Abstract
This experiment aimed to explore whether glutamate (Glu) had beneficial effects on intestinal injury caused by Escherichia coli LPS challenge via regulating mTOR, TLRs, as well as NODs signaling pathways. Twenty-four piglets were allotted to 4 treatments including: (1) control group; (2) LPS group; (3) LPS + 1.0% Glu group; (4) LPS + 2.0% Glu group. Supplementation with Glu increased jejunal villus height/crypt depth ratio, ileal activities of lactase, maltase and sucrase, and RNA/DNA ratio and protein abundance of claudin-1 in jejunum and ileum. In addition, the piglets fed Glu diets had higher phosphorylated mTOR (Ser2448)/total mTOR ratio in jejunum and ileum. Moreover, Glu decreased TNF-α concentration in plasma. Supplementation with Glu also decreased mRNA abundance of jejunal TLR4, MyD88, IRAK1, TRAF6, NOD2 and increased mRNA abundance of ileal Tollip. These results indicate that Glu supplementation may be closely related to maintaining mTOR and inhibiting TLR4 and NOD signaling pathways, and concomitant improvement of intestinal integrity under an inflammatory condition.
Introduction
The intestine is not only critical for the digestion and absorption of nutrients, but also interacts with a complex external milieu1. As the largest component of innate immune system, the intestine employs a number of unique strategies to defend against bacterial-derived endogenous and exogenous harmful agents2. However, the intestinal health status could be hazarded by a lot of factors including infection and inflammation3,4. Inflammation can potentially leads to intestinal injury and dysfunction which can result in decreased animal performance and health5. Therefore, maintaining intestinal health is vital for humans and animals.
In weaned piglets, conditions that compromise the integrity of the small intestine may be related to processes of the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α)6, which could be induced by activation of inflammatory signaling pathways such as toll-like receptors (TLRs) and nucleotide-binding oligomerization domain proteins (NODs)7,8. TLRs and NODs are important protein families that participate in recognizing pathogen-associated molecular patterns (PAMPs) and modulating congenital antibacterial and inflammatory responses7,9. In the TLRs family, TLR4 is a critical receptor in transduction of the inflammatory response and plays an important role in intestinal homeostasis10. Meanwhile, NOD1 and NOD2 are prominent members in NODs family, whose roles in innate immunity and inflammatory diseases have been studied11, particularly in intestine12,13. After interacting with their specific PAMPs, activated TLRs or NODs trigger downstream signaling pathways that lead to activation of nuclear factor-κB (NF-κB), which further motivates the expression of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6 and TNF-α7,8. Therefore, exploring nutrients to improve intestinal integrity through inhibiting intestinal TLR4 and NOD signaling pathways further reducing inflammatory response has been a research hotspot in the human or animal nutrition areas.
Mammalian target of rapamycin (mTOR) is a serine-threonine kinase which is related to several important aspects of mammalian cell function14. The activity of mTOR is regulated by various extracellular and intracellular factors such as amino acids, energy and hormones, in turn, mTOR changes rates of translation, transcription, protein synthesis and degradation, cell signaling, metabolism as well as cytoskeleton dynamics15–17. Many researchers have presented that activation of mTOR signaling pathway could improve intestinal health status including enhanced intestinal barrier function in Caco-2 cells18, and improved cell proliferation and protein synthesis in intestinal porcine epithelial cells-J2 (IPEC-J2)15,19. Therefore, exploring a nutritional additive to improve intestinal integrity by activating mTOR signaling is also a critical issue which needs to be solved.
Glutamate (Glu), as an acidic amino acid, is traditionally classified as a non-essential amino acid. As the research developed, some studies have shown that Glu has some important functions in nutrition, metabolism and organ health status. Firstly, Glu is one of the major metabolic fuels for small intestine to maintain function and protect mucosal integrity20. In addition, it is important in stabilizing the protein structure21 and activating taste receptors in the digestive tract21,22. Moreover, Glu is a member of the “arginine family”, which comprises arginine, glutamine, glutamate, proline, aspartate, asparagine, ornithine, and citrulline. These amino acids are interconvertible via complex interorgan metabolism to play important roles in the body23. Some studies showed that dietary supplementation with Glu improved intestinal health status23,24. Rezaei et al. reported that monosodium glutamate supplementation maintained the small-intestinal structure and function in weaned pigs24. Jiao et al. reported that Glu enhanced barrier and antioxidative functions in intestinal porcine epithelial cells25. These studies are mostly focused on the beneficial effect of Glu on intestinal morphology, barrier and antioxidative capacity, however, few reports about the mechanisms underlying the protective effect of Glu are found.
Accordingly, we hypothesized that Glu may ameliorate intestinal injury induced by lipopolysaccharide (LPS) through regulating mTOR, TLR4 as well as NOD signaling pathways. The aim of our experiment was to explore whether Glu could attenuate LPS-induced intestinal injury, and to reveal its molecular mechanism(s).
Results
Intestinal morphology
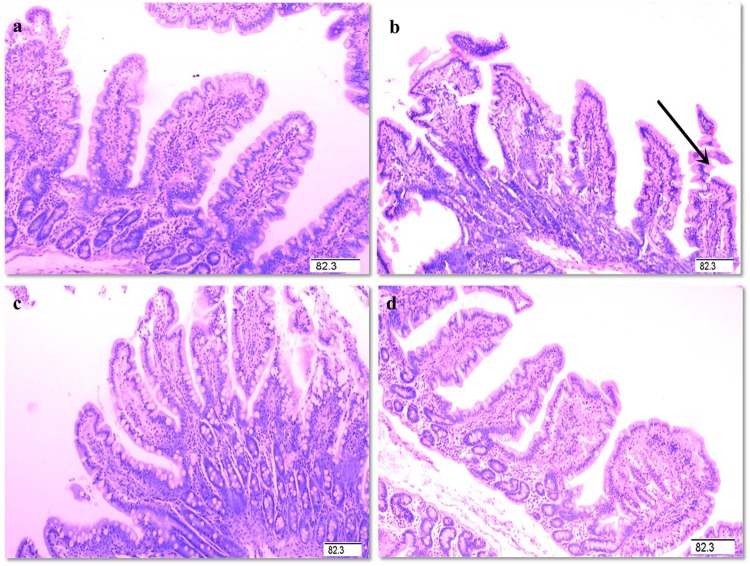
The intestinal mucosal condition in control piglets was good (Fig. 1). However, the LPS-challenged pigs exhibited intestinal injury. After supplementation with Glu, the intestinal mucosal injury was alleviated. Compared with the pigs in LPS group, supplementation with 2.0% Glu increased villus height/crypt depth ratio (VCR, Table 1) in jejunum (p < 0.05).
Figure 1.
Histological appearance of jejunal mucosa (hematoxylin and eosin). (a) control. (b) LPS. Arrow represents the damaged intestinal mucosa in the LPS-challenged pigs. (c) LPS + 1.0% Glu. (d) LPS + 2.0% Glu. Original magnification 100×. Scale bars = 82.6 μm.
Table 1.
Effects of dietary supplementation with Glu on intestinal morphology of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| Villus height (μm) | 289 | 285 | 285 | 274 | 14 | 0.731 |
| Crypt depth (μm) | 106 | 102 | 95 | 94 | 6 | 0.148 |
| Villus height/crypt depth ratio | 2.79c | 2.82bc | 2.89ab | 2.94a | 0.05 | 0.014 |
| Ileum | ||||||
| Villus height (μm) | 241 | 240 | 265 | 232 | 17 | 0.285 |
| Crypt depth (μm) | 83 | 81 | 87 | 78 | 6 | 0.487 |
| Villus height/crypt depth ratio | 2.92 | 2.91 | 3.03 | 2.97 | 0.05 | 0.081 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Intestinal protein, DNA and RNA contents
LPS challenge reduced the ratio of RNA to DNA in ileum compared with the pigs in control group (p < 0.05, Table 2). Among the LPS-challenged pigs, supplementation of 2.0% Glu increased the ratio of RNA to DNA in jejunum (p < 0.05). Supplementation with 1.0% Glu increased the ratio of RNA to DNA in ileum (p < 0.05).
Table 2.
Effects of dietary supplementation with Glu on intestinal mucosal protein, DNA and RNA concentrations of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| Protein (mg/g tissue) | 84.8 | 82.8 | 89.4 | 86.6 | 5.7 | 0.691 |
| RNA/DNA (μg/μg) | 3.2b | 3.3b | 4.1ab | 5.2a | 0.6 | 0.026 |
| Protein/DNA (mg/μg) | 0.198 | 0.168 | 0.220 | 0.205 | 0.021 | 0.125 |
| Ileum | ||||||
| Protein (mg/g tissue) | 58.2 | 61.8 | 60.6 | 64.5 | 3.2 | 0.287 |
| RNA/DNA (μg/μg) | 10.2a | 7.6b | 9.9a | 8.4ab | 0.9 | 0.018 |
| Protein/DNA (mg/μg) | 0.310 | 0.240 | 0.303 | 0.250 | 0.033 | 0.101 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Intestinal disaccharidase activities
LPS challenge decreased the activity of maltase in jejunum, as well as the activities of maltase and sucrase in ileum of the piglets (p < 0.05, Table 3). However, supplementation with 1.0% Glu increased the activities of maltase and sucrase in ileum compared with pigs in LPS group (p < 0.05). Moreover, supplementation with 2.0% Glu increased the activities of lactase compared with other groups (p < 0.05).
Table 3.
Effects of dietary supplementation with Glu on disaccharidase activities of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| Lactase (U/mg protein) | 15.6 | 14.6 | 7.9 | 12.9 | 5.1 | 0.459 |
| Maltase (U/mg protein) | 169a | 75b | 92b | 97b | 19 | <0.001 |
| Sucrase (U/mg protein) | 47.3 | 38.7 | 25.2 | 29.6 | 10 | 0.160 |
| Ileum | ||||||
| Lactase (U/mg protein) | 1.6b | 1.3b | 2.7b | 4.4a | 0.7 | 0.001 |
| Maltase (U/mg protein) | 85a | 56b | 74a | 51b | 8 | 0.001 |
| Sucrase (U/mg protein) | 11.6a | 7.7b | 10.3a | 7.2b | 1.1 | 0.002 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
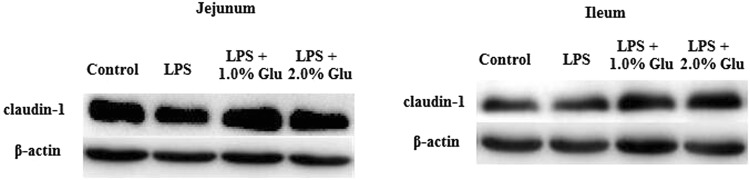
Intestinal claudin-1 protein expression
No difference was observed in intestinal claudin-1 protein expression between control and LPS-challenged piglets (Table 4). Supplementation with Glu increased the protein expression of claudin-1 both in jejunum and ileum compared with the pigs in LPS group (Fig. 2, p < 0.05).
Table 4.
Effects of dietary supplementation with Glu on tight junction protein expression in the intestinal mucosa of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| Claudin-1/β-actin | 0.202b | 0.246b | 0.331a | 0.328a | 0.033 | 0.002 |
| Ileum | ||||||
| Claudin-1/β-actin | 0.531c | 0.653bc | 0.730b | 0.974a | 0.087 | 0.002 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Figure 2.
Western blotting analysis of the expression of clauding-1 in the jejunal and ileal mucosa. n = 6.
Plasma TNF-α concentration
Compared with control piglets, LPS challenge increased TNF-α concentration in plasma (p < 0.05, Table 5). Supplementation with 2.0% Glu decreased plasma TNF-α concentration in piglets compared with LPS group piglets (p < 0.05).
Table 5.
Effects of dietary supplementation with Glu on TNF-α concentration in plasma after 4 h Escherichia coli LPS challenge in weaned pigs.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Plasma TNF-α, pg/ml | 49c | 2793a | 1578ab | 1029bc | 679 | 0.006 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
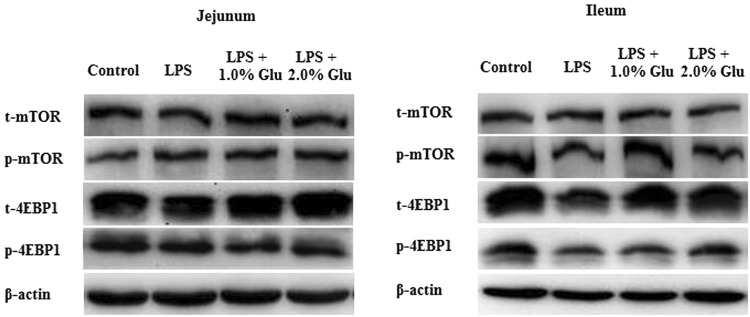
Intestinal protein abundances of key signaling molecules in mTOR signaling pathway
The piglets challenged with LPS had decreased phosphorylated mTOR (Ser2448) (p-mTOR, p < 0.05) and phosphorylated 4E-binding protein 1 (Thr70) (p-4EBP1, p < 0.05) protein expression in ileum (Table 6, Fig. 3). The ratio of p-4EBP1/t-4EBP1 protein expression in ileum of LPS-challenged pigs was also reduced compared with pigs in control group (p < 0.05). Compared with the pigs in LPS group, supplementation with 2.0% Glu increased jejunal ratio of p-mTOR/t-mTOR (p < 0.05) and supplementation with 1.0% Glu increased ileal ratio of p-mTOR/t-mTOR (p < 0.05).
Table 6.
Effects of dietary supplementation with Glu on protein expression of proteins related to mTOR signaling pathway in the intestinal mucosa of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| t-mTOR | 42.2 | 37.6 | 45.2 | 50.0 | 7.9 | 0.473 |
| p-mTOR | 21.7 | 22.3 | 25.5 | 27.9 | 4.9 | 0.557 |
| p-mTOR/t-mTOR | 0.54b | 0.58b | 0.48b | 0.73a | 0.07 | 0.006 |
| t-4EBP1 | 21.0 | 27.9 | 26.3 | 23.5 | 5.6 | 0.634 |
| p-4EBP1 | 10.0 | 8.0 | 5.8 | 5.8 | 2.6 | 0.338 |
| p-4EBP1/t-4EBP1 | 0.34 | 0.27 | 0.18 | 0.28 | 0.08 | 0.276 |
| Ileum | ||||||
| t-mTOR | 12.7 | 11.1 | 10.2 | 11.1 | 2.1 | 0.673 |
| p-mTOR | 15.6a | 11.8b | 14.5ab | 12.3b | 1.5 | 0.062 |
| p-mTOR/t-mTOR | 1.29b | 1.11b | 1.79a | 1.27b | 0.23 | 0.042 |
| t-4EBP1 | 30.1 | 21.2 | 30.3 | 31.8 | 4.8 | 0.145 |
| p-4EBP1 | 36.0a | 16.4b | 19.3b | 28.1ab | 6.2 | 0.017 |
| p-4EBP1/t-4EBP1 | 1.22a | 0.77b | 0.62b | 0.90ab | 0.17 | 0.020 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Figure 3.
Western blotting analysis of the expression of mTOR and 4EBP1 in the jejunal and ileal mucosa. n = 6.
Intestinal mRNA expression of key signaling molecules in TLR4 and NODs signaling pathways
The LPS-challenged pigs had increased mRNA expression of TLR4, Myeloid differentiation protein 2 (MD2), cluster of differentiation 14 (CD14), myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase 1 (IRAK1), TNF receptor associated factor 6 (TRAF6), NOD2, receptor-interacting serine/threonine-protein kinase 2 (RIPK2), NF-κB in jejunum, and NOD2 and RIPK2 in ileum compared with control piglets (p < 0.05, Table 7). Compared with pigs in LPS group, supplementation with 2.0% Glu reduced mRNA expression of jejunal TLR4, MyD88, IRAK1, TRAF6, and NOD2 (p < 0.05).
Table 7.
Effects of dietary supplementation with Glu on mRNA expression of key genes related to TLR4 and NODs signaling pathways in the intestinal mucosa of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| TLR4 | 1.00c | 1.85a | 1.64ab | 1.33bc | 0.21 | 0.004 |
| MD2 | 1.00b | 1.97a | 1.91a | 1.83a | 0.21 | 0.001 |
| CD14 | 1.00b | 2.16a | 2.75a | 2.32a | 0.41 | 0.003 |
| LBP | 1.00 | 0.88 | 0.84 | 0.60 | 0.22 | 0.336 |
| MyD88 | 1.00b | 1.25a | 1.30a | 0.98b | 0.09 | 0.004 |
| IRAK1 | 1.00bc | 1.16a | 1.03ab | 0.87c | 0.06 | 0.002 |
| TRAF6 | 1.00bc | 1.32a | 1.17ab | 0.93c | 0.09 | 0.003 |
| NOD1 | 1.00a | 0.70ab | 0.87a | 0.46b | 0.14 | 0.007 |
| NOD2 | 1.00b | 2.32a | 1.97a | 1.33b | 0.21 | <0.001 |
| RIPK2 | 1.00b | 1.99a | 1.83a | 1.64a | 0.17 | <0.001 |
| NF-κB | 1.00b | 1.41a | 1.29a | 1.18ab | 0.11 | 0.012 |
| Ileum | ||||||
| TLR4 | 1.00 | 1.28 | 1.25 | 1.12 | 0.13 | 0.152 |
| MD2 | 1.00b | 1.33a | 1.21ab | 1.32a | 0.14 | 0.087 |
| CD14 | 1.00b | 1.13b | 1.44ab | 1.69a | 0.22 | 0.022 |
| LBP | 1.00 | 1.18 | 1.13 | 0.62 | 0.35 | 0.388 |
| MyD88 | 1.00b | 1.10ab | 1.20a | 0.97b | 0.06 | 0.010 |
| IRAK1 | 1.00 | 0.87 | 0.90 | 0.91 | 0.06 | 0.261 |
| TRAF6 | 1.00b | 1.12ab | 1.32a | 1.12ab | 0.11 | 0.062 |
| NOD1 | 1.00 | 1.07 | 0.89 | 0.83 | 0.12 | 0.241 |
| NOD2 | 1.00b | 2.06a | 2.19a | 1.69ab | 0.35 | 0.013 |
| RIPK2 | 1.00b | 2.01a | 2.02a | 1.85a | 0.19 | < 0.001 |
| NF-κB | 1.00 | 1.13 | 1.11 | 1.04 | 0.09 | 0.513 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Intestinal mRNA expression of TLR4 and NODs negative regulators
Compared with the pigs in control group, LPS challenge decreased mRNA expression of toll-interacting protein (Tollip), and Erbb2 interacting protein (ERBB2IP) in ileum (p < 0.05, Table 8). Supplementation with 2.0% Glu enhanced mRNA expression of Tollip in ileum (p < 0.05). However, the mRNA expressions of ERBB2IP in jejunum were decreased with the supplementation with 2.0% Glu (p < 0.05).
Table 8.
Effects of dietary supplementation with Glu on mRNA expression of negative regulating factors related to TLR4 and NODs signaling pathways in the intestinal mucosa of weanling piglets after 4 h Escherichia coli LPS challenge.
| Item | Control | LPS | LPS + 1.0% Glu | LPS + 2.0% Glu | SEM | p value |
|---|---|---|---|---|---|---|
| Jejunum | ||||||
| RP105 | 1.00 | 2.09 | 2.19 | 1.44 | 0.62 | 0.210 |
| SOCS1 | 1.00b | 2.98a | 2.56a | 2.21a | 0.46 | 0.002 |
| Tollip | 1.00 | 0.61 | 0.74 | 0.93 | 0.18 | 0.145 |
| SIGIRR | 1.00 | 1.00 | 0.93 | 0.97 | 0.06 | 0.634 |
| ERBB2IP | 1.00a | 0.96a | 0.91a | 0.74b | 0.08 | 0.014 |
| ACAP1 | 1.00a | 0.84ab | 0.88a | 0.66b | 0.10 | 0.018 |
| Ileum | ||||||
| RP105 | 1.00 | 0.76 | 0.89 | 1.01 | 0.23 | 0.663 |
| SOCS1 | 1.00b | 1.11b | 1.56a | 1.07b | 0.19 | 0.028 |
| Tollip | 1.00a | 0.64b | 0.90ab | 1.13a | 0.15 | 0.025 |
| SIGIRR | 1.00 | 1.07 | 1.07 | 0.96 | 0.10 | 0.632 |
| ERBB2IP | 1.00a | 0.80b | 0.93ab | 0.88ab | 0.07 | 0.049 |
| ACAP1 | 1.00 | 0.78 | 0.78 | 0.80 | 0.10 | 0.113 |
Data are the means of 6 replicates with 1 piglet per replicate. Means with the same row without common superscripts differ (p < 0.05).
Discussion
Glu is one of the most abundant amino acids in alimentary proteins26. The majority of Glu molecule is either oxidized as energy or metabolized into other nonessential amino acids via transamination27. Recently, some studies showed that Glu plays an important role in maintaining physiological function as well as energy level in intestine28. In the present study, Glu improved the intestinal integrity and function via maintaining mTOR, as well as suppressing TLR4 and NOD signaling pathways in the piglets under an inflammation condition.
The intestinal healthy status is often reflected by both structural and functional integrity4. Villus height, crypt depth and VCR have been used to quantify the intestinal morphology29. Mucosal protein content is an important indicator for cell metabolism, and protein deficiency negatively affects the intestinal epithelial barrier of piglets30. RNA/DNA ratio reflects the cell capacity for protein synthesis31. Protein/DNA ratio in mucosa has been employed as an indicator of intestinal growth and repair4. Mucosal disaccharidases, namely lactase, maltase and sucrase, reflect intestinal digestive function32. Claudins are major constituents of tight junctions33. In present study, the piglets challenged with LPS had decreased RNA/DNA ratio, and maltase and sucrase activities, which is similar to the findings of Liu et al. and Wang et al.4,34. These results indicate that injection of LPS caused intestinal injury in weaned pigs. Glu supplementation to the LPS-challenged pigs increased jejunal VCR. Besides, supplementation with Glu increased RNA/DNA ratio as well as protein expression of claudin-1 in jejunum and ileum. Furthermore, the pigs fed Glu diets had enhanced ileal disaccharidase activities. These results indicate that Glu had a significant effect on protecting intestinal mucosa and improving intestinal repair. Similarly, there were several studies that showed results in accordance with our findings. Rezaei et al. reported that monosodium glutamate supplementation increased villus height and DNA content in weaned pigs24. In addition, Lin et al. found that Glu supplementation increased the relative mRNA expression of occludin and zonula occludens protein-1 in jejunal mucosa in weaning piglets35.
mTOR is a serine-threonine kinase which is related to several important aspects of mammalian cell function. The activity of mTOR is modulated through various extracellular and intracellular factors (such as amino acids, energy, hormones), in turn, mTOR changes rates of translation, transcription, protein synthesis degradation, cell signaling, metabolism, and cytoskeleton dynamics14. Specially, mTOR signaling pathway plays a key role in maintaining intestinal health15,18,19. 4EBP1, as one of the most well-known substrates of mTOR, is involved in regulation of the rate of protein synthesis36. Phosphorylation of 4EBP1 by mTOR promotes dissociation of 4EBP1 from Eukaryotic initiation factor 4E (EIF4E), enabling EIF4E to induce protein translation37. Recent studies have shown that amino acids as important signaling regulators for intestinal protein synthesis and cell growth via mTOR, could regulate downstream signal 4EBP115,16. In this experiment, supplementation with Glu increased jejunal and ileal ratio of p-mTOR/t-mTOR in the LPS-challenged pigs. These results indicate that Glu activated mTOR signaling pathway, further to improve protein synthesis, which is consistent with increased intestinal RNA/DNA. We speculated that the protective effect of Glu on intestinal mTOR signaling pathway might be due to the following mechanisms. Firstly, Glu phosphorylated mTOR directly. There were reports showed that Glu activated mTOR as neurotransmitter, or the activation of both ionotropic and metabotropic glutamate receptors (mGluR) induced mTOR14. Glu supplementation increased the relative mRNA expression of the jejunal mucosa mGluR1 and mGluR4 in weaning piglets35. Secondly, Glu could be oxidized directly to produce energy which further affects mTOR. Glu has been demonstrated to be one of the major sources of energy in mammalian enterocytes via mitochondrial oxidation21. In addition, Glu might exert its beneficial effects through transforming into arginine and glutamine23. Abundant evidences have shown that arginine and glutamine participate in regulating mTOR and their downstream signals in intestine15,38. For example, there was study demonstrating that arginine-dependent cell survival and protein synthesis signaling in IPEC-J2 cells were mediated by mTOR15. Xi et al. reported that glutamine could regulate protein synthesis in intestinal cells through the mTOR signaling pathway38.
TLR4 and NOD signaling pathways are activated to defense against pathogens invading via triggering the production of pro-inflammatory cytokines and inflammatory response. In our present study, supplementation with 2.0% Glu reduced mRNA expressions of key genes in TLR4 (jejunal TLR4, MyD88, IRAK1 and TRAF6) and NOD (jejunal NOD2) signaling pathways in the pigs challenged with LPS, which is in consistent with the decreased TNF-α concentration in plasma. However, there are few studies on Glu regulating TLR4, NODs and their downstream signals. In the central nervous system, Glu is the major excitatory neurotransmitter26, and its metabolism is involved with inflammation39. So it is possible that Glu exerted a protective effect on inhibiting TLR4 and NOD signaling pathways as an excitatory neurotransmitter, further reducing inflammation in intestine. In addition, as a member of the “arginine family”, Glu can convert into other amino acids (arginine, glutamine, aspartate, asparagine, proline, ornithine and citrulline) via complex interorgan metabolism in most mammals, including the pig23. Glu might exert its beneficial effects through transforming into arginine, asparagine and aspartate23. Some previous evidence has shown that arginine, aspartate, and asparagine participate in regulating TLRs, NOD and their downstream signals in weaned piglets40–42.
The aberrant activation of inflammatory signaling pathways (TLR4 and NOD) elicits collateral host tissue injury43. In order to prevent excessive inflammatory responses, many mechanisms can negatively control inflammatory signaling pathways. So far, several negative regulators of TLR4 (such as SOCS1 and Tollip) and NOD (such as ERBB2IP and ACAP1) signaling pathways have been identified and characterized44–46. Our data reflected that the LPS-challenged pigs had reduced mRNA expressions of ileal ERBB2IP and Tollip, which is in agreement with the study of Wang et al.40. Our results indicate that LPS challenge down-regulated the gene expressions of intestinal negative regulators in TLR4 and NOD signaling pathways, which is in consistent with increased mRNA expressions of intestinal key genes in TLR4 and NOD signaling-related genes. Thus, the up-regulation of key genes in TLR4 and NOD signaling-related genes might be related to the decrease of their negative regulators. In the present study, supplementation with 2.0% Glu increased mRNA expression of ileal Tollip, decreased gene expression of jejunal ERBB2IP. Wu et al. demonstrated that Tollip down-regulated the TLR4 signaling pathway via bounding to IL-1 receptor-associated kinase (IRAK) and inhibiting IRAK phosphorylation44. This indicates that supplementation with Glu could inhibit the activity of IRAK through increasing the gene expression of Tollip, leading to impair the signaling from TLR4 to downstream pathways and reduce the synthesis of pro-inflammatory cytokines. Curiously, Glu inhibited mRNA expressions of ERBB2IP. It is possible that Glu inhibited TLR4 and NOD signaling pathways directly or through other non-negative regulatory pathways to reduce excessive release of inflammatory cytokines, which resulted in less mRNA expression of ERBB2IP, but this needs to be further studied.
In conclusion, supplementation with Glu alleviates intestinal damage and improves intestinal repair in LPS-challenged piglets. The protective effects of Glu on the intestine may be associated with maintaining mTOR and inhibiting TLR4 and NOD signaling pathways.
Methods
Animal care and experimental design
The animal experiment was carried out in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocol, and was approved by the Animal Care and Use Committee of Wuhan Polytechnic University. A total of 24 crossbred pigs (Duroc × Large White × Landrace, initial body weight of 7.02 ± 0.21 kg) were used and randomly allotted into 4 treatments. Every treatment included 6 replicate pens. Piglets were housed in 1.80 × 1.10 m2 pens and had free access to drinking water and their diets individually. The basal diet was formulated according to National Research Council requirements for all nutrients (Table 9)47. Ambient temperature was maintained at 25–27 °C.
Table 9.
Ingredient composition of experimental diets (%, as-fed basis).
| Ingredients | Nutrients levelb | ||
|---|---|---|---|
| Corn | 56.40 | Digestible energy (MJ/kg) | 13.70 |
| Soybean meal, 44% crude protein | 22.40 | Crude protein | 19.20 |
| Wheat middling | 5.00 | Calcium | 0.89 |
| Fish meal | 3.60 | Total phosphorus | 0.67 |
| Soy protein concentrate | 1.40 | Total aspartate + asparagine | 1.65 |
| Fat powder | 2.00 | Total glutamate + glutamine | 2.97 |
| Defatted milk-replacer power | 3.00 | Arginine | 0.96 |
| Dicalcium phosphate | 1.22 | Lysine | 1.02 |
| Limestone | 0.94 | Serine | 0.90 |
| Salt | 0.34 | Threonine | 0.74 |
| Alanine | 1.21 | Proline | 1.11 |
| Cornstarch | 0.79 | Glycine | 0.70 |
| Acidifier | 0.20 | Alanine | 2.00 |
| L-Lysine HCl, 78% | 0.27 | Histidine | 0.51 |
| DL-Methionine, 99% | 0.10 | Leucine | 1.45 |
| L-Threonine, 98% | 0.08 | Isoleucine | 0.55 |
| Butylated hydroquinone | 0.05 | Tyrosine | 0.42 |
| Vitamin and mineral premixa | 1.00 | Phenylalanine | 0.71 |
| Total | 100.00 | Valine | 0.63 |
aPremix supplied per kg diet: retinyl acetate, 5512 IU; cholecalciferol, 2200 IU; DL-α-tocopheryl acetate, 30 IU; menadione sodium bisulfite complex, 4 mg; riboflavin, 5.22 mg; D-calcium-pantothenate, 20 mg; niacin, 26 mg; vitamin B12, 0.01 mg; Mn (MnSO4 · H2O), 40 mg; Fe (FeSO4 · H2O), 75 mg; Zn (ZnSO4 · 7H2O), 75 mg; Cu (CuSO4 · 5H2O), 100 mg; I (CaI2), 0.3 mg; Se (Na2SeO3), 0.3 mg.
bThe nutrients level was analyzed value except digestible energy which is calculated value.
The 4 treatments were set as follows: (1) control (basal diet + 0.9% NaCl solution); (2) LPS (basal diet + E. coli LPS (E. coli serotype 055: B5; Sigma Chemical Inc., St Louis, MO, USA); (3) LPS + 1.0% Glu (1.0% Glu diet + LPS); (4) LPS + 2.0% Glu (2.0% Glu diet + LPS).
The doses of Glu (purity > 99%; Amino Acid Bio-Chemical, Wuhan, China) were used according to Rezaei’s studies24. In order to obtain isonitrogenous diets, 1.21, 0.61 and 0% alanine (purity > 99%; Amino Acid Bio-Chemical, Wuhan, China) were added to the control, 1.0% Glu and 2.0% Glu diets, respectively. On day 28, pigs were injected intraperitoneally with either LPS at 100 μg/kg body weight or the same amount of 0.9% (w/v) saline. The LPS was dissolved in sterile 0.9% NaCl solution (500 mg LPS /L saline).
Sample collection
At 4 h post-injection, we collected blood samples into heparinized vacuum tubes from the anterior vena cava and centrifuged (3000 r/min, 15 min, 4 °C) to separate plasma. Plasma was stored at −80 °C until analysis of TNF-α. Following blood collection, piglets were harvested using pentobarbital at 80 mg/kg body weight, and 2 gut samples were taken from the mid-jejunum (3 cm and 10 cm) as well as mid-ileum (3 cm and 10 cm). The collection methods of intestinal samples were referred to Liu et al.4. The 3 cm sections were rinsed gently with 0.9% ice-cold saline, and then kept in 4% paraformaldehyde in PBS waiting to determine intestinal morphology. The 10 cm sections were opened and luminal contents were removed and rinsed. Mucosal samples were collected using a sterile glass slide, then immediately packaged and put in liquid nitrogen to froze, and stored at −80 °C for subsequent analysis.
Intestinal morphology
Intestinal samples were dehydrated, embedded in paraffin, sectioned, and stained with haematoxylin and eosin after fixation in 4% paraformaldehyde for 24 h4. Then jejunal and ileal samples were analyzed on histologic slides by a microscope (Olympus, Japan) at 10× magnification using Image-Pro Plus software. Villus height and crypt depth were measured referred to the previous methods reported by Nunez et al.48. Briefly, a minimum of 10 well-oriented and intact villi were chosen. Villus height was defined as the distance from the villus tip to crypt mouth, and crypt depth was measured from crypt mouth to base.
Concentrations of mucosal protein, DNA and RNA
Intestinal mucosal samples were homogenized, and then centrifuged to harvest the supernatant for determining the concentrations of protein, DNA and RNA. The analysis procedures were performed according to the previous reports4. Protein concentration of mucosal homogenates was determined by a detergent-compatible protein assay (Bio-Rad Laboratories, Hercules, CA, USA) and using bovine serum albumin as standards. Mucosal DNA content was measured by a fluorometric assay. Mucosal RNA content was measured by spectrophotometry with a modified Schmidt-Tannhauser method.
Disaccharidase activities
Disaccharidase activities in the supernatant of intestinal mucosa were determined referred to previous study reported by Hou et al.49 using glucose kits (#A082–1 for lactase, #A082-2 for sucrase and #A082-3 for maltase; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Plasma TNF-α concentration
The TNF-α concentration in plasma was measured by using a commercially available porcine ELISA kit (R&D Systems, Minneapolis, MN, USA, REF: PTA00)40.
mRNA abundance analysis
Intestinal total RNA was extracted using RNAiso Plus (TaKaRa, 9108/9109, Dalian, China), and dissolved in RNase-free water. A total of 1 μg of RNA, as templates, was reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa, RR047A, Dalian, China). The reaction was carried out for 15 min at 37 °C, 5 sec at 85 °C, ∞ at 4 °C. RT-PCR was performed on an ABI 7500 Real Time PCR system (Applied Biosystems, Foster City, CA, USA) using SYBR® Premix Ex Taq™ (TaKaRa, Dalian, China). We used specific primers to measure the mRNA abundance. GAPDH was used as a housekeeping gene to normalize the target gene transcript levels. Primer sequences are listed in Table 10. The relative mRNA expression of genes was calculated as a ratio of the target gene to the control gene depending on the the 2−ΔΔCT method4.
Table 10.
Primer sequences used for real-time PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product length (bp) | Accession numbers |
|---|---|---|---|---|
| TLR4 | TCAGTTCTCACCTTCCTCCTG | GTTCATTCCTCACCCAGTCTTC | 166 | GQ503242.1 |
| MD2 | TGCAATTCCTCTGATGCAAG | CCACCATATTCTCGGCAAAT | 227 | NM_001104956.1 |
| CD14 | CGTTTGTGGAGCCTGGAAG | TGCGGATGCGTGAAGTTG | 226 | NM_001097445.2 |
| LBP | GAACACAGCCGAATGGTCTAC | GGAAGGAGTTGGTGGTCAGT | 151 | NM 001128435.1 |
| MyD88 | GATGGTAGCGGTTGTCTCTGAT | GATGCTGGGGAACTCTTTCTTC | 148 | AB292176.1 |
| IRAK1 | CAAGGCAGGTCAGGTTTCGT | TTCGTGGGGCGTGTAGTGT | 115 | XM_003135490.1 |
| TRAF6 | CAAGAGAATACCCAGTCGCACA | ATCCGAGACAAAGGGGAAGAA | 122 | NM_001105286.1 |
| RP105 | CGAGGCTTCTGACTGTTGTG | GGTGCTGATTGCTGGTGTC | 245 | AB190767.1 |
| SOCS1 | GCGTGTAGGATGGTAGCA | GAGGAGGAGGAGGAGGAAT | 101 | NM_001204768.1 |
| Tollip | GCAGCAGCAACAGCAGAT | GGTCACGCCGTAGTTCTTC | 133 | AB490123.1 |
| SIGIRR | ACCTTCACCTGCTCCATCCA | TTCCGTCATTCATCTCCACCTC | 205 | AB490122.1 |
| NOD1 | CTGTCGTCAACACCGATCCA | CCAGTTGGTGACGCAGCTT | 57 | AB187219.1 |
| NOD2 | GAGCGCATCCTCTTAACTTTCG | ACGCTCGTGATCCGTGAAC | 66 | AB195466.1 |
| RIPK2 | CAGTGTCCAGTAAATCGCAGTTG | CAGGCTTCCGTCATCTGGTT | 206 | XM_003355027.1 |
| NF-κB | AGTACCCTGAGGCTATAACTCGC | TCCGCAATGGAGGAGAAGTC | 133 | EU399817.1 |
| ERBB2IP | ACAATTCAGCGACAGAGTAGTG | TGACATCATTGGAGGAGTTCTTC | 147 | GU990777.1 |
| ACAP1 | GAAGCCGAAGTGTCCGAATT | AGGTCACAGATGCCAAGAATG | 125 | XM_003358258.2 |
| GAPDH | CGTCCCTGAGACACGATGGT | GCCTTGACTGTGCCGTGGAAT | 194 | AF017079.1 |
Protein abundance analysis
The protein abundance of intestinal mucosa was determined according to the procedures of Wang et al.34. Specific primary antibodies included rabbit anti-claudin-1 (1:1000) from Invitrogen (Invitrogen Technology Inc., Danvers, MA, USA), rabbit anti-mTOR (1:1000), rabbit anti-phosphorylated mTOR (Ser2448; 1:1000), rabbit anti-4EBP1 (1:1000), rabbit anti-phosphorylated 4EBP1 (Thr70; 1:1000) from Cell Signaling (Cell Signaling Technology Inc., Danvers, MA, USA), and mouse anti-β-actin (1:10,000) from Sigma Aldrich (Sigma Aldrich Inc., St. Louis, MO, USA). The protein expression of claudin-1 was expressed as the ratio of claudin-1/β-actin. Phosphorylated forms of mTOR and 4EBP1 were normalized against the total protein content of each protein.
Statistical analysis
Data were analyzed by the GLM procedure of SAS (SAS Inst. Inc., Cary, NC, USA). The differences among group means were compared using Duncan Multiple Comparison based on the variance derived from ANOVA. Individual piglet was used as an experimental unit for the data. Results were expressed as means and pooled SEM. Significant differences were considered at p < 0.05.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31772615), the Project of the Hubei Provincial Department of Education (T201508), State’s Key Project of Research and Development Plan (2016YFD0501210), and the Project of Hangzhou Qianjiang Distinguished Expert (3122).
Author Contributions
Y.L. obtained financial support and conceived and oversaw this study. Y.L., H.Z. and Y.H. designed the animal experiment. Q.Q., X.X., X.W., H.W., B.D. and X.L. performed the animal experiment and generated the tissue samples, prepared testing sample and work with the analysis of data. Y.L., Q.Q. and X.X. wrote the manuscript with the contribution from the other authors. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qin Qin and Xiao Xu contributed equally.
References
- 1.Wang WW, Qiao SY, Li DF. Amino acids and gut function. Amino Acids. 2008;37:105–110. doi: 10.1007/s00726-008-0152-4. [DOI] [PubMed] [Google Scholar]
- 2.Mowat, A. M. The Intestinal Immune System. Encyclopedia of Immunobiology. 1–12 (2016).
- 3.Liu Y. Fatty acids, inflammation and intestinal health in pigs: a review. J Anim Sci Biotechno. 2015;6:41. doi: 10.1186/s40104-015-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, et al. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100:552–560. doi: 10.1017/S0007114508911612. [DOI] [PubMed] [Google Scholar]
- 5.Wang YJ, et al. Irradiation induced injury reduces energy metabolism in small intestine of Tibet minipigs. PLoS One. 2013;8:e58970. doi: 10.1371/journal.pone.0058970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen J, LaCasse EC, Seidelin JB, Coskun M, Nielsen OH. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20:652–665. doi: 10.1016/j.molmed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Rafa H, et al. All-Trans Retinoic Acid Modulates TLR4/NF-κB Signaling Pathway Targeting TNF-α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer. Mediators Inflamm. 2017;2017:7353252. doi: 10.1155/2017/7353252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pashenkov MV, Balyasova LS, Dagil YA, Pinegin BV. The Role of the p38-MNK-eIF4E Signaling Axis in TNF Production Downstream of the NOD1 Receptor. J Immunol. 2017;198:1638–1648. doi: 10.4049/jimmunol.1600467. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, et al. A novel crosstalk between TLR4- and NOD2-mediated signaling in the regulation of intestinal inflammation. Sci Rep. 2015;5:12018. doi: 10.1038/srep12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SZ, et al. Ischemic Preconditioning-Induced SOCS-1 Protects Rat Intestinal Ischemia Reperfusion Injury via Degradation of TRAF6. Dig Dis Sci. 2017;62:105–114. doi: 10.1007/s10620-016-4277-0. [DOI] [PubMed] [Google Scholar]
- 11.Correa RG, Snezana M, Reed JC. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parlato M, Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al NZ, Dietrich G, Hugot JP, Barreau F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Switon K, Kotulska K, Januszkaminska A, Zmorzynska J, Jaworski J. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112–153. doi: 10.1016/j.neuroscience.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Bauchart-Thevret C, Cui L, Wu G, Burrin DG. Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab. 2010;299:E899–909. doi: 10.1152/ajpendo.00068.2010. [DOI] [PubMed] [Google Scholar]
- 16.Nakajo T, et al. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem & Biophys Res Commun. 2005;326:174–180. doi: 10.1016/j.bbrc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Suryawan A, Davis TA. Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci. 2011;16:1445–1460. doi: 10.2741/3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, et al. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J Nutr Biochem. 2017;43:18–26. doi: 10.1016/j.jnutbio.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Xia X, Li T, Yin Y. Ethanolamine enhances the proliferation of intestinal epithelial cells via the mTOR signaling pathway and mitochondrial function. In Vitro Cell Dev Biol Anim. 2016;52:562–567. doi: 10.1007/s11626-016-0002-8. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, et al. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44:1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- 21.Wu G. Functional amino acids in nutrition and health. Amino Acids. 2013;45:407–411. doi: 10.1007/s00726-013-1500-6. [DOI] [PubMed] [Google Scholar]
- 22.San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest. 2009;56(Suppl):209–217. doi: 10.2152/jmi.56.209. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, et al. Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci. 2007;112:8–22. doi: 10.1016/j.livsci.2007.07.003. [DOI] [Google Scholar]
- 24.Rezaei R, et al. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids. 2013;44:911–923. doi: 10.1007/s00726-012-1420-x. [DOI] [PubMed] [Google Scholar]
- 25.Jiao N, et al. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J Nutr. 2015;145:2258–2264. doi: 10.3945/jn.115.217661. [DOI] [PubMed] [Google Scholar]
- 26.Brosnan JT, Brosnan ME. Glutamate: a truly functional amino acid. Amino Acids. 2013;45:413–418. doi: 10.1007/s00726-012-1280-4. [DOI] [PubMed] [Google Scholar]
- 27.Burrin DG, Janeczko MJ, Stoll B. Emerging aspects of dietary glutamate metabolism in the developing gut. Asia Pac J Clin Nutr. 2008;17(Suppl 1):368–371. [PubMed] [Google Scholar]
- 28.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr. 2009;90:850S–856S. doi: 10.3945/ajcn.2009.27462Y. [DOI] [PubMed] [Google Scholar]
- 29.Xun W, et al. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int Immunopharmacol. 2015;27:46–52. doi: 10.1016/j.intimp.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Vlasova AN, et al. Protein Malnutrition Modifies Innate Immunity and Gene Expression by Intestinal Epithelial Cells and Human Rotavirus Infection in Neonatal Gnotobiotic Pigs. mSphere. 2017;2:e00046–17. doi: 10.1128/mSphere.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith GI, et al. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperaminoacidemia-hyperinsulinemia in healthy young and middle aged men and women. Clin Sci. 2011;121:267–278. doi: 10.1042/CS20100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinheiro DF, et al. Maternal protein restriction affects gene expression and enzyme activity of intestinal disaccharidases in adult rat offspring. Brazilian Journal of Medical & Biological Research. 2013;46:287–292. doi: 10.1590/1414-431X20122561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, et al. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br J Nutr. 2015;114:553–565. doi: 10.1017/S0007114515001877. [DOI] [PubMed] [Google Scholar]
- 35.Lin M, et al. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS One. 2014;9:e111950. doi: 10.1371/journal.pone.0111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma XM, Blenis J. Molecular mechanisms of mTOR mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson E, et al. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed, and associated with a poor prognosis and endocrine resistance in breast cancer: a retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res. 2013;15:R96. doi: 10.1186/bcr3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi P, et al. Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. 2012;23:1012–1017. doi: 10.1016/j.jnutbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Haroon E, Miller AH. Inflammation Effects on Brain Glutamate in Depression: Mechanistic Considerations and Treatment Implications. Curr Top in Behav. Neurosci. 2016;31:173–198. doi: 10.1007/7854_2016_40. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, et al. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur J Nutr. 2017;56:1433–1443. doi: 10.1007/s00394-016-1189-x. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, et al. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun. 2016;22:577–587. doi: 10.1177/1753425916664124. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, et al. Dietary arginine supplementation alleviates immune challenge induced by Salmonella enterica serovar Choleraesuis bacterin potentially through the Toll-like receptor 4-myeloid differentiation factor 88 signalling pathway in weaned piglets. Br J Nutr. 2012;108:1069–1076. doi: 10.1017/S0007114511006350. [DOI] [PubMed] [Google Scholar]
- 43.Coll RC, O’Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 44.Wu W, et al. Bifidobacterium adolescentisprotects against necrotizing enterocolitis and upregulates TOLLIP and SIGIRR in premature neonatal rats. BMC Pediatr. 2017;17:1. doi: 10.1186/s12887-016-0759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piñeros Alvarez AR, et al. SOCS1 is a negative regulator of metabolic reprogramming during sepsis. JCI Insight. 2017;2:92530. doi: 10.1172/jci.insight.92530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu, V. W. Downregulation of ErbB2 by Perturbing its Endocytic Recycling. Researchgate. https://www.researchgate.net/publication/235119665 (2007).
- 47.National Research Council. Nutrient Requirements of Swine (10th ed.). Washington, DC: National Academic Press. (1998).
- 48.Nunez MC, et al. Dietary restriction induces biochemical and morphometric changes in the small intestine of nursing piglets. J Nutr. 1996;126:933–944. doi: 10.1093/jn/126.4.933. [DOI] [PubMed] [Google Scholar]
- 49.Hou YQ, Liu YL, Hu J, Shen WH. Effects of lactitol and tributyrin on growth performance, small intestinal morphology and enzyme activity in weaned pigs. Asian-Aust J Anim Sci. 2006;19:1470–1477. doi: 10.5713/ajas.2006.1470. [DOI] [Google Scholar]