Abstract
Systemic inflammatory response has been implicated as a contributor to the onset of febrile seizures (FS). The four novel indices of the inflammatory response such as, neutrophil-to-lymphocyte ratio (NLR), mean platelet volume (MPV), platelet count (PLT) ratio and red blood cell distribution width (RDW) have been investigated in FS susceptibility and FS types (simple febrile seizure and complex febrile seizure). However, the potential role of these inflammatory markers and MPV/PLT ratio (MPR) in Chinese children with FS has yet to be fully determined. This study investigated the relevance of NLR, MPV, PLT, MPR and RDW in febrile children with and without seizures. 249 children with FS and 249 age matched controls were included in this study. NLR and MPR were calculated from complete blood cell counts prior to therapy. Differences in age, gender and these inflammatory markers between the FS group and the control group were evaluated using the chi-square test, t-test or logistic regression analysis. Receiver Operating Characteristic (ROC) curve was used to determine the optimal cut-off value of NLR and MPR for FS risk. Interactions between NLR and MPR on the additive scale were calculated by using the relative excess risk due to interaction (RERI), the proportion attributable to interaction (AP), and the synergy index (S). It has been shown that the elevated NLR and MPR levels were associated with increased risk of FS. The optimal cut-off values of NLR and MPR for FS risk were 1.13 and 0.0335 with an area under the curve (AUC) of 0.768 and 0.689, respectively. Additionally, a significant synergistic interaction between NLR and MPR was found on an additive scale. The mean levels of MPV were lower and NLR levels were higher in complex febrile seizure (CFS) than simple febrile seizure (SFS), and the differences were statistically significant. ROC analysis showed that the optimal cut-off value for NLR was 2.549 with 65.9% sensitivity and 57.5% specificity. However, no statistically significant differences were found regarding average values of MPR and RDW between CFS and SFS. In conclusion, elevated NLR and MPR add evidence to the implication of white cells subsets in FS risk, and our results confirmed that NLR is an independent, albeit limited, predictor in differentiating between CFS and SFS. Moreover, NLR and MPR may have a synergistic effect that can influence the occurrence of FS.
Introduction
Febrile seizures (FS), also known as febrile convulsions, affecting approximately 2–5% of children aged six months to five years, are typically divided into two types, i.e., simple febrile seizures (SFS) and complex febrile seizures (CFS)1,2. FS are associated with rapidly rising fever without evidence of intracranial infection, metabolic disturbance, or history of afebrile seizures1,2. Although fever is a common symptom in children, only some children with fever experience FS, and it is not well understood how fever generates FS. Fever is induced by pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α during infections3. To date, many studies have suggested that inflammation, which is intrinsic to the fever response, is involved in the generation of FS3–9. These studies suggested that inflammatory cytokines, especially IL-1β, IL-6 and TNF-α can play important role in the generation of FS. Although inflammatory cytokines are useful biomarkers, their increased cost and limited availability are drawbacks.
Peripheral blood neutrophil-to-lymphocyte ratio (NLR), mean platelet volume (MPV) and red blood cell distribution width (RDW) are three novel indices for inflammation. NLR is an indicator of systemic inflammatory response and has been implicated in the pathogenesis of a number of diseases, especially in cardiac diseases and malignancies10–14. MPV is a machine-calculated measurement of the average size of platelets. It reflects the platelet size and the rate of platelet production in bone marrow, and may be used as an indicator of platelet activation and severity of inflammation15–17. Activated platelets release a large number of substances that are crucial mediators of inflammation18. Moreover, several studies have demonstrated an inverse relationship between MPV and platelet counts in critically ill patients19,20. Emerging evidence suggests that the combination of platelet count and MPV may be more clinically significant than platelet count or MPV alone19,21–23. In addition, the interaction between neutrophils and activated platelets also occurs during the inflammatory response in the blood24. RDW has been reported to be positively correlated with inflammatory markers such as the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels and inflammatory cytokines in various diseases25,26. Therefore, as widely-used, low-cost host inflammatory response markers, NLR, MPV, PLT and RDW have gained increasing attention as independent predictors associated with FS susceptibility and FS types27–33.
Clinical studies have attempted to address the relationship in the blood of febrile children with and without seizures by comparing levels of MPV, NLR, PLT and RDW, but these studies have reported inconsistent results. And it is unknown whether these inflammatory markers and MPR are associated with FS susceptibility and FS types in Chinese children. It is even unknown whether the interaction between NLR and MPR can affect FS susceptibility. Here, to address these questions, we analyzed the association between these inflammatory factors and FS in Chinese children.
Material and Methods
Patients and Methods
The study was approved by the Ethics Committee of Southern Medical University Affiliated Maternal & Child Health Hospital, Foshan (China). All experiments and methods were performed in accordance with the relevant guidelines and regulations. A retrospective study was conducted in the department of pediatrics, Southern Medical University Affiliated Maternal & Child Health Hospital, Foshan (China). The hospital admits approximately 440,000 children annually. At recruitment, informed consent was obtained from parents of each child.
A total of 867 children (aged 5 months to 6 years) presented to the department of pediatrics or emergency department because of fever with seizures between January 1, 2015, and December 31, 2017. Of them, 416 (48.0%) patients who not had blood routine test (BRT) obtained within 2 hours after FS were excluded. To control the potential confounding factors and ensure accurate identification of all eligible children, subjects with conditions known or suspected to cause seizures without fever were systematically excluded using sequential, stringent exclusion criteria from the final analysis. Exclusion criteria included prematurity (age at birth <37 weeks and age under one year); a history of FS or epilepsy; a family history of genetic and neurological diseases; subsequent epilepsy after FS; abnormalities of amino acids, organic acid analyses and brain magnetic resonance imaging; electrolyte disorders; static encephalopathy; hydrocephalus; meningitis; viral central nervous system (CNS) infection; previous intracranial infection; mental retardation; stroke; demyelinating disease or ventricular shunt. Patients with acute or chronic systemic disease, eg, cancer and hematologic or rheumatologic disorders, were also excluded. Of these, 133 (15.3%) met sequential exclusion criteria. 69(8.0%) patients without informed consent and were also excluded. The remaining 249 (28.7%) subjects and 249 age matched healthy controls were included in the final analysis. Patients were divided into two groups: one group consisting of 249 children (82 children with complex febrile seizure and 167 children with simple febrile seizure) with febrile seizure and a control group of 249 children with fever of unknown etiology without seizures.
A diagnosis of FS was determined according to the International Classification of Diseases, Ninth Revision (ICD-9) codes (ICD-9 780.31, 780.32).
Laboratory Analysis
White blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), red blood cell distribution width (RDW), mean platelet volume (MPV), platelet count (PLT), monocytes, neutrophil and lymphocyte counts and percentages, were measured from peripheral venous blood samples collected in EDTA tubes during admission. The blood samples were taken and measured 2 h after FS. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. MPR was calculated by dividing the MPV by the platelet count.
Statistical analysis
Statistical analysis was performed by using the SPSS Statistics 19.0 for Windows (SPSS Inc., Chicago, IL, USA) program. Parametric data (quantitative) was expressed as numbers and percentages; qualitative data were expressed as a mean ± standard deviation. Independent samples t-test was used for comparison of independent data. The Mann-Whitney U-test was used for evaluation of parametric data without binomial distribution. When appropriate, X2-test was used for the comparison of categorical data. Receiver operating characteristic (ROC) curve analysis was used for calculating the optimal cut-off values, sensitivity, and specificity of NLR and MPR. The optimal cut-off value was determined by Youden’s index. The NLR and MPR were dichotomized according to their optimal cut-off values of ROC curve. In addition, logistic regression was used to calculate the odds ratios (ORs) and their relative 95% confidence intervals (CIs) for risk estimation. Significant associations were defined as P < 0.05.
Then, we explored the additive interaction between MPR and NLR according to the following strategy34. Among cases and controls, a binary classification was used both for MPR (High vs. Low) and NLR (High vs. Low). The risk for FS for a given MPR and NLR was expressed by the index (i) and (j), where the first index (i) indicated the MPR status coded as 0 for Low subjects and 1 for High subjects, and the second index (j) indicated the NLR, which was coded as 0 for Low subjects and 1 for High subjects. Subjects who were Low MPR and Low NLR were considered as the reference group, and their FS risk was coded as OR00 = 1. The relative ORs were obtained by logistic regression. The CIs were calculated by the regression coefficients and corresponding covariance matrix35. Deviation from an additive model was calculated as the relative excess risk due to interaction (RERI), the proportion attributable due to interaction (AP), and the synergy index (S). Biological interactions in the regression models were tested as departure from additivity. Based on the adjusted ORs obtained in the logistic regression models, an Excel spreadsheet (www.epinet.se) was used to calculate RERI, AP and S on an additive scale and its corresponding CIs35. RERI and AP values (95% CI) that does not cross 0, and S value (95% CI) that does not cross 0 indicates a biological interaction36. In addition, RERI > 0 or AP > 0 or S > 1 indicates a positive interaction or more than additivity and RERI < 0 or AP < 0 or S < 1 indicates a negative interaction or less than additivity37,38.
Results
Characteristics of the study population
A total of 498 patients were included in the study. The mean age of the FS group (n = 249, 69.1% males) was 27.3 ± 14.8 months. The FS group was further divided into two groups such as the SFS group (n = 167, 66.5% males) and the CFS group (n = 82, 74.4% males). The mean age of the SFS group and the CFS group were 26.9 ± 13.5 months and 28.1 ± 17.3 months, respectively. The mean age of the control group (n = 249, 58.2% males) was 25.0 ± 16.5 months. As shown in Table 1, there were no significant differences between the groups in term of age (P > 0.05). However, the frequency of FS was significantly higher in males than controls (P < 0.05).
Table 1.
Age and gender of the patients.
| Variables | Control group (n = 249) | FS group (n = 249) | SFS group (n = 167) | CFS group (n = 82) | P value | |
|---|---|---|---|---|---|---|
| FS VS. Control | CFS VS. SFS | |||||
| Age(months) | 25.0 ± 16.5 | 27.3 ± 14.8 | 26.9 ± 13.5 | 28.1 ± 17.3 | 0.099 | 0.566 |
| Gender | ||||||
| Male | 145 (58.2%) | 172 (69.1%) | 111 (66.5%) | 61 (74.4%) | 0.012 | 0.204 |
| Female | 104 (41.8%) | 77 (30.9%) | 56 (33.5%) | 21 (25.6%) | ||
Values are expressed as mean ± standard deviation. FS: febrile seizure. SFS: simple febrile seizure, CFS: complex febrile seizure.
Association between laboratory test results and febrile seizures
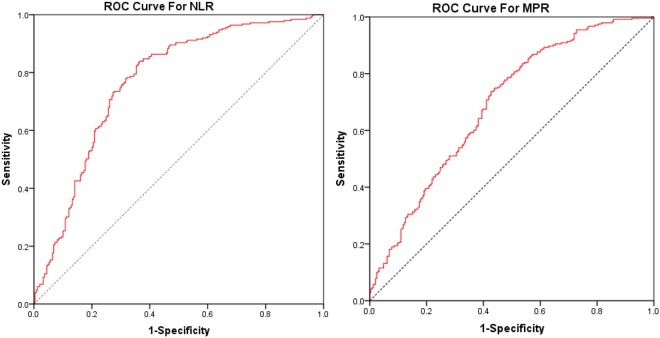
Then, we assessed the selected laboratory results in febrile children with or without seizures. The results showed that the mean levels of NLR, MPV, MPR, and the number and percentage of neutrophils were significantly higher, while PLT and the number and percentage of lymphocytes were significantly lower in the FS group, compared to the control group (Table 2). The best NLR and MPR cut-off values were ≥1.13 vs. <1.13 and ≥0.0335 vs. <0.0335, respectively, as identified by ROC curve analysis (Fig. 1). Logistic regression analysis revealed that after adjusting for age and gender, NLR ≥ 1.13 was associated with a 10.92-fold increased FS risk compared with the NLR < 1.13 (95% CI: 5.93–13.88, P < 0.001); and MPR ≥ 0.0335 was associated with a 3.76-fold increased FS risk compared with the MPR < 0.0335 (95% CI: 2.55–5.53, P < 0.001) (Table 3).
Table 2.
Laboratory tests performed in children in the FS group and the control group.
| Laboratory parameters | Control group (n = 249) | FS group (n = 249) | P value |
|---|---|---|---|
| WBC (103/mm3) | 10.2 ± 3.7 | 10.6 ± 4.0 | 0.241 |
| RBC (106/mL) | 4.5 ± 0.4 | 4.5 ± 0.4 | 0.759 |
| Hb (g/dl) | 11.8 ± 1.1 | 11.8 ± 1.0 | 0.437 |
| Hct (%) | 36.0 ± 3.0 | 35.6 ± 3.0 | 0.114 |
| MCV (fl) | 79.8 ± 6.1 | 79.2 ± 6.1 | 0.253 |
| MPV (fL) | 9.6 ± 0.9 | 9.8 ± 1.1 | 0.010 |
| PLT (10 9 /L) | 310.6 ± 112.6 | 236.2 ± 74.8 | <0.001 |
| MPR | 0.036 ± 0.015 | 0.047 ± 0.020 | <0.001 |
| Neutrophils (%) | 42.4 ± 19.7 | 61.1 ± 15.8 | <0.001 |
| Neutrophils count (x10 3 /mm 3 ) | 4.6 ± 3.4 | 6.7 ± 3.5 | <0.001 |
| Lymphocytes (%) | 46.0 ± 19.1 | 27.8 ± 14.3 | <0.001 |
| Lymphocytes count (x10 3 /mm 3 ) | 4.4 ± 2.3 | 2.8 ± 1.7 | <0.001 |
| NLR | 1.6 ± 1.9 | 3.2 ± 2.4 | <0.001 |
| Monocytes (%) | 9.5 ± 3.8 | 10.1 ± 3.9 | 0.095 |
| Monocytes count (x103/mm3) | 0.95 ± 0.52 | 1.02 ± 0.46 | 0.142 |
| RDW (%) | 13.7 ± 1.4 | 13.5 ± 1.4 | 0.096 |
| CRP (mg/L) | 14.0 ± 23 | 9.4 ± 12 | 0.007 |
Values are expressed as mean ± standard deviation. SFS: simple febrile seizure, CFS: complex febrile seizure. CRP: C-reactive protein. Bold text indicates statistical significance.
Figure 1.
ROC curves of NLR (A) and MPR (B) for predicting febrile seizure in children with fever.
Table 3.
Logistic regression analysis of the association of factors (NLR, MPR) and FS.
| Control group | FS group | OR(95% CI) | P | ORadjusted (95% CI)a | P a value | ||||
|---|---|---|---|---|---|---|---|---|---|
| NO. | % | NO. | % | ||||||
| NLR | <1.13 | 158 | 63.5 | 40 | 16.1 | 1 (Ref) | N/A | 1 (Ref) | N/A |
| ≥1.13 | 91 | 36.5 | 209 | 83.9 | 9.07 (5.93–13.88) | <0.001 | 10.92 (5.93–13.88) | <0.001 | |
| MPR | <0.0335 | 141 | 56.6 | 63 | 25.3 | 1(Ref) | N/A | 1(Ref) | N/A |
| ≥0.0335 | 108 | 43.4 | 186 | 74.7 | 3.85 (2.64–5.64) | <0.001 | 3.76 (2.55–5.53) | <0.001 | |
OR, odds ratio; NO, number of subjects; CI, confidence interval; aAdjusted for age and gender; N/A, Not applicable; Bold text indicates statistical significance.
MPR-NLR interaction for Febrile Seizure susceptibility
Additionally, the interactive effects of MPR and NLR based on an additive scale were also evaluated. According to the RERI, AP and S indexes, a significant synergistic interaction was found between MPR and NLR (AP = 0.57, 95% CI = 0.36–0.77; S = 2.35, 95% CI = 1.43–3.87) (Table 4).
Table 4.
The interaction of risk estimates between MPR and NLR based on the additive scale.
| MPRb | NLRc | Cases (%) | Controls (%) | OR (95% CI) | P a value |
|---|---|---|---|---|---|
| − | − | 33 (13.3) | 66 (26.5) | 1 | |
| + | − | 7 (2.8) | 92 (36.9) | 6.69 (2.87–15.58) | <0.001 |
| − | + | 168 (67.5) | 50 (20.1) | 18.75 (8.02–43.85) | <0.001 |
| + | + | 41 (16.5) | 41 (16.5) | 56.15 (24.21–130.21) | <0.001 |
| RERI (95%CI) = 31.71 (−0.41~63.84) | |||||
| AP (95%CI) = 0.57 (0.36~0.77) | |||||
| S (95%CI) = 2.35 (1.43~3.87) | |||||
aAdjusted for age (months) and gender; bMPR (−<0.0335; +≥0.0335); cNLR (−<1.13; +≥1.13); OR, odds ratio; CI, confidence interval; RECI, excess risk due to interaction; AP, attributable proportion due to interaction; S, the synergy index; Bold values are statistically significant.
Differences in the selected laboratory results between Children with Simple and Complex Febrile Seizures
Then, the selected laboratory results were also assessed between the SFS group and the CFS group. The results showed that NLR was significantly lower (SFS vs. CFS: 2.9 ± 2.4 vs. 3.7 ± 2.5), while MPV was significantly higher (SFS vs. CFS: 10.0 ± 1.0 vs. 9.4 ± 1.1) in the SFS group, compared with the CFS group (Table 5). However, no statistically significant differences were found regarding average values of MPR and RDW between CFS and SFS. According to ROC curve analysis in differentiating between SFS and CFS (Fig. 2), the optimal cut-off value for NRL was found to be 2.549 (65.9% sensitivity, 57.5% specificity, AUC: 0.620) (Table 6).
Table 5.
Mean values and standard deviations of MPV, PLT, MPR, NLR, and RDW among SFS and CFS patients.
| Variables | SFS group (n = 167) | CFS group (n = 82) | P value |
|---|---|---|---|
| MPV (fL) | 10.0 ± 1.0 | 9.4 ± 1.1 | <0.001 |
| PLT (109/L) | 233.3 ± 75.2 | 242.0 ± 74.1 | 0.392 |
| MPR | 0.048 ± 0.019 | 0.044 ± 0.023 | 0.175 |
| NLR | 2.9 ± 2.4 | 3.7 ± 2.5 | 0.014 |
| RDW (%) | 13.5 ± 1.3 | 13.4 ± 1.5 | 0.762 |
| CRP | 9.4 ± 11 | 9.5 ± 13 | 0.936 |
Values are expressed as mean ± standard deviation. SFS: simple febrile seizure, CFS: complex febrile seizure. CRP: C-reactive protein. Bold text indicates statistical significance.
Figure 2.
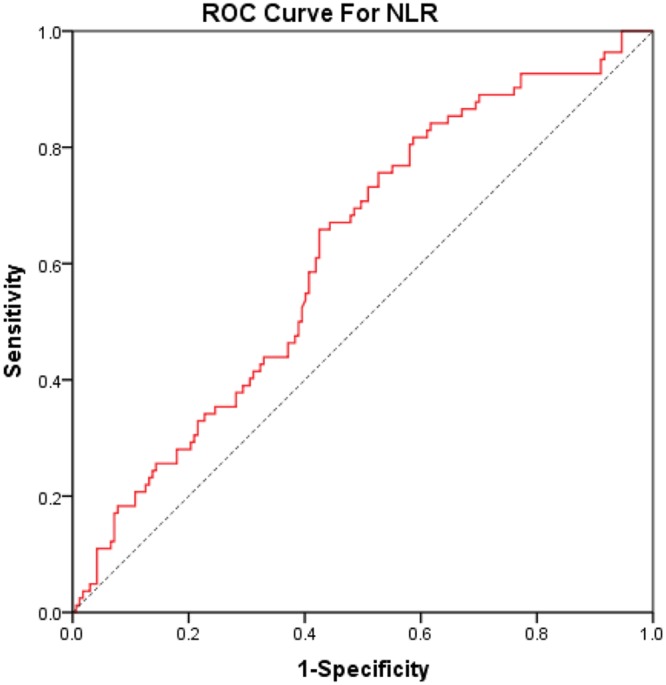
ROC curve analysis of neutrophil lymphocyte ratio (NLR) in differentiating between simple and complex febrile seizures. Area under the curve (AUC, 0.665; confidence interval CI, 0.573–0.756).
Table 6.
ROC curve analysis results of NLR in SFS and CFS patients.
| Variables | Cut-off value | FN | TP | TN | FP | Sensitivity | Specificity | AUC, 95% CI |
|---|---|---|---|---|---|---|---|---|
| NLR | 2.549 | 28 | 54 | 96 | 71 | 65.9% | 57.5% | 0.620 (0.548–0.692) |
FN: false negative, TP: true positive, TN: true negative, FP: false positive, AUC: Area Under the Curve.
Discussion
Although numerous studies have been performed on the identification of factors causing FS in children, the pathophysiology of FS is incompletely understood39,40. Accumulating evidence suggests a link between FS and inflammation5,41–43. Since FS occur during the course of a high body temperature or a rapidly rising fever in susceptible individuals, various factors associated with fever generation could be involved in the possible causative mechanism of FS. Fever generation involves many cytokines and endogenous mediators, including IL-1β, IL-6, TNF-α, IL-1Ra, and IL-10, in response to exogenous pyrogens. Of these, IL-1β and TNF-α are the important cytokines in the development of FS41,44. One of the important role of IL-1β and TNF-α is direct and indirect modulating effects on neurons and neurotoxic neurotransmitters released during excitation or inflammation45.
Additionally, inflammation is a mechanism of innate immunity, this process involve the major subtypes of immune system cells, including monocytes, macrophages, neutrophils, lymphocytes, basophils, eosinophils, dendritic cells, and mast cells. Immune system activation is of evident importance in patients with FS46. So, this question was raised to us; what is the role of subtypes of immune system cells in FS? Previous studies supporting the hypothesis that associated increased cytokines play an important role in the development of FS indicate that during infections, immune cells such as macrophages, and lymphocytes are stimulated and consequently secrete proinflammatory cytokines such as IL-1β, TNF-α, and IL-647–49. Helminen et al.50 and Matsuo et al.51 studied peripheral blood monocytes in children with FS and found that peripheral blood mononuclear cells obtained at the time of FS were observed to produce more IL-1β than peripheral blood mononuclear cells obtained from control subjects. Matsuo et al.51 also showed that induction of leukocytes by double-stranded RNA resulted in a large-scale production of IL-1β in FS patients as compared to that in controls. IL-1β also stimulates the secretion of cortisol52–54. Cortisol induces leukocytosis, neutrophilia, and lymphopenia55. Woiciechowsky et al.56 also reported that intracerebroventricular infusion of IL-1β (but not TNF-α) dramatically increased peripheral neutrophil counts, whereas lymphocytes dropped. Romanowska et al.28 showed that children with FS had statistically significant higher neutrophils level compared to those with fever without seizures and the number of lymphocytes was lower in children with FS than in children with fever without seizures. In addition, Oguz et al.57 showed that children with FS had lower blood CD3 and CD4 values than healthy children. Except for the above studies, there is no information on the role of other subtypes of immune system cells in the development of FS, still this question remains unanswered for us, whether subtypes of immune system cells related to the effect of seizure attack during infection? According to my knowledge and literature review there is no document now to have satisfactory answer for this question. Responding to this question, more extensive study including FS group and febrile control group with the values of subtypes of immune system cells and IL-1β should be performed to clarify this point.
In this study, our main objective is to identify peripheral blood markers of FS and for that purpose; we are looking at the profile of white blood cells subsets focusing on monocytes, neutrophils, and lymphocytes. We did not include eosinophils and basophils, because they are granulocytic white blood cells that are rare in humans. The role of eosinophils in immunity remains enigmatic. They have been recognized as crucial players in allergic inflammation, but there is no document now to reveal an association between them and FS. As a result, we found children with FS had statistically significant higher neutrophils level and lower lymphocytes level in children with FS than in children with fever without seizures. And, no significant association was found between monocytes and FS.
NLR is a measure of the proportion of systemic neutrophils and lymphocytes, and may serve as an emerging parameter that reflects systemic inflammation of various diseases. In recent years, NLR has been suggested as one of practical predictor for differentiating FS types29,31. In this study, we found that febrile children with FS had statistically significant higher NLR levels compared to those without seizures. Romanowska et al.28, also found the similar results, which is the only related report in the literature. These findings suggested that elevated NLR is independently and strongly associated with increased risk of FS in Chinese children. Although the mechanism underlying the association is complex and remains to be elucidated, it may be related to increased neutrophil-dependent inflammation, and reduced lymphocyte mediated anti-inflammation response58,59. First, neutrophils are specialist cells of the innate immune system that play a major role in host defense through phagocytosis and generation of reactive oxygen species (ROS)60,61. Previous studies have demonstrated that there exists a cause and effect relationship between ROS production and epileptic seizures62–64. Second, neutrophils are the first cells to migrate into the area of injury as part of the host defense system, and can induce the secretion of several inflammatory cytokines associated with the risk of FS, especially IL-1β and TNF-α play an important role in the pathogenesis of FS41,65. The inflammation, thus triggered by these molecules can lead to further inflammation due to cell dysfunction61,66,67. Third, recent studies have reported that the voltage-gated sodium channels can comprise a family of nine alpha subunits (referred to as NaV1.1 through NaV1.9), which are variably expressed in leukocytes. A subset of neutrophils recruited to the site of injury has been demonstrated to express NaV1.3 sodium channels68,69. NaV1.3 channels can recover from inactivation rapidly and sustain high-frequency firing70. These channels can be activated during slow ramp depolarizations, and can produce persistent sodium current71–74. Finally, a low lymphocyte count specifies that the body’s resistance to fight infection is substantially reduced. Thus, neutrophils and lymphocytes play important role in the process of FS. Elevated NLR is associated with increased risk of FS.
Platelet activation has been reported as a common phenomenon in cardiovascular diseases and several malignancies, and inhibition of platelet activation could ameliorate inflammation75–78. MPV and platelet count are two main characteristics to evaluate platelet activation79. Elevated MPV is an indicator of larger, more reactive platelets resulting from an increased platelet turnover, and it may be used as an indicator of platelet activation and severity of inflammation16. Recent studies have shown that the platelet count was significantly lower28,30,33 and MPV was significantly higher30 in children with FS compared to febrile children without FS. In the present study, we also found that the MPV value was higher in the FS group; however, the value of platelet count was lower. Therefore, we suspected that there is an association between the FS risk and platelet activation, and MPR (measured by MPV and platelet count) could serve as a marker for FS risk. Our findings demonstrated that MPR may be a reliable predictive marker for FS risk. Although the underlying mechanisms are still to be elucidated, several hypotheses can be proposed. Recent study has suggested that platelet activation maybe a consequence of enhanced bacterial lipopolysaccharide (LPS) circulating levels80. Bacteria may activate platelets81; thus, the association between FS caused by the bacterial infection and in vivo platelet activation is biologically plausible. On the other hand, platelets can store a number of pro-inflammatory (IL-1α, IL-1β, and TGF-β1) and regulatory mediators (serotonin, dopamine, epinephrine, histamine, and GABA) in their granules, and are promptly released at the sites of inflammation or tissue injury during platelet activation79,82. Moreover, activated platelets may bind to neutrophils after neutrophils have adhered to activated endothelium83. Platelet–neutrophils interactions can also stimulate adhesion molecules and activate cytokine expression in neutrophils. Further activating platelets may stimulate entry of neutrophils into lesions and neutrophils that interact with platelets phagocytose bacteria more readily than unbound neutrophils. Furthermore, it has been shown that activated platelets can express P-selectin24 and E-selectin that are absolutely required for the delivery of neutrophils to the inflamed brain84. These generate the hypothesis that there is an interaction between platelets and neutrophils in relation to the risk of FS. Additionally, our study found a synergistic effect between MPR and NLR on an additive scale.
C-reactive protein (CRP), an acute-phase reactant secreted by the liver during inflammation, is considered one of the peaks of inflammatory markers. In this study, we found that CRP levels were significantly lower in children with FS compared to children without seizures and similar results were found by Romanowska et al.28. It can be suspected that children with FS develop inflammatory processes quickly enough that CRP levels do not reach their highest values. In children with fever without seizures, the inflammatory process was increasing slow enough to get CRP to higher levels28,85. Therefore, CRP is not identified as a factor affects the susceptibility of FS in this study.
We observed a statistically significant difference in NLR and MPV between the SFS group and the CFS group, whereas, no significant differences in the MPR, PLT and RDW values were observed between the two groups. To date, several studies have investigated the relationship between the inflammatory markers (NLR, PLT, MPV and RDW) and FS types, and suggested that NLR may be a practical predictor for differentiating FS types29,31. Consistently, in our study, NLR was significantly higher in the CFS group compared with the SFS group. Therefore, we believed that more inflammatory processes may have occurred in the brain of the patients with CFS. Particularly, ROC curve analysis of NLR in differentiating FS types was performed, and the optimal cut-off value of NLR was found to be 2.549. The sensitivity and specificity were 65.9% and 57.5%, respectively. The results are consistent with the previous studies29,31. Therefore, we agreed with the suggestion that NLR may provide clinicians with an insight into differentiating between simple and complex FS. Nevertheless, it is clear that the current sensitivity and specificity levels are moderate, far from assuring a definitive and objective differentiation.
The history for investigating MPV as a predictor for differentiating FS types is not long, but the evidence is increasing27,29,31,32. Nevertheless, the relationship between these factors and FS types was not consistent in different studies. For instance, Ozaydin et al.32, demonstrated that the MPV levels were significantly higher in the SFS group compared with the CFS group. However, Goksugur et al.29, and Yigit et al.31, revealed that there was no significant difference in MPV values between the two groups. Thus, the role of MPV in the differential diagnosis of two FS types is controversial. Although, we found that MPV was significantly higher in the SFS group than the CFS group in Chinese children, whether MPV will ever become a predictor for differentiating FS types remain to be seen. The physiological mechanisms of PLT and MPV in SFS and CFS will be explored first.
One limitation in our study should be addressed, because our study is a retrospective study, and in that serum cytokines such as IL-1β and TNF-α level were not measured normally in patients with FS. So there is no information on the levels of cytokines in our study. Experimental studies have concluded that IL-1β plays an important role in the pathogenesis of FS46,50,51,86,87. Several case-control studies also performed to measure the concentration of cytokines in the serum of seizure patients compared with that of healthy controls without seizures. However, conflicting results have been reported88,89. Choi et al. and Tutuncuoglu et al. reported that the serum IL-1β levels were significantly higher in FS patients than in the controls3,42. Haspolat et al.65 showed serum IL-1β and TNF-α level in patients with febrile seizures as compared to those in controls was not significantly different. Mahyar et al.89 showed serum IL-1β and TNF-α level in the simple and complex febrile seizure groups were significant lower than those in the control group. Another study also showed that the serum levels of the IFN-γ, IL-6, and IL-8 pro-inflammatory cytokines and the serum levels of the IL-10 and IL-1Ra anti-inflammatory cytokines were significantly higher in the FS children, however, serum IL-1β and TNF-α level in the two groups were not significantly different88. According above studies, IL-1β is an important factor influence the pathogenesis of FS. It does not mean any children with FS have higher level of IL-1β, we hypothesized that complex interactions among the above cytokines activation are involved in the pathogenesis of FS.
In conclusion, elevated NLR and MPR add evidence to the implication of white cells subsets in FS risk. Our results confirmed that NLR is an independent, albeit limited, predictor in differentiating between CFS and SFS. Moreover, NLR and MPR may have a synergistic effect that can influence the occurrence of FS.
Author Contributions
X.G.Y. and Z.G.L. designed the study. X.F.H., J.B., Z.W.P., X.X.L., D.N.Y. and H.P.Z. collected data. W.R.P. and X.G.Y. contributed the statistical analyses and discussion. Z.G.L., X.X.L. and M.P.Z. drafted the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhigang Liu, Xiangxin Li and Meipin Zhang contributed equally.
Contributor Information
Ruiping Wan, Email: imrp@163.com.
Xingguang Ye, Email: yestar1989@163.com.
References
- 1.Patterson JL, Carapetian SA, Hageman JR, Kelley KR. Febrile seizures. Pediatr Ann. 2013;42:249–254. doi: 10.3928/00904481-20131122-09. [DOI] [PubMed] [Google Scholar]
- 2.Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89:751–756. doi: 10.1136/adc.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation. 2011;8:135. doi: 10.1186/1742-2094-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dube CM, Brewster AL, Baram TZ. Febrile seizures: mechanisms and relationship to epilepsy. Brain Dev. 2009;31:366–371. doi: 10.1016/j.braindev.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy M, Dube CM, Ehrengruber M, Baram TZ. Inflammatory processes, febrile seizures, and subsequent epileptogenesis. Epilepsy Curr. 2014;14:15–22. doi: 10.5698/1535-7511-14.s2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saghazadeh A, Gharedaghi M, Meysamie A, Bauer S, Rezaei N. Proinflammatory and anti-inflammatory cytokines in febrile seizures and epilepsy: systematic review and meta-analysis. Rev Neurosci. 2014;25:281–305. doi: 10.1515/revneuro-2013-0045. [DOI] [PubMed] [Google Scholar]
- 7.Yu HM, Liu WH, He XH, Peng BW. IL-1beta: an important cytokine associated with febrile seizures? Neurosci Bull. 2012;28:301–308. doi: 10.1007/s12264-012-1240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon A, et al. Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure. 2018;59:5–10. doi: 10.1016/j.seizure.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan I, Karaman K, Sonmez B, Celik S, Turker O. Relationship between serum neutrophil count and infarct size in patients with acute myocardial infarction. Nucl Med Commun. 2009;30:797–801. doi: 10.1097/MNM.0b013e32832e3a16. [DOI] [PubMed] [Google Scholar]
- 11.Templeton AJ, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama Y, et al. Usefulness of the neutrophil/lymphocyte ratio measured preoperatively as a predictor of peritoneal metastasis in patients with advanced gastric cancer. Surg Today. 2014;44:2146–2152. doi: 10.1007/s00595-014-0917-1. [DOI] [PubMed] [Google Scholar]
- 13.Tasoglu I, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann Vasc Surg. 2014;28:606–613. doi: 10.1016/j.avsg.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139:220–231. doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 15.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–161. doi: 10.1097/00001721-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 17.Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med. 1993;13:937–950. doi: 10.1016/S0272-2712(18)30418-9. [DOI] [PubMed] [Google Scholar]
- 18.Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi: 10.3389/fimmu.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. Combination of Mean Platelet Volume/Platelet Count Ratio and the APACHE II Score Better Predicts the Short-Term Outcome in Patients with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy. Kidney Blood Press. Res. 2018;43:479–489. doi: 10.1159/000488694. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Garcia AC, et al. Platelet count and mean platelet volume predict outcome in adults with Eisenmenger syndrome. Heart. 2018;104:45–50. doi: 10.1136/heartjnl-2016-311144. [DOI] [PubMed] [Google Scholar]
- 21.Shin DH, et al. An Increase in Mean Platelet Volume/Platelet Count Ratio Is Associated with Vascular Access Failure in Hemodialysis Patients. PLoS One. 2017;12:e0170357. doi: 10.1371/journal.pone.0170357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, et al. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016;37:9323–9331. doi: 10.1007/s13277-015-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Zhang H, Zhang B, Zhang L, Wang C. Prognostic value of combination of preoperative platelet count and mean platelet volume in patients with resectable non-small cell lung cancer. Oncotarget. 2017;8:15632–15641. doi: 10.18632/oncotarget.14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maugeri N, et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- 25.Lippi G, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–632. doi: 10.1043/1543-2165-133.4.628. [DOI] [PubMed] [Google Scholar]
- 26.Yesil A, et al. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. 2011;5:460–467. doi: 10.5009/gnl.2011.5.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikkhah A, Salehiomran MR, Asefi SS. Differences in Mean Platelet Volume and Platelet Count between Children with Simple and Complex Febrile Seizures. Iran J Child Neurol. 2017;11:44–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Gontko-Romanowska K, et al. The assessment of laboratory parameters in children with fever and febrile seizures. Brain Behav. 2017;7:e00720. doi: 10.1002/brb3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goksugur SB, Kabakus N, Bekdas M, Demircioglu F. Neutrophil-to-lymphocyte ratio and red blood cell distribution width is a practical predictor for differentiation of febrile seizure types. Eur Rev Med Pharmacol Sci. 2014;18:3380–3385. [PubMed] [Google Scholar]
- 30.Abuhandan M, et al. Evaluation of selenium levels and mean platelet volume in patients with simple febrile convulsion. Iran J Pediatr. 2014;24:401–405. [PMC free article] [PubMed] [Google Scholar]
- 31.Yigit Y, et al. The role of neutrophil-lymphocyte ratio and red blood cell distribution width in the classification of febrile seizures. Eur Rev Med Pharmacol Sci. 2017;21:554–559. [PubMed] [Google Scholar]
- 32.Ozaydin E, et al. Differences in iron deficiency anemia and mean platelet volume between children with simple and complex febrile seizures. Seizure. 2012;21:211–214. doi: 10.1016/j.seizure.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Jun YS, Bang HI, Yu ST, Shin SR, Choi DY. Relationship between iron deficiency anemia and febrile convulsion in infants. Korean Journal of Pediatrics. 2010;53:392. doi: 10.3345/kjp.2010.53.3.392. [DOI] [Google Scholar]
- 34.Tunesi S, et al. Gene-asbestos interaction in malignant pleural mesothelioma susceptibility. Carcinogenesis. 2015;36:1129–1135. doi: 10.1093/carcin/bgv097. [DOI] [PubMed] [Google Scholar]
- 35.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 36.Muthuri SG, et al. Gene-environment interaction between body mass index and transforming growth factor beta 1 (TGFbeta1) gene in knee and hip osteoarthritis. Arthritis Res Ther. 2013;15:R52. doi: 10.1186/ar4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 38.Knol MJ, et al. Estimating measures of interaction on an additive scale for preventive exposures. Eur J Epidemiol. 2011;26:433–438. doi: 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jan MM, Girvin JP. Febrile seizures. Update and controversies. Neurosciences (Riyadh) 2004;9:235–242. [PubMed] [Google Scholar]
- 40.French JA. Febrile seizures: possible outcomes. Neurology. 2012;79:e80–82. doi: 10.1212/WNL.0b013e31826aa902. [DOI] [PubMed] [Google Scholar]
- 41.Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002;43:920–923. doi: 10.1046/j.1528-1157.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 42.Tutuncuoglu S, et al. Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int. 2001;43:235–239. doi: 10.1046/j.1442-200x.2001.01389.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang W, et al. TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain. Brain Behav. Immun. 2015;48:68–77. doi: 10.1016/j.bbi.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins SJ. The pathophysiological role of cytokines. Leg Med (Tokyo) 2003;5(Suppl 1):S45–57. doi: 10.1016/S1344-6223(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 45.Tomoum HY, Badawy NM, Mostafa AA, Harb MY. Plasma interleukin-1beta levels in children with febrile seizures. J Child Neurol. 2007;22:689–692. doi: 10.1177/0883073807304007. [DOI] [PubMed] [Google Scholar]
- 46.Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol. 2005;57:152–155. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuhas Y, et al. Involvement of tumor necrosis factor alpha and interleukin-1beta in enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae. Infect Immun. 1999;67:1455–1460. doi: 10.1128/iai.67.3.1455-1460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain Res. 1995;692:244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- 49.Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/S0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 50.Helminen M, Vesikari T. Increased interleukin-1 (IL-1) production from LPS-stimulated peripheral blood monocytes in children with febrile convulsions. Acta Paediatr Scand. 1990;79:810–816. doi: 10.1111/j.1651-2227.1990.tb11559.x. [DOI] [PubMed] [Google Scholar]
- 51.Matsuo M, Sasaki K, Ichimaru T, Nakazato S, Hamasaki Y. Increased IL-1beta production from dsRNA-stimulated leukocytes in febrile seizures. Pediatr Neurol. 2006;35:102–106. doi: 10.1016/j.pediatrneurol.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 53.Dinarello CA. Biology of interleukin 1. FASEB J. 1988;2:108–115. doi: 10.1096/fasebj.2.2.3277884. [DOI] [PubMed] [Google Scholar]
- 54.Lang CH, Cooney R, Vary TC. Central interleukin-1 partially mediates endotoxin-induced changes in glucose metabolism. Am J Physiol. 1996;271:E309–316. doi: 10.1152/ajpendo.1996.271.2.E309. [DOI] [PubMed] [Google Scholar]
- 55.Tonnesen E, Christensen NJ, Brinklov MM. Natural killer cell activity during cortisol and adrenaline infusion in healthy volunteers. Eur J Clin Invest. 1987;17:497–503. doi: 10.1111/j.1365-2362.1987.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 56.Woiciechowsky C, et al. Brain IL-1beta increases neutrophil and decreases lymphocyte counts through stimulation of neuroimmune pathways. Neurobiol Dis. 1999;6:200–208. doi: 10.1006/nbdi.1999.0242. [DOI] [PubMed] [Google Scholar]
- 57.Tuncer O, et al. Lymphocytes subsets in children with febrile convulsions. Int J Neurosci. 2007;117:919–925. doi: 10.1080/00207450600910713. [DOI] [PubMed] [Google Scholar]
- 58.Hernberg M, et al. The prognostic role of blood lymphocyte subset distribution in patients with resected high-risk primary or regionally metastatic melanoma. J Immunother. 2007;30:773–779. doi: 10.1097/CJI.0b013e31814e0898. [DOI] [PubMed] [Google Scholar]
- 59.Brandau S, Dumitru CA, Lang S. Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol. 2013;35:163–176. doi: 10.1007/s00281-012-0344-6. [DOI] [PubMed] [Google Scholar]
- 60.Zheng J, et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: a Meta-Analysis and Systematic Review. Cell Physiol Biochem. 2017;44:967–981. doi: 10.1159/000485396. [DOI] [PubMed] [Google Scholar]
- 61.Mantovani A, Cassatella M, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 62.Abuhandan M, et al. The oxidative and antioxidative status of simple febrile seizure patients. J Pak Med Assoc. 2013;63:594–597. [PubMed] [Google Scholar]
- 63.Choi BH. Oxygen, antioxidants and brain dysfunction. Yonsei Med J. 1993;34:1–10. doi: 10.3349/ymj.1993.34.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Sobaniec W, et al. Evaluation of the influence of antiepileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J Child Neurol. 2006;21:558–562. doi: 10.1177/08830738060210070501. [DOI] [PubMed] [Google Scholar]
- 65.Haspolat S, et al. Interleukin-1beta, tumor necrosis factor-alpha, and nitrite levels in febrile seizures. J Child Neurol. 2002;17:749–751. doi: 10.1177/08830738020170101501. [DOI] [PubMed] [Google Scholar]
- 66.Avcil, S. Evaluation of the neutrophil/lymphocyte ratio, platelet/lymphocyte ratio and mean platelet volume as inflammatory markers in children with attention-deficit/hyperactivity disorder. Psychiatry Clin. Neurosci (2018). [DOI] [PubMed]
- 67.Nikolaus S, et al. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998;42:470–476. doi: 10.1136/gut.42.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinberg Benjamin Ethan. Neutrophils. Anesthesiology. 2018;128(6):1060–1061. doi: 10.1097/ALN.0000000000002205. [DOI] [PubMed] [Google Scholar]
- 69.Poffers Marit, Bühne Nathalie, Herzog Christine, Thorenz Anja, Chen Rongjun, Güler Faikah, Hage Axel, Leffler Andreas, Echtermeyer Frank. Sodium Channel Nav1.3 Is Expressed by Polymorphonuclear Neutrophils during Mouse Heart and Kidney Ischemia In Vivo and Regulates Adhesion, Transmigration, and Chemotaxis of Human and Mouse Neutrophils In Vitro. Anesthesiology. 2018;128(6):1151–1166. doi: 10.1097/ALN.0000000000002135. [DOI] [PubMed] [Google Scholar]
- 70.Cummins TR, et al. Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J Neurosci. 2001;21:5952–5961. doi: 10.1523/JNEUROSCI.21-16-05952.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Estacion M, Gasser A, Dib-Hajj SD, Waxman SG. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp Neurol. 2010;224:362–368. doi: 10.1016/j.expneurol.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Sun GC, Werkman TR, Battefeld A, Clare JJ, Wadman WJ. Carbamazepine and topiramate modulation of transient and persistent sodium currents studied in HEK293 cells expressing the Na(v)1.3 alpha-subunit. Epilepsia. 2007;48:774–782. doi: 10.1111/j.1528-1167.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 73.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen YH, et al. Cloning, distribution and functional analysis of the type III sodium channel from human brain. Eur J Neurosci. 2000;12:4281–4289. [PubMed] [Google Scholar]
- 75.Venturinelli ML, et al. Platelet activation in different clinical forms of the coronary artery disease (role of P-selectin and others platelet markers in stable and unstable angina) Arq Bras Cardiol. 2006;87:446–450. doi: 10.1590/S0066-782X2006001700008. [DOI] [PubMed] [Google Scholar]
- 76.Kapoor, J. R. Platelet activation and atherothrombosis. N Engl J Med 358, 1638; author reply 1638–1639, 10.1056/NEJMc080056 (2008). [DOI] [PubMed]
- 77.Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41:302–310. doi: 10.1053/j.seminoncol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Stegner D, Dutting S, Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014;133(Suppl 2):S149–157. doi: 10.1016/S0049-3848(14)50025-4. [DOI] [PubMed] [Google Scholar]
- 79.Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22:1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 80.Raparelli V, et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017;65:571–581. doi: 10.1002/hep.28853. [DOI] [PubMed] [Google Scholar]
- 81.Cangemi R, et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol. 2014;64:1917–1925. doi: 10.1016/j.jacc.2014.07.985. [DOI] [PubMed] [Google Scholar]
- 82.Ponomarev E. Fresh Evidence for Platelets as Neuronal and Innate Immune Cells: Their Role in the Activation, Differentiation, and Deactivation of Th1, Th17, and Tregs during Tissue Inflammation. Front Immunol. 2018;9:406. doi: 10.3389/fimmu.2018.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sreeramkumar V, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carvalho-Tavares J, et al. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.RES.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 85.Calkosinski I, et al. [Characterization of an inflammatory response] Postepy Hig Med Dosw (Online) 2009;63:395–408. [PubMed] [Google Scholar]
- 86.Dube CM, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 2010;30:7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukuda M, et al. Postnatal interleukin-1beta enhances adulthood seizure susceptibility and neuronal cell death after prolonged experimental febrile seizures in infantile rats. Acta Neurol Belg. 2014;114:179–185. doi: 10.1007/s13760-013-0246-y. [DOI] [PubMed] [Google Scholar]
- 88.Kim K, et al. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures. J Neuroinflammation. 2017;14:200. doi: 10.1186/s12974-017-0974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahyar A, et al. Serum interleukin-1beta and tumor necrosis factor-alpha in febrile seizures: is there a link? Korean J Pediatr. 2014;57:440–444. doi: 10.3345/kjp.2014.57.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.