenges for EPO patient studies, interactions between GSK3β and EPO/EPOR also require further examination using large scale, well powered healthy participant populations. In vivo work examining the functional effects of EPO/EPOR and GSK3β will also be an important avenue for further investigation.
The evidence to date suggests that EPO has potential clinical utility to reduce cognitive deficits in patients with depression. There is no known pharmacokinetic drug–drug interaction and no adverse events were observed in the recent EPO clinical trials33,34. Nevertheless, significant adverse events for EPO treatment have been reported including tumour progression and thromboembolic events. Given these potential risks of EPO treatment, extensive screening is necessary prior to starting EPO therapy and EPO-treated patients must also be closely monitored (for details, see ref. 74). Furthermore, the long-term benefits and use of EPO in patients need to be demonstrated in clinical studies with longer-term follow-up times regarding its potential benefits and risks. Specifically, studies using six months follow-up assessments of cognition and functioning are highly warranted given the short (6 weeks) follow-up times in the recent trials in BD and MDD. Knowing the biological and molecular genetic mechanisms, and pharmacogenetics underlying the effects of EPO, may guide clinicians and patients in understanding who will tolerate and respond to EPO treatment. This will allow clinicians to choose the best medications for each individual patient for precision medical care.
Given the highly pleiotropic effects of GSK3β in triggering multiple pathways and processes, including cancer development and tumour growth174, it is important to extensively investigate molecular targets that act with less potency and greater GSK3β downstream specificity (i.e., its numerous substrates). However, in vivo and in vitro evidence is currently limited and many unknown putative GSK3β substrates may exist. The identification of more GSK3β substrates is therefore of great importance for understanding the larger impact GSK3β plays in hippocampal volume of individuals with mood disorders. Algorithms that estimate the likelihood of proteins binding to GSK3β should facilitate this work175. Furthermore, exploration of protein kinases required for priming phosphorylation prior to GSK3β protein docking should also be explored, as is being explored in cancer research174. Also crucial will be harnessing the power of statistical methods, such as machine learning, to better understand how genotypic combinations combinations interact across these substrates and upstream regulating proteins as part of GSK3β’s larger genetic network. The identification of GSK3β-related genotypic combinations that specifically influence hippocampal volume in MDD may help guide further exploration for additional and more specific therapeutic combinations of treatment targets58.
Issues that require further exploration include disease onset and specificity. It is unclear how such factors relate to addressing issues of disease susceptibility and disease progression. While researchers have started to address complex interactions with disease onset176, future studies are needed, including longitudinal studies involving risk populations, to further our understanding and to further elucidate the causal pathway of antidepressant actions in the context of mood disorders. Growing evidence for dynamic neurodevelopmental patterns of GSK3β poses many additional research questions177. In this review, we have shown evidence that EPO may have generalizable effects spanning across illnesses; therefore, much work in this area is required to understand comorbidity and disease specificity. Furthermore, EPO neuroprotection is only partially reduced by the inhibition of pro-survival PI3K/AKT signalling45. In addition, GSK3β inhibition suppresses pro-inflammatory responses involving the NFκB pathway178; however, EPO may activate neuroinflammation signalling pathways (and other unknown molecules) using different mechanisms of action from GSK3β. Likewise, the complex relationship between EPO, GSK3β and MAPK179 needs further mapping. Overall, it is highly warranted to further delineate the EPO-GSK3β pathways involved in different aspects of treatment and illness.
The important role of EPO in cognition and mood disorders sheds light on the longstanding treatment challenge in psychiatric patients who suffer from chronic cognitive deficits. Unearthing the cellular pathways governed by EPO may enhance translation of targeted therapeutic strategies for mood disorders and other related conditions. Further work that explores molecular mechanisms related to the inhibition of GSK3β function and the promotion of EPO function within a mood disorder and cognition framework is highly warranted.
Restoration of neuroplasticity, including upregulation of neurogenesis and BDNF, may be an important mechanism of chronic antidepressant treatment. Mechanistically distinct compounds, such as EPO, which directly increases cellular resilience and plasticity hold great promise as novel faster acting treatments of depression. To assess the importance of such mechanisms for the cognitive and potential antidepressant effects of EPO, it would be a conceptually important next step to assess the effects of hippocampal irradiation, which impedes upregulation of BDNF and neurogenesis and blocks the behavioural effects of antidepressant drugs180. The associations presented in this review are only beginning to scratch the surface of the upstream and downstream events that may be changing as a result of EPO administration in relation to GSK3β and much more work is needed in this important area.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Becky Inkster and Gwyneth Zai
Contributor Information
Becky Inkster, Email: becky.inkster@gmail.com.
Gemma Lewis, Email: gemma.lewis@ucl.ac.uk.
References
- 1.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Isometsa E. Suicidal behaviour in mood disorders--who, when, and why? Can. J. Psychiatry. 2014;59:120–130. doi: 10.1177/070674371405900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global burden of mental disorders and the need for a comprehensive, coordinated response from health and social sectors at the country level. EB130/9:1-6 (2011). http://apps.who.int/gb/ebwha/pdf_files/EB130/B130_R8-en.pdf?TSPD_101_R0=b25342eb151f7aee4bba7ff412b1a4a9wq300000000000000026421b256ffff00000000000000000000000000005bb7b87b0099e5c58.
- 4.Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Moreno J, et al. Functioning and disability in bipolar disorder: an extensive review. Psychother. Psychosom. 2009;78:285–297. doi: 10.1159/000228249. [DOI] [PubMed] [Google Scholar]
- 6.Michalak EE, Murray G, Young AH, Lam RW. Burden of bipolar depression: impact of disorder and medications on quality of life. Cns. Drugs. 2008;22:389–406. doi: 10.2165/00023210-200822050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Miller S, Dell’Osso B, Ketter TA. The prevalence and burden of bipolar depression. J. Affect Disord. 2014;169(Suppl 1):S3–S11. doi: 10.1016/S0165-0327(14)70003-5. [DOI] [PubMed] [Google Scholar]
- 8.Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv. 2014;65:977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- 9.Papakostas GI, Culpepper L. Understanding and managing cognition in the depressed patient. J. Clin. Psychiatry. 2015;76:418–425. doi: 10.4088/JCP.13086ah1c. [DOI] [PubMed] [Google Scholar]
- 10.Depp CA, et al. Neurocognitive impairment in middle-aged and older adults with bipolar disorder: comparison to schizophrenia and normal comparison subjects. J. Affect Disord. 2007;101:201–209. doi: 10.1016/j.jad.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etkin A, Gyurak A, O’Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialog-. Clin. Neurosci. 2013;15:419–429. doi: 10.31887/DCNS.2013.15.4/aetkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinberg M, Miskowiak KW, Kessing LV. Impairment of executive function and attention predicts onset of affective disorder in healthy high-risk twins. J. Clin. Psychiatry. 2013;74:e747–e753. doi: 10.4088/JCP.12m08258. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger J, Berns S, Uzelak S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145:39–48. doi: 10.1016/j.psychres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Depp CA, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14:217–226. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B. CDBE2010 study group, European Brain Council. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt RJ, Henter I. (1995): An economic evaluation of manic-depressive illness--1991. Soc. Psychiatry Psychiatr. Epidemiol. 1995;30:213–219. doi: 10.1007/BF00789056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol. Learn. Mem. 2011;96:553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Raust A, et al. Neurocognitive performance as an endophenotype for bipolar disorder. Front Biosci. (Elite Ed.) 2014;6:89–103. doi: 10.2741/e694. [DOI] [PubMed] [Google Scholar]
- 19.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J. Clin. Psychiatry. 2008;69:1122–1130. doi: 10.4088/JCP.v69n0712. [DOI] [PubMed] [Google Scholar]
- 20.Canales-Rodriguez EJ, et al. Structural abnormalities in bipolar euthymia: a multicontrast molecular diffusion imaging study. Biol. Psychiatry. 2014;76:239–248. doi: 10.1016/j.biopsych.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 21.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Marsden WN. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol. Med. 2013;43:2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 24.Bourne C, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr. Scand. 2013;128:149–162. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- 25.Dias VV, et al. Pharmacological approaches in bipolar disorders and the impact on cognition: a critical overview. Acta Psychiatr. Scand. 2012. 2012;126:315–331. doi: 10.1111/j.1600-0447.2012.01910.x. [DOI] [PubMed] [Google Scholar]
- 26.Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol. Med. 2013;43:1187–1196. doi: 10.1017/S0033291712001948. [DOI] [PubMed] [Google Scholar]
- 27.Goeldner C, et al. Cognitive impairment in major depression and the mGlu2 receptor as a therapeutic target. Neuropharmacology. 2013;64:337–346. doi: 10.1016/j.neuropharm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int. J. Neuropsychopharmacol. 2016;24:pyw055. doi: 10.1093/ijnp/pyw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaser M, et al. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:115–122. doi: 10.1016/j.bpsc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety. 2006;23:482–484. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- 31.Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol. Psychiatry. 2013;73:646–651. doi: 10.1016/j.biopsych.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Yatham LN, et al. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: a randomised, open-label, pilot study. Lancet Psychiatry. 2017;4:208–217. doi: 10.1016/S2215-0366(17)30046-9. [DOI] [PubMed] [Google Scholar]
- 33.Miskowiak KW, et al. Recombinant human erythropoietin for treating treatment-resistant depression: a double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology. 2014;39:1399–1408. doi: 10.1038/npp.2013.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miskowiak KW, Ehrenreich H, Christensen EM, Kessing LV, Vinberg M. Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder: a double-blind, randomized, placebo-controlled phase 2 trial. J. Clin. Psychiatry. 2014;75:1347–1355. doi: 10.4088/JCP.13m08839. [DOI] [PubMed] [Google Scholar]
- 35.Miskowiak KW, Ott CV, Petersen JZ, Kessing LV. Systematic review of randomized controlled trials of candidate treatments for cognitive impairment in depression and methodological challenges in the field. Eur. Neuropsychopharmacol. 2016;26:1845–1867. doi: 10.1016/j.euroneuro.2016.09.641. [DOI] [PubMed] [Google Scholar]
- 36.Miskowiak KW, Carvalho AF, Vieta E, Kessing LV. Cognitive enhancement treatments for bipolar disorder: A systematic review and methodological recommendations. Eur. Neuropsychopharmacol. 2016;26:1541–1561. doi: 10.1016/j.euroneuro.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho AF, et al. Cognitive dysfunction in depression - pathophysiology and novel targets. Cns. Neurol. Disord. Drug. Targets. 2014;13:1819–1835. doi: 10.2174/1871527313666141130203627. [DOI] [PubMed] [Google Scholar]
- 38.Porter RJ, Bowie CR, Jordan J, Malhi GS. Cognitive remediation as a treatment for major depression: a rationale, review of evidence and recommendations for future research. Aust. N. Z. J. Psychiatry. 2013;47:1165–1175. doi: 10.1177/0004867413502090. [DOI] [PubMed] [Google Scholar]
- 39.Demant KM, Vinberg M, Kessing LV, Miskowiak KW. Effects of short-term cognitive remediation on cognitive dysfunction in partially or fully remitted individuals with bipolar disorder: results of a randomised controlled trial. PLoS ONE. 2015;2015:e0127955. doi: 10.1371/journal.pone.0127955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miskowiak KW, et al. Effects of erythropoietin on hippocampal volume and memory in mood disorders. Biol. Psychiatry. 2015;78:270–277. doi: 10.1016/j.biopsych.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson LO, Goldwasser E, Fried W, Plzak LF. Studies on erythropoiesis. VII. The role of the kidney in the production of erythropoietin. Trans. Assoc. Am. Physicians. 1957;70:305–317. [PubMed] [Google Scholar]
- 42.Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics 2009. 2009;6:108–127. doi: 10.1016/j.nurt.2008.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kastner A, et al. Common variants of the genes encoding erythropoietin and its receptor modulate cognitive performance in schizophrenia. Mol. Med. 2012;18:1029–1040. doi: 10.2119/molmed.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best. Pract. Res. Clin. Anaesthesiol. 2010;24:573–594. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit. Rev. Oncol. Hematol. 2007. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma C, et al. Erythropoietin pathway: a potential target for the treatment of depression. Int. J. Mol. Sci. 2016;17:E677. doi: 10.3390/ijms17050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. Int. J. Mol. Sci. 2012;13:11102–11129. doi: 10.3390/ijms130911102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marti HH, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 49.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat. Rev. Neurosci. 2005. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 50.Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byts N, Sirén AL. Erythropoietin: a multimodal neuroprotective agent. Exp. Transl. Stroke Med. 2009;1:4. doi: 10.1186/2040-7378-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girgenti MJ, et al. Erythropoietin induction by electroconvulsive seizure, gene regulation, and antidepressant-like behavioral effects. Biol. Psychiatry. 2009;66:267–274. doi: 10.1016/j.biopsych.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Leconte C, et al. Comparison of the effects of erythropoietin and its carbamylated derivative on behaviour and hippocampal neurogenesis in mice. Neuropharmacology. 2011;60:354–364. doi: 10.1016/j.neuropharm.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Ge XH, Zhu GJ, Geng DQ, Zhang ZJ, Liu CF. Erythropoietin attenuates 6-hydroxydopamine-induced apoptosis via glycogen synthase kinase 3beta-mediated mitochondrial translocation of Bax in PC12 cells. Neurol. Sci. 2012;33:1249–1256. doi: 10.1007/s10072-012-0959-3. [DOI] [PubMed] [Google Scholar]
- 55.Li YP, et al. Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid β42 in mice. Brain Res. 2015;1618:159–167. doi: 10.1016/j.brainres.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 56.Inkster B, et al. Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch. Gen. Psychiatry. 2009;66:721–728. doi: 10.1001/archgenpsychiatry.2009.70. [DOI] [PubMed] [Google Scholar]
- 57.Inkster B, et al. Pathway-based approaches to imaging genetics association studies: Wnt signaling, GSK3beta substrates and major depression. Neuroimage 2010. 2010;53:908–917. doi: 10.1016/j.neuroimage.2010.02.065. [DOI] [PubMed] [Google Scholar]
- 58.Inkster, B. et al. Unravelling the GSK3β-related genotypic interaction network influencing hippocampal volume in recurrent major depressive disorder. PG-D-17-00038 accepted, Psychiatric Genetics (2018). [DOI] [PMC free article] [PubMed]
- 59.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl Acad. Sci. USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catania MA, et al. Erythropoietin prevents cognition impairment induced by transient brain ischemia in gerbils. Eur. J. Pharmacol. 2002;437:147–150. doi: 10.1016/S0014-2999(02)01292-X. [DOI] [PubMed] [Google Scholar]
- 61.Mogensen J, et al. Erythropoietin improves place learning in fimbria-fornix-transected rats and modifies the search pattern of normal rats. Pharmacol. Biochem. Behav. 2004;77:381–390. doi: 10.1016/j.pbb.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Siren AL, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–489. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- 63.Ehrenreich H, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol. Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- 64.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–2588. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 65.Miskowiak K, O’Sullivan U, Harmer CJ. Erythropoietin enhances hippocampal response during memory retrieval in humans. J. Neurosci. 2007;27:2788–2792. doi: 10.1523/JNEUROSCI.5013-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen P1, et al. Effects of erythropoietin administration on cerebral metabolism and exercise capacity in men. Appl. Physiol. 2010;109:476–483. doi: 10.1152/japplphysiol.00234.2010. [DOI] [PubMed] [Google Scholar]
- 67.Wüstenberg T1, et al. Recombinant human erythropoietin delays loss of gray matter in chronic schizophrenia. Mol. Psychiatry. 2011;16:26–36. doi: 10.1038/mp.2010.51. [DOI] [PubMed] [Google Scholar]
- 68.Nekoui A, Blaise G. Erythropoietin and Nonhematopoietic Effects. Am. J. Med Sci. 2017. 2017;353:76–81. doi: 10.1016/j.amjms.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Mikati MA, El Hokayem JA, El Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at P10. Epilepsia. 2007;48:175–181. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 70.Miskowiak K, O’Sullivan U, Harmer CJ. Erythropoietin reduces neural and cognitive processing of fear in human models of antidepressant drug action. Biol. Psychiatry. 2007;62:1244–1250. doi: 10.1016/j.biopsych.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Miskowiak KW, et al. Effects of erythropoietin on memory-relevant neurocircuitry activity and recall in mood disorders. Acta Psychiatr. Scand. 2016;134:249–259. doi: 10.1111/acps.12597. [DOI] [PubMed] [Google Scholar]
- 72.Miskowiak KW, et al. Neural correlates of improved executive function following erythropoietin treatment in mood disorders. Psychol. Med. 2016;46:1679–1691. doi: 10.1017/S0033291716000209. [DOI] [PubMed] [Google Scholar]
- 73.Miskowiak K, et al. Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology. 2008;33:611–618. doi: 10.1038/sj.npp.1301439. [DOI] [PubMed] [Google Scholar]
- 74.Miskowiak KW, et al. Effects of erythropoietin on depressive symptoms and neurocognitive deficits in depression and bipolar disorder. Trials. 2010;11:97. doi: 10.1186/1745-6215-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ostergaard, S. D., Bech, P., Miskowiak, K. W. Fewer study participants needed to demonstrate superior antidepressant efficacy when using the Hamilton melancholia subscale (HAM-D) as outcome measure. J. Affect Disord. pii: S0165-S0327 00678-8 (2014). [DOI] [PubMed]
- 76.Hieronymus F, Emilsson JF, Nilsson S, Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol. Psychiatry. 2016;21:523–530. doi: 10.1038/mp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat. Rev. Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell. Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359:1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Morales-Garcia JA, et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem. Neurosci. 2012;3:963–971. doi: 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br. J. Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molz S, et al. Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/ glycogen synthase kinase 3beta pathway activation and inducible nitric oxide synthase inhibition. J. Neurosci. Res. 2011;89:1400–1408. doi: 10.1002/jnr.22681. [DOI] [PubMed] [Google Scholar]
- 84.Shaw PC, et al. Isolation and chromosomal mapping of human glycogen synthase kinase-3 alpha and -3 beta encoding genes. Genome. 1998;41:720–727. [PubMed] [Google Scholar]
- 85.Kwok JB, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Ann. Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 86.Zhang K, et al. Genetic association of the interaction between the BDNF and GSK3B genes and major depressive disorder in a Chinese population. J. Neural Transm. 2010;117:393–401. doi: 10.1007/s00702-009-0360-4. [DOI] [PubMed] [Google Scholar]
- 87.Yang C, et al. The combined effects of the BDNF and GSK3B genes modulate the relationship between negative life events and major depressive disorder. Brain Res. 2010;1355:1–6. doi: 10.1016/j.brainres.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 88.Ronai Z, et al. Glycogen synthase kinase 3 beta gene structural variants as possible risk factors of bipolar depression. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2014;165B:217–222. doi: 10.1002/ajmg.b.32223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S, et al. Possible association of the GSK3beta gene with the anxiety symptoms of major depressive disorder and P300 waveform. Genet. Test. Mol. Biomark. 2012;16:1382–1389. doi: 10.1089/gtmb.2012.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serretti A, et al. Association between GSK-3beta -50T/C polymorphism and personality and psychotic symptoms in mood disorders. Psychiatry Res. 2008;158:132–140. doi: 10.1016/j.psychres.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 91.Saus E, et al. A haplotype of glycogen synthase kinase 3beta is associated with early onset of unipolar major depression. Genes. Brain. Behav. 2010;9:799–807. doi: 10.1111/j.1601-183X.2010.00617.x. [DOI] [PubMed] [Google Scholar]
- 92.Benedetti F, et al. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci. Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 93.Yoon HK, Kim YK. Association between glycogen synthase kinase-3beta gene polymorphisms and major depression and suicidal behavior in a Korean population. Prog Neuropsychopharmacol. Biol. Psychiatry. 2010;34:331–334. doi: 10.1016/j.pnpbp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Chen J, et al. The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese population. J. Affect Disord. 2015;185:149–155. doi: 10.1016/j.jad.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 95.Tsai SJ, Liou YJ, Hong CJ, Yu YW, Chen TJ. Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. Pharm. J. 2008;8:384–390. doi: 10.1038/sj.tpj.6500486. [DOI] [PubMed] [Google Scholar]
- 96.Benedetti F, et al. Long-term response to lithium salts in bipolar illness is influenced by the glycogen synthase kinase 3-beta -50 T/C SNP. Neurosci. Lett. 2005;376:51–55. doi: 10.1016/j.neulet.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 97.Adli M, et al. Response to lithium augmentation in depression is associated with the glycogen synthase kinase 3-beta -50T/C single nucleotide polymorphism. Biol. Psychiatry. 2007;62:1295–1302. doi: 10.1016/j.biopsych.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 98.Verchinski, B. A. et al. Effects of a common variant in GSK3β on hippocampal volume in healthy human volunteers. Human Brain Mapping conference 2010; abstract.
- 99.Liu Z, et al. A combined study of GSK3β polymorphisms and brain network topological metrics in major depressive disorder. Psychiatry Res. 2014;223:210–217. doi: 10.1016/j.pscychresns.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Dietsche B, et al. Altered neural function during episodic memory encoding and retrieval in major depression. Hum. Brain. Mapp. 2014;35:4293–4302. doi: 10.1002/hbm.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hall J, et al. Hippocampal function in schizophrenia and bipolar disorder. Psychol. Med. 2010;40:761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- 102.Carlson PJ, Singh JB, Zarate CA, Jr, Drevets WC, Manji HK. Neural circuitry and neuroplasticity in mood disorders: insights for novel therapeutic targets. NeuroRx. 2006;3:22–41. doi: 10.1016/j.nurx.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 104.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gold PW, Licinio J, Pavlatou MG. Pathological parainflammation and endoplasmic reticulum stress in depression: potential translational targets through the CNS insulin, klotho and PPAR-gamma systems. Mol. Psychiatry. 2013;18:154–165. doi: 10.1038/mp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borcel E, et al. Chronic stress in adulthood followed by intermittent stress impairs spatial memory and the survival of newborn hippocampal cells in aging animals: prevention by FGL, a peptide mimetic of neural cell adhesion molecule. Behav. Pharmacol. 2008;19:41–49. doi: 10.1097/FBP.0b013e3282f3fca9. [DOI] [PubMed] [Google Scholar]
- 107.Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Ann. N. Y. Acad. Sci. 2004;1018:323–327. doi: 10.1196/annals.1296.039. [DOI] [PubMed] [Google Scholar]
- 108.Yun J, et al. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: possible involvement of a brain-specific transcription factor Npas4. J. Neurochem. 2010;114:1840–1851. doi: 10.1111/j.1471-4159.2010.06893.x. [DOI] [PubMed] [Google Scholar]
- 109.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol. Psychiatry. 1999;46:1181–1191. doi: 10.1016/S0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 110.Schmaal L, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elvsashagen T, et al. Evidence for reduced dentate gyrus and fimbria volume in bipolar II disorder. Bipolar Disord. 2013;15:167–176. doi: 10.1111/bdi.12046. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol. Psychiatry. 2013;74:62–68. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Tae WS, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am. J. Neuroradiol. 2011;32:671–676. doi: 10.3174/ajnr.A2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma R, et al. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009;56:1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Somervaille TC, Linch DC, Khwaja A. Growth factor withdrawal from primary human erythroid progenitors induces apoptosis through a pathway involving glycogen synthase kinase-3 and Bax. Blood. 2001;98:1374–1381. doi: 10.1182/blood.V98.5.1374. [DOI] [PubMed] [Google Scholar]
- 116.Ma Shengli, Chen Juwu, Chen Chen, Wei Na, Xu Jingjing, Yang Guohui, Wang Nan, Meng Yu, Ren Jia, Xu Zongchao. Erythropoietin Rescues Memory Impairment in a Rat Model of Chronic Cerebral Hypoperfusion via the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β Pathway. Molecular Neurobiology. 2017;55(4):3290–3299. doi: 10.1007/s12035-017-0568-5. [DOI] [PubMed] [Google Scholar]
- 117.Maurer U, Preiss F, Brauns-Schubert P, Schlicher L, Charvet C. GSK-3 – at the crossroads of cell death and survival. J. Cell. Sci. 2014;127:1369–1378. doi: 10.1242/jcs.138057. [DOI] [PubMed] [Google Scholar]
- 118.Hassouna I, et al. Revisiting adult neurogenesis and the role of erythropoietin for neuronal and oligodendroglial differentiation in the hippocampus. Mol. Psychiatry. 2016;21:1752–1767. doi: 10.1038/mp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Z, Dai J, Liao Y, Wang T. Sox9 Protects against Human Lung Fibroblast Cell Apoptosis Induced by LPS through Activation of the AKT/GSK3β Pathway. Biochem. (Mosc.) 2017;82:606–612. doi: 10.1134/S000629791705008X. [DOI] [PubMed] [Google Scholar]
- 120.Topol L, Chen W, Song H, Day TF, Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J. Biol. Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chew LJ, et al. SRY-box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells. J. Neurosci. 2011;31:13921–13935. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xue Q, et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci. Rep. 2016;6:26781. doi: 10.1038/srep26781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roy B, Dunbar M, Shelton RC, Dwivedi Y. Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology. 2017;42:864–875. doi: 10.1038/npp.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Higuchi F, et al. Hippocampal MicroRNA-124 Enhances Chronic Stress Resilience in Mice. J. Neurosci. 2016;36:7253–7267. doi: 10.1523/JNEUROSCI.0319-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jo HR, Kim YS, Son H. Erythropoietin and carbamylated erythropoietin promote histone deacetylase 5 phosphorylation and nuclear export in rat hippocampal neurons. Biochem. Biophys. Res. Commun. 2016;470:220–225. doi: 10.1016/j.bbrc.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 126.Tong Q, et al. TRPC3 is the erythropoietinregulated calcium channel in human erythroid cells. J. Biol. Chem. 2008;283:10385–10395. doi: 10.1074/jbc.M710231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohori K, et al. Ser9 phosphorylation of mitochondrial GSK-3beta is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2079–H2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- 128.Nishihara M, et al. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3 beta. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- 129.Ozalp SS, Eren CY, Bostancioglu RB, Koparal AT. Induction of apoptosis and inhibition of cell proliferation by the cyclooxgenase enzyme blocker nimesulide in the Ishikawa endometrial cancer cell line. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;164:79–84. doi: 10.1016/j.ejogrb.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 130.Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc. Natl Acad. Sci. USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Numakawa T, et al. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 132.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 133.Duman RS. Synaptic plasticity and mood disorders. Mol. Psychiatry. 2002;7:S29–S34. doi: 10.1038/sj.mp.4001016. [DOI] [PubMed] [Google Scholar]
- 134.Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mai L, Jope RS, Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J. Neurochem. 2002;82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 136.Foulstone EJ, Tavare JM, Gunn-Moore FJ. Sustained phosphorylation and activation of protein kinase B correlates with brain-derived neurotrophic factor and insulin stimulated survival of cerebellar granule cells. Neurosci. Lett. 1999;264:125–128. doi: 10.1016/S0304-3940(99)00166-4. [DOI] [PubMed] [Google Scholar]
- 137.Oh DH, Park YC, Kim SH. Increased glycogen synthase kinase-3beta mRNA level in the hippocampus of patients with major depression: a study using the stanley neuropathology consortium integrative database. Psychiatry Investig. 2010;7:202–207. doi: 10.4306/pi.2010.7.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ho JJ, Man HS, Marsden PA. Nitric oxide signaling in hypoxia. J. Mol. Med (Berl.). 2012;90:217–231. doi: 10.1007/s00109-012-0880-5. [DOI] [PubMed] [Google Scholar]
- 139.Flügel D, Görlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol. Cell. Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2011;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 141.Sani G, et al. The wnt pathway in mood disorders. Curr. Neuropharmacol. 2012;10:239–253. doi: 10.2174/157015912803217279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chong ZZ1, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3β, and β-catenin to foster vascular integrity during experimental diabetes. Curr. Neurovasc. Res. 2011;8:103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang Y, et al. Peroxisome proliferator-activated receptor γ is inhibited by histone deacetylase 4 in cortical neurons under oxidative stress. J. Neurochem. 2011;118:429–439. doi: 10.1111/j.1471-4159.2011.07316.x. [DOI] [PubMed] [Google Scholar]
- 144.Ge Z, et al. Erythropoietin alleviates hepatic insulin resistance via PPARγ-dependent AKT activation. Sci. Rep. 2015;5:17878. doi: 10.1038/srep17878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 146.Yanagita Toshihiko, Nemoto Takayuki, Satoh Shinya, Yoshikawa Norie, Maruta Toyoaki, Shiraishi Seiji, Sugita Chihiro, Murakami Manabu. Mood Disorders. 2013. Neuronal Insulin Receptor Signaling: A Potential Target for the Treatment of Cognitive and Mood Disorders. [Google Scholar]
- 147.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry. 2011;16:1068–1070. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim AJ, Shi Y, Austin RC, Werstuck GH. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell. Sci. 2005;118:89–99. doi: 10.1242/jcs.01562. [DOI] [PubMed] [Google Scholar]
- 149.Vosahlikova M, Svoboda P. Lithium - therapeutic tool endowed with multiple beneficiary effects caused by multiple mechanisms. Acta Neurobiol. Exp. (Wars.) 2016;76:1–19. doi: 10.21307/ane-2017-001. [DOI] [PubMed] [Google Scholar]
- 150.Freland L, Beaulieu JM. Inhibition of GSK3 by lithium, from single molecules to signalling networks. Front. Mol. Neurosci. 2012;5:14. doi: 10.3389/fnmol.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malhi GS, Outhred T. Therapeutic mechanisms of lithium in bipolar disorder: recent advances and current understanding. Cns. Drugs. 2016;30:931–949. doi: 10.1007/s40263-016-0380-1. [DOI] [PubMed] [Google Scholar]
- 152.Noble W, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. Usa. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 154.Iadarola ND, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Costemale-Lacoste JF, Guilloux JP, Gaillard R. The role of GSK-3 in treatment-resistant depression and links with the pharmacological effects of lithium and ketamine: a review of the literature. Encephale. 2016;42:156–164. doi: 10.1016/j.encep.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 156.Yi, Z., Za, X. & Zhi, Z. Erythropoietin protects neuron against ketamine induced injuries. 88, 876–879 (2008). [PubMed]
- 157.Hayley S, Litteljohn D. Neuroplasticity and the next wave of antidepressant strategies. Front. Cell. Neurosci. 2013;7:218. doi: 10.3389/fncel.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu JR, Baek C, Han XH, Shoureshi P, Soriano SG. Role of glycogen synthase kinase-3beta in ketamine-induced developmental neuroapoptosis in rats. Br. J. Anaesth. 2013;110:i3–i9. doi: 10.1093/bja/aet057. [DOI] [PubMed] [Google Scholar]
- 159.Delimbeuf N, Petit A, Karila L, Lejoyeux M. Ketamine: psychiatric indications and misuses. Rev. Med. Liege. 2014;69:434–440. [PubMed] [Google Scholar]
- 160.Li Y, et al. Effects of ketamine on levels of inflammatory cytokines IL-6, IL-1β, and TNF-α in the hippocampus of mice following acute or chronic administration. Front. Pharmacol. 2017;8:139. doi: 10.3389/fphar.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yeung LY, et al. Hyperphosphorylated tau in the brains of mice and monkeys with long-term administration of ketamine. Toxicol. Lett. 2010;193:189–193. doi: 10.1016/j.toxlet.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 162.Becker A, et al. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 163.Razoux F, Garcia R, Léna I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- 164.Takahashi T, et al. The effect of ketamine anesthesia on the immune function of mice with postoperative septicemia. Anesth. Analg. 2010;111:1051–1058. doi: 10.1213/ANE.0b013e3181ed12fc. [DOI] [PubMed] [Google Scholar]
- 165.Ward JL, Harting MT, Jr CoxCS, Mercer DW. Effects of ketamine on endotoxin and traumatic brain injury induced cytokine production in the rat. J. Trauma. 2011;70:1471–1479. doi: 10.1097/TA.0b013e31821c38bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang N, et al. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups. J. Med. Sci. 2015;120:241–248. doi: 10.3109/03009734.2015.1060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanacora G, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 168.Singh I, et al. Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry. 2017;4:419–426. doi: 10.1016/S2215-0366(17)30102-5. [DOI] [PubMed] [Google Scholar]
- 169.Newport DJ, et al. APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 170.Beaulieu JM, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li X, et al. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lipoma TV, et al. Inhibition of glycogen synthase kinase 3 prevents synaptic long term depression and facilitates cognition in C57bI/6J mice. Opera Med Physiol. 2016;2:87–102. [Google Scholar]
- 173.O’Leary O, Nolan Y. Glycogen synthase kinase-3 as a therapeutic target for cognitive dysfunction in neuropsychiatric disorders. Cns. Drugs. 2015;29:1–15. doi: 10.1007/s40263-014-0213-z. [DOI] [PubMed] [Google Scholar]
- 174.McCubrey JA, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–2911. doi: 10.18632/oncotarget.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Linding R, et al. NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 2008;36:D695–D699. doi: 10.1093/nar/gkm902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baare WF, et al. Hippocampal volume changes in healthy subjects at risk of unipolar depression. J. Psychiatr. Res. 2010;44:655–662. doi: 10.1016/j.jpsychires.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 177.Beurel E, Mines M, Song L, Jope RS. Glycogen synthase kinase-3 levels and phosphorylation undergo large fluctuations in mouse brain during development. Bipolar Disord. 2012;14:822–830. doi: 10.1111/bdi.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Orellana AM, et al. Age-related neuroinflammation and changes in AKT-GSK-3β and WNT/ β-CATENIN signaling in rat hippocampus. Aging. 2015;7:1094–1108. doi: 10.18632/aging.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bikkavilli RK, Malbon CC. Mitogen-activated protein kinases and Wnt/β-catenin signaling molecular conversations among signaling pathways. Commun. Integr. Biol. 2009;2:46–49. doi: 10.4161/cib.2.1.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]