Abstract
Vascular tumours of the liver represent an underrated chapter of medical and surgical hepatology. These tumours cover a wide spectrum ranging from the frequent and most benign hepatic haemangioma (HH), via the rare and intermediately aggressive hepatic epithelioid haemangioendothelioma (HEHE) to the rare and most malignant hepatic haemangiosarcoma (HHS). In contrast to the treatment algorithms for hepatocellular and cholangiocellular cancer, the diagnostic and therapeutic approaches to HEHE and HHS are not well developed. The related uncertainty is explained by their rare occurrence and their protean clinical, morphological (imaging) and histopathological presentation and behaviour. This article gives an update about these particular tumours based on the analysis of the recent literature and of the studies on vascular tumours published by the European Liver Intestine Transplantation Association (ELITA)-European Liver Transplant Registry (ELTR). It focuses also on the place of liver transplantation (LT) in the respective therapeutic algorithms. The differential diagnosis between these vascular and other tumour types may be very difficult. Correct diagnosis is of utmost importance and is based on a high index of clinical suspicion and on the integration of clinical, radiological, histological [including immunohistochemistry (IHC) and molecular biology findings]. Surgery, be it partial or total hepatectomy (LT), should be proposed whenever possible, because it is the therapeutic mainstay. In HEHE, LT provides excellent results, with long-term disease-free survivals (DFS) reaching 75%. Good results can be obtained even in case of (frequent) extrahepatic spread. Based on the extensive ELITA-ELTR study a HEHE-LT prognostic score has been proposed in order to estimate the risk of recurrence after LT. In contrast, results of surgery and LT are extremely poor for HHS, for the almost invariably rapid recurrence (within 6 months) and related death within 2 years. LT remains a contraindication for HHS. Due to the still important recurrence rate after surgical resection (25% in HEHE and almost 100% in HHS), there is an urgent need to develop pharmacological treatments targeting angiogenic and non-VEGF angiogenic pathways. To date, some prospective pilot studies and case reports have shown some short-term stabilisation of the disease in small groups of patients. In order to make progress, combination of surgery, anti-angiogenic and immunotherapy seems worthwhile. To complete the panel of vascular liver tumours, infantile haemangioendothelioma, haemangiopericytoma, nodular regenerative hyperplasia (NRH) and hepatic small vessel neoplasms (HSVN) are also discussed.
Keywords: Liver neoplasms, vascular tissue, liver transplantation, hepatectomy, hepatic haemangioma, hepatic haemangiomatosis, hepatic haemangiosarcoma, hepatic epithelioid haemangioendothelioma, hepatic infantile haemangioendothelioma, haemangiopericytoma, hepatic small vessel neoplasms, nodular regenerative hyperplasia
Introduction
Liver tumours arise, in decreasing order of frequency, from epithelial (hepatocytes, cholangiocytes) and mesenchymal (endothelial) cells. Unlike hepatocellular and cholangiocellular cancers, diagnostic and therapeutic algorithms of vascular tissue neoplasms are far from being standardized (1,2). This uncertainty is explained by the protean clinical, morphological and histopathological presentation and also by the limited awareness of the medical community about this “orphan” disease (defined as a disease occurring in less than 6/pmp) (3-9). Not surprisingly, many debates concerning the optimal management are on-going. This article aims to give, based on data from recent literature and the European Liver Registry (ELTR), an up-to-date overview about this particular group of liver tumours, looking thereby also to the place of liver transplantation (LT) in their treatment (10-13). To complete the panel of vascular liver tumours, this review also addresses haemangiomatosis, infantile haemagioendothelioma, nodular regenerative hyperplasia (NRH) and hepatic small vessel neoplasms (HSVN).
The broken “vascular tumour continuum”
Vascular tumours have been traditionally described as a continuum going from the most benign tumour in humans, the hepatic haemangioma (HH), via the intermediate type, the hepatic epithelioid haemangioendothelioma (HEHE) to the most aggressive tumour in humans, the hepatic haemangiosarcoma (HHS). Recently, molecular biology has revealed that HEHE and HHS result from very different mutations. However, HHS present HRAS, KRAS, NRAS and PTPRB mutations, whereas the t(1;3)(p36;q25) translocation leads to the EHE-specific fusion oncogene WWTR1-CAMTA1; a small subset (6%) of patients bears the YAP1-TFE3 fusion oncogene (14,15). Only one (0.6%) of the 149 patients registered in the ELTR presented simultaneously HH, HEHE and HHS in the hepatectomy specimen.
HH, giant or cavernous haemangioma
Cavernous haemangioma is the most common soft tissue tumour of the liver. Its incidence ranges from 0.4% to 20% in autopsy series. Most hepatic haemangioma (HH) have a diameter less than 4 cm, are solitary (90%), asymptomatic and are diagnosed in adults during occasional or hepatological check-ups. If the diameter exceeds 5 cm, they are called giant haemangioma. Most HH are asymptomatic; some HH become symptomatic due to thrombosis, mass syndrome and more rarely severe thrombocytopenia, eventually leading to disseminated intravascular coagulopathy [Kasabach-Merritt syndrome (KMS)] (16). Spontaneous bleeding and rupture are very rare. Ultrasound and magnetic resonance imaging (MRI, with vascular reconstruction in case of giant HH) are the best non-invasive diagnostic studies. It should be kept in mind that the larger the HH is, the more HH typical characteristics (such as posterior shadowing and centripetal filling) are lost. Unclear imaging warrants further investigation, in order to exclude HHS or other malignant tumours. The therapeutic attitude is clear. Small HH do not require treatment and surgery should be restricted to very symptomatic patients, who present huge abdominal mass, pain due to intra-tumour thrombosis, continuous tumour growth responsible for compression of surrounding organs, or severe post-traumatic bleeding (16,17). Surgery mostly consists of enucleation. Modern energy devices such as ultrasonic dissection make this surgery safe(r) by identifying the plan between tumour and normal liver tissues. Intermittent clamping of the hepatoduodenal ligament (Pringle manoeuvre) is useful to avoid major blood losses. Partial hepatectomy is seldom necessary and LT is indicated in exceptional circumstances, mainly when facing KMS, post-traumatic uncontrollable bleeding, or in the presence of huge invalidating lesions. Medical treatment, using steroids and beta-blockers, has been proposed in case of infantile haemangioma symptomatic tumours (18-20).
Haemangiomatosis
Liver haemangiomatosis is a rare and atypical presentation of haemangiomas, largely more frequent in women than in men. The liver parenchyma is diffusely replaced by ill-defined, multiple haemangiomatous lesions, with a diameter ranging from a few millimetres to several centimetres. Unlike haemangioma, this disease is rarely asymptomatic. The differential diagnosis with HEHE and HHS should be considered. LT has been exceptionally reported for hepatic haemangiomatosis leading to painful hepatomegaly, liver failure, heart failure or portal hypertension because of hyperdynamic flow (10,12,21,22).
HEHE
The epithelioid haemangioendothelioma (EHE) is a rare vascular tumour with an epithelioid and histiocytoid appearance, originating from vascular endothelial or pre-endothelial cells and representing less than 1% of all vascular tumours. HEHE is a rare (<1/pmp), low-grade malignancy featuring an intermediate behaviour between HH and HHS (3,23,24). This tumour was first recognized in 1975 by Dail and Liebow as a lung lesion; later, in soft tissues, head and neck region, pleura, bones and in many other organs. Ishak described in 1984 this tumour in the liver of 32 patients (23). Makhlouf extended these series to 137 cases (25). The Hemangioendothelioma, Epithelioid hemangioendothelioma And Related vascular Disorders (HEARD) Support Group observed that the most common HEHE presentations are liver alone (21%), liver and lung (18%), lung alone (12%) and bone alone (14%) (26). The pulmonary and hepatic EHE forms seem to carry similar characteristics in relation to presentation and behaviour (24). HEHE is more frequent in middle-aged women (female/male: 4/1). The tumour is exceptional in children (27). No definitive etiological factor has been identified. Recently, it has been suggested that there is a causal relationship between chronic Bartonella infection and the development of HEHE trough the induction of vasoproliferation (28).
The clinical manifestation is highly variable and non-specific, ranging from the absence of symptoms to hepatic failure (nonetheless rare). In the ELTR database, including 149 patients, the most frequent symptoms were upper abdominal discomfort or pain (60%), weight loss (20%), impaired general condition, due to weakness and fatigue (20%), and dyspnoea (5%). One fourth of patients were asymptomatic. Hepatosplenomegaly (30%) was the most frequent clinical sign. Jaundice, portal hypertension and Budd-Chiari syndrome, caused by tumour compression or venous infiltration, were reported in 5% of patients. One third of patients presented cholestasis and cytolytic activity. Serum tumour markers are always normal, in the absence of an underlying liver disease. KMS has been reported exceptionally.
At MRI, two different but typical patterns are identified in accordance with tumour progression. The early phase is characterized by the presence of peripheral, nodular, usually bilobar and subcapsular lesions (“peripheral pattern”), the later phase shows multiple confluent lesions (“diffuse pattern”) and eventual invasion of the greater vessels and modification of the non-tumorous liver. The most frequent features of the HEHE lesions are a ring-like arterial enhancement, a core pattern, a hyperintense rim on T1-weighted imaging and a capsular retraction (29). Focal calcifications can be present as a consequence of central tumour necrosis. Sometimes, angiography is made in the context of a difficult differential diagnosis and reveals only moderate vascularisation with displacement of the intrahepatic vascular tree by the tumour masses, indicating that liver biopsy is not contraindicated. A complete assessment, including thoracic CT-scan, scintigraphy and FDG-PET scanning, is mandatory, especially when LT is planned, in order to exclude extrahepatic (especially thoracic) localisations (30). Chest lesions mostly (60%) present as bilateral multiple nodular opacities or solitary nodules, measuring up to 5 cm, in 10% to 20% of cases. Bone metastases appear as osteolytic lesions (31).
The diagnosis of HEHE is based on a high index of suspicion and on the integration of radiological and clinical findings, such as the occurrence in a young (female) adult presenting numerous intrahepatic tumours but retaining a good condition notwithstanding a long-lasting clinical history (9,10,12). Both histopathology, including both H&E staining and IHC, and molecular biology are necessary to confirm the diagnosis. Although it has been shown that cytology can provide the right diagnosis, histology, on samples procured during laparoscopic exploration when appropriate, is preferred because of the frequently difficult differential diagnosis with HHS, secondary malignancies, and some other tumours (9,32,33). Macroscopic examination shows multi-focal, fibrous masses with characteristic central zoning (due to necrosis), while microscopic examination shows pleomorphic, medium and large size, epithelioid cells spreading within sinusoids and small veins but with preservation of acinar and portal tract landmarks. Notably, lymph nodes can be negative on H&E staining, but positive on IHC. Cellular atypia, nuclear fission, presence of spindle cells, necrotic tumour changes, and Ki-67 index >10–15% have been shown to be indicators of more aggressive HEHE (24,32,34,35). Though the disease was reported to be more aggressive in a European pluricentric, paediatric cohort, this observation was not confirmed in the UNOS survey (36). The ultimate diagnosis should be confirmed through IHC, especially looking at the expression of vascular endothelial markers, like factor VIII-related antigen, Fli-1 (a protein expressed by the endothelium), the more specific CD31, CD34, and ERG (an ETS family transcription factor expressed on endothelial cells) (14,37). Clinical, radiologic, histopathological (vascular differentiation), cytological and IHC (vascular markers expression) findings should be correlated in order to ensure the correct HEHE diagnosis.
Four findings are helpful to make the differential diagnosis with HHS: (I) HHS is more frequent in men; (II) may be due, in up to 25% of patients, to environmental, toxic factors such as vinyl chloride, cyclophosphamide, thorium dioxide (Thorotrast) and arsenic; (III) HHS is more aggressive, as shown by the more frequently compromised liver function (lower albumin, elevated INR, anaemia and thrombocytopenia) and (IV) at histological level, by the loss of the typical hepatic lobular and portal landmarks (11,13,32,38). It is known that the delay between toxic exposure and HHS development may be extremely long (35 to 65 years) (39,40).
The treatment algorithm of HEHE and pulmonary EHE is difficult not only because of its rarity but also because of the largely unpredictable behaviour and prognosis. Indeed, the well documented spontaneous, long-term survivals (up to 28 years), the usual absence of symptoms (in up to 25% of cases), the common extra-hepatic disease localisation, at the time of diagnosis (up to 45%), the lack of solid predictive clinical or histological criteria, and, finally, the high incidence (up to 33%) of (sometimes even very late) recurrence have made difficult the acceptance of LT as a valid treatment of HEHE (10,12,24,25,36). Before the publication of the “HEHE-ELITA-ELTR” series, detailed data analysis and long-term follow-up after LT performed for HEHE were lacking (10,12,13). The largest institutional series from Pittsburgh (16 patients) showed that LT offered 5-year patient (PS) and disease-free survival (DFS) rates of 71% and 60% (38). In the Canadian multicentre experience, including 11 patients, 5-year PS and DFS reached 82% and 69% (41). In the UNOS study, including 110 patients, 5 years PS reached 64%. Information about DFS lacked (36). The 2006 review by Mehrabi, including 286 patients, clearly favoured surgery, in form of partial or total (i.e., LT) resection, as the treatment of choice (42-44). Five-year PS rates after partial resection, LT, local or systemic chemo- and radiotherapy and no treatment reached 75%, 55%, 30% and 4.5%. This review indicated that partial resection is only possible in the most favourable cases (single or few lesions), representing 9% of patients. The Mayo group obtained 1- and 5-year survivals of 86% and 62% after partial resection in 11 patients (44). The assessment of non-surgical approaches, such as radiotherapy, local tumour ablation, trans-arterial (chemo-)embolization [TA(C)E], hormone treatment, systemic or locoregional radio-chemotherapy, and anti-angiogenic or anti-tumour pharmacotherapy is difficult because of the lack of uniform treatment modalities and of long-term follow-up. Radiotherapy is of value in local pain control.
The 2007 and 2017 “ELITA-ELTR HEHE” papers undoubtedly modified the outlook of patients affected by HEHE (10,12,13). These studies were the first to show results based on both an in-depth analysis (216 items per patient) of a large cohort (149 patients) and on long-term follow-up (median from moment of LT of 7.6 years and from moment of diagnosis of 10.5 years). They allowed to improve the diagnostic and therapeutic approach to HEHE, and crucially raised the awareness of the hepatological and transplant community about this disease. Indeed, during the period 1989–2004, 57 patients were transplanted for HEHE. Following the first publication, 92 patients were transplanted during the time span 2008–2015. Moreover, many patient records, from all over the world, were sent by e-mail in order to obtain advice. Five- and 10-year post-transplant PS rates reached 81% and 77%. DFS rates were 79% and 73%. Pre-LT treatment (28% of cases), lymph node invasion (27% of cases) and limited extrahepatic disease (26.8% of cases) did not significantly influence outcome, whereas micro- and macrovascular invasion (present in 13% and 48% of patients) did. These results indicate that long-term DFS can be obtained for multifocal disease only if an aggressive surgical, even preventive, approach is undertaken. For multifocal chest and liver disease, Desie et al. even reported about successful sequential (lung after liver, with reported 10 and 8 years post-transplant survival, despite pleural and diaphragmatic invasion at transplantation) and simultaneous liver-lung transplantation (with 7 and 1 years post-transplant survival, without signs of disease progression despite bone metastases at moment of transplantation) (45). Recurrent disease in and outside the graft was recorded in one fourth of patients and, thus, it still remains of concern. If present, it should be treated aggressively, as prolonged, sometimes even disease-free, survival can be obtained. The role of Re-LT, considered in case of isolated intra-hepatic recurrence, remains till now unclear (32).
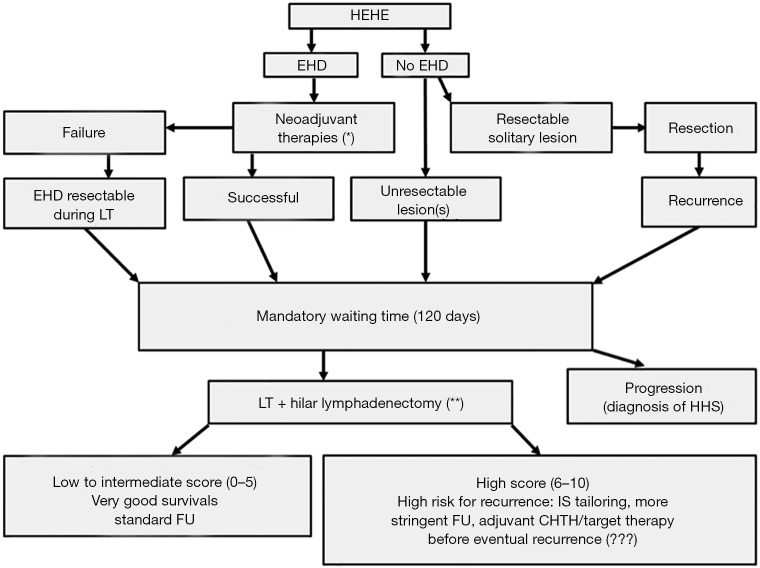
The high recurrence rate, along with the high incidence of extrahepatic disease localisation, should lead to the development of prognostic scores as well as efficacious neo- and adjuvant oncologic therapies. Lau et al. identified presence of pulmonary lesions, multi-organ involvement, disease progression, ascites, age ≥55 years and male gender as poor prognostic factors (26). The high number of HEHE patients included in the second ELITA-ELTR-HEHE study allowed to build, for the first time, a prognostic score on the following formula: 5× (pathological MaVi) +3× (WT ≤120 days) + 2× (pathological invasion hilar LN). This HEHE-LT score well stratified the patients according to their risk of post-LT recurrence: a low score (score 0–2) correlated with had an excellent 5-year DFS of 94%, an intermediate one (score 3–5) with good DFS (77%), and a “high score” (score 6–10) with worse rates (38.5%). According to these results, a therapeutic algorithm for the treatment of HEHE was proposed, as shown in Figure 1 (13). It is worth noticing that the waiting time of some months is useful in relation to the differential diagnosis between HEHE and HHS. Indeed, HHS usually progresses rapidly, in contrast to HEHE, within some months from diagnosis.
Figure 1.
Therapeutic algorithm for hepatic epithelioid haemangioendothelioma. *, non-standardized neo-adjuvant approach; **, histological examination combining immunohistochemistry and H&E staining. HEHE, hepatic epithelioid haemangioendothelioma; CHTH, chemotherapy; EHD, extrahepatic disease; FU, follow-up; IS, immunosuppression; HHS, hepatic haemangiosarcoma; LT, liver transplantation.
During the recent years, several agents have all applied to small numbers of patients harbouring EHE, HHS and other sarcomas. Some of them target angiogenic pathways, like bevacizumab, oral tyrosine-kinase inhibitors (sorafenib, sunitinib, pazopanib), and paclitaxel. Some others tackle non-VEGF angiogenic pathways, like angiopoietin peptibody, PDGFR and Endoglin inhibitor. Several other vascular target agents (thalidomide, lenalidomide, interferon and beta-blockers), chemotherapies (cyclophosphamide, doxorubicin, carboplatin-etoposide) have been tried. As these tumours contain VEGF and VEGF receptors, treatments based on anti-VEGF antibodies seem logical. There are some suggestions that a higher content of these receptors might improve outcome (46-52). Beta-blockers have been also used, because vascular tumours contain high levels of beta-adrenergic receptors (53). Recently, the French sarcoma group has reported the outcome of sorafenib in a series of 15 HEHE patients, and the Eastern Cooperative Oncology Group has released the results of bevacizumab in a series of 7 HEHE patients. Both studies were phase II trials including advanced, non-resectable, metastatic diseases. Both drugs were able to stabilize the disease up to 10 months in 20% to 40% of patients; partial response was around 10% up to 6 months (54,55).
Better knowledge about the pathological and biological behaviour of the tumour, based on the study of mitotic index and pleomorphism, along with new molecular and genetic markers, are necessary to improve outcomes, to monitor the efficacy of the emerging neo- and adjuvant treatments and to discriminate the aggressive HEHE subtypes (42,56,57). Until recently, it has been still questioned, in case of multiple lesions, if it is about a multicentric tumour or a primary lesion metastasizing to other tissues. The identification of the specific HEHE oncogenes has confirmed the monoclonal nature of all the different lesions of a multifocal HEHE in a same patient. Thus, multifocality and extrahepatic spread can be seen as metastatic implants of the same neoplastic clone rather than synchronous occurrence of multiple different clones, allowing therefore an equally effective neo-adjuvant therapy on both hepatic and extra-hepatic lesions (58). Unfortunately, no reports are yet available on clonality of simultaneous hepatic and extra-hepatic lesions. Such genetic diagnosis is urgently needed in order to manage HEHE patients, especially those with extrahepatic spread at the time of transplantation.
Hepatic infantile haemangioendothelioma (HIHE)
HIHE is the most common tumour of the liver in the infant age (<3 years) group, which is usually diagnosed during the first 6 months of life. HIHE is more frequent in females and presents with (a)symptomatic hepatosplenomegaly, failure to thrive, congestive cardiac failure (15%), due to intratumoral arteriovenous shunting, and cutaneous haemangiomas (20–40%). It appears as a histological benign tumour, which may have a poor outcome due to complications such as heart failure (19,42). Histology identifies two types of HIHE. The more pleomorphic character of type II seems to have a better prognosis. The differentiation between HEHE and HIHE is important because the latter does not metastasize. Several lesions, present simultaneously in different organs such as spleen, lungs and bone, are probably separate lesions. HEHE can be differentiated from HIHE on the basis of their different, age-related, clinical and pathological features. The natural history of HIHE is variable but up to two thirds of symptomatic patients die of tumour complications. Several reported cases mention the presence of HHS foci. The treatment modalities include anti-angiogenic therapies (using or combining high-dose steroids, interferon, chemo- or radiotherapy), interventional radiological and surgical interventions (19,59,60). Partial hepatectomy is indicated if lesions are confined to one liver lobe; diffuse lesions can only be managed by LT. The Boston group developed a clear algorithm for the treatment of these children (19). Diffuse HIHE, resistant to steroid therapy, requires LT.
HHS
HHS is the most common primary sarcoma in the liver and accounts for up to 2% of all primary liver tumours. HHS occurs most in the sixth and seventh decades of life and more frequently in males (male-to-female ratio: 3/1). It is rarely seen in children (42,61-63). HHS received much attention due to the association with many environmental carcinogens (Thorotrast, vinyl chloride monomer, radium, pesticides, external radiation, cyclophosphamide, arsenical compounds, use of androgenic/anabolic steroids and iron) (9,23,39,40). Most cases are sporadic. The diagnosis can be very difficult, even using modern imaging and liver biopsy (6,38,64-66). Macroscopically, HHS presents as ill-defined spongy haemorrhagic nodule(s) involving the whole liver. Four types of growth patterns have been described: multiple nodules, large dominant mass, multiple nodules and dominant mass and more rarely diffusely infiltrating macro-nodular tumour (3,21,23). At diagnosis, up to 40% of patients exhibit extra-hepatic disease mostly in lungs, spleen, bones and adrenal glands. HHS brings about a more pronounced sickness state compared to HEHE. Signs of portal hypertension and liver failure are often present. Hepatosplenomegaly, thrombocytopenia, pain, jaundice, ascites, peripheral oedema, acute abdomen, due to the rather frequent tumour rupture, can all be present (11,61).
Again, the “ELITA-ELTR HHS study”, based on detailed data analysis of 20 HHS liver recipients, gave a better insight in the disease and, especially, in the differential diagnosis with HEHE and the place of LT in its treatment. All, but one, patients were symptomatic. Weakness and fatigue, upper abdominal pain and discomfort, anorexia and nausea were respectively reported in 75%, 60% and 50% of the patients. Hepatomegaly was present in 80%, weight loss in 60%, jaundice and ascites in 45% of patients. Portal hypertension and acute liver failure were frequent (25% and 10% respectively). At the moment of LT, 15% of patients exhibited metastases.
The disease was correctly identified only in one third of cases before surgery, indicating the level of difficulty in establishing the right diagnosis before LT (67). The pathologic specimen showed diffuse bilobar involvement in all cases. Outcome after surgery, either partial or total hepatectomy (LT), is extremely disappointing. All patients invariably died after a median of six/seven months due to tumour recurrence. Similar results have been shown in and outside the transplant setting (61,68-74). Both European and American experiences confirm that HHS is an absolute contra-indication to LT (11,72).
The combination of surgery and adjuvant chemotherapy allows to extend survival, reaching 84 months for resectable solitary or multinodular but confined tumours. It is even possible to obtain limited but favourable survivals in case of multinodular tumours with metastasis using chemotherapy (70). TAE is an effective treatment of bleeding after tumour rupture (75). Radiotherapy is not helpful since HHS is a radio-resistant tumour (73).
Similar to HEHE, the search for effective medical therapy, based on the expression of different angiogenic growth factors receptors and other vascular-targeted agents, is a priority in order to improve the outcome of this very aggressive disease. Different drug combinations and immunotherapy using anti-PD1, occasionally combined with classic chemotherapies, have been used with sporadic (partial) response (61,76,77).
Haemangiopericytoma (HPC)
HPC is an uncommon vascular tumour comprising less than 2% of soft tissue sarcomas. This tumour arises from the pericytes of Zimmermann, the small oval cells that surround the capillaries and is usually seen in adults of both genders, as a painless mass. Lower extremities, abdominal cavity and retroperitoneal space are most commonly affected districts, while liver involvement is rare. They can present as solitary and multiple lesions. Half of them are malignant: tumour size (>20 cm), more than four mitosis per 10 high-power fields, pleomorphic cells, with chromatin pattern, and presence of central necrosis or intra-tumour haemorrhage indicate malignant transformation.
These tumours are hypervascular and tumorous cells are mostly spindle-shaped. Histology and IHC allow to differentiate from other sarcomas (78-80).
Paraneoplastic syndromes display at presentation or at the time of development of metastases. Hypoglycaemia, secondary to the release of the insulin-like growth factors, appears in the later stage of the disease. Aggressive surgery is the treatment of choice for the primary and metastatic lesions. The role of chemo- and radiotherapy is uncertain. Even after R0 resection, two thirds of patients recur. The detection of these recurrences is difficult because of the diversity of the tumour size and the lack of specific markers. PET-scan is very useful for diagnosis and follow-up of these patients if the initial tumour had tracer uptake.
Four patients were recorded in the ELITA-ELTR study, two of them with primary and metastatic HPC. Only one (metastatic) patient had a DFS of 12 years after LT but after multiple abdominal, thoracic and orthopaedic surgeries (81).
After R0 resection, the 5-year DFS is 50%. Long-term follow-up remains necessary as 10% of tumours recur after 5 years. Repetitive surgery may be useful in case of appearance of paraneoplastic syndromes, in particular, of invalidating hypoglycaemia (80).
NRH
NRH of the liver is a rare condition, which can cause intrahepatic portal hypertension in the absence of cirrhosis (82). Its prevalence is estimated between 0.7% and 2.6% in autopsy series (83). NRH is frequently asymptomatic, while the presence of signs of portal hypertension qualifies for NRH syndrome (84,85). Indeed, NRH is the major cause of non-cirrhotic portal hypertension. These lesions develop as a consequence of a vasculopathy, with alterations in the hepatic blood flow consequent to obliterative vasculopathy and/or secondary damage of the sinusoids. When a critical proportion of portal venules are affected, portal hypertension develops. The formation of usually small (<1 cm) non-fibrotic parenchymal nodules, which are not separated by fibrotic septa, allows to differentiate NRH from micronodular cirrhosis. Haemodynamic investigation shows presinusoidal and, sometimes, sinusoidal portal hypertension, in association with a patent portal vein. NRH has been associated with a variety of systemic disorders. The list includes myelo- and lymphoproliferative diseases, autoimmune diseases, inflammatory conditions, immunodeficiency disorders, primary biliary cholangitis, Budd-Chiari syndrome, collagen-vascular diseases, and congenital and acquired hepatic macrovascular abnormalities (Rendu-Osler-Weber, Abernethy diseases and HEHE) as well as certain medications, such as highly active anti-retroviral therapy, platinum-based chemotherapy and thiopurines, azathioprine and thioguanine (4,85,86). NRH has also been described in liver allografts (87). Familial transmission, occurring without underlying or associated systemic disease, has been described. These cases have poorer clinical course and are often associated with progressive renal failure (88). To make the diagnosis, detailed imaging and liver biopsy are necessary (83,89). In the ELITA-ELTR study, six cases of NRH are reported, of whom four did well and two died due to heart failure. Five larger series and some case reports about successful LT for this condition have been reported in literature (82,86-88,90). In the Dutch LT series, 7 out of 11 patients (64%) had underlying disorders or drug exposure. Five-year PS was 73%. Three patients presenting hepatopulmonary syndrome died during follow-up (82). The review of the literature by Meijer (comprising 34 patients and 11 own patients) and Manzia (comprising 26 patients) confirm that NRH is a rare (0.6% of all LT in the Netherlands) but good indication for LT, yielding 78% 5-year PS rates (82,91). All patients have been transplanted because of portal hypertension-related complications. Only one recurrence has been reported (91).
NRH has been also described as a complication of chronic immunosuppression using azathioprine, leading even to re-LT, as well as in the context of living-donor LT with small-for-size grafts and in recipients transplanted for biliary tract disease complicated with severe non-cirrhotic portal hypertension (88,92).
Hepatic small vessels neoplasm (HSVN)
Recently, HSVN has been described as an infiltrative neoplasm of the liver. Their infiltrative nature can mimic HHS but these lesions are considered benign or low-grade tumours because they lack cellular atypia and increased proliferation (93). Diagnosis can be made by detailed histological examination and molecular biology. Apparently, these lesions share GNAQ and GNA14 mutations, which are also seen in several other vascular lesions (such as several types of HHS and Kaposiform HE) (94). Resection is the proposed treatment. Given the rarity of the condition, the infiltrative nature and the insufficient follow-up in literature, the benign versus low-grade nature of this tumour type is still uncertain at the moment (95).
Conclusions
Vascular tumours are many times a diagnostic and therapeutic challenge for clinicians, mainly because of their rarity (except for haemangioma) and their usually protean clinical, morphological and histopathological manifestation. The complex differential diagnosis between the different tumour types can now be refined using immunohistochemistry and molecular biology. The extensive review of the actual literature and, even more, the ELITA-ELTR vascular tumour study has helped not only to better understand these diseases but also to raise awareness of the medical and transplant community about these orphan diseases. Surgery is the mainstay in the therapeutic algorithm. Partial hepatectomy is possible only in a minority of patients due to tumour multifocality and total hepatectomy requires subsequent LT. In case of HEHE, surgery, preventive or therapeutic, obtains excellent 5-year DFS (75%), even in the presence of extrahepatic disease at the time of transplantation. The HEHE-LT score, based on macrovascular invasion, lymph node involvement, and waiting time, is a tool to better estimate the risk of recurrence after surgery.
The outlook of patients harbouring HHS remains dismal, although some progress has been done with aggressive surgery and chemotherapy. However, LT remains contraindicated due to the universal rapid recurrence (mostly within 6 months) and short-term survival (24 months). The development of several agents, targeting angiogenic, non-VEGF angiogenic pathways, and other vascular targets, are warranted, along with neo- and adjuvant chemotherapies. New drugs are expected to improve the outcomes of both HEHE and HHS and to reduce the incidence of post-LT HEHE recurrence, still reaching 25%.
LT is anecdotally indicated in case of giant haemangioma (to overcome KMS), of haemangiopericytoma (to improve quality of life) and in case of NRH (to treat severe complications of non-cirrhotic portal hypertension). A longer follow-up is necessary for the recently described small vessel neoplasm, in order to define its possible malignant nature.
Acknowledgements
The authors thank Luca Giustozzi, for his kind linguistic revision. S Iesari also thanks the Hepatotransplant Association and the Euroliver Foundation, for their unrestricted grants.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. 10.1016/j.jhep.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Ishak K, Goodman Z, Stocker J. Tumors of the liver and intrahepatic bile ducts. Atlas of tumour pathology. Third series, fascicle 31. Armed Forces Institute of Pathology, Washington, 1999. [Google Scholar]
- 4.Sempoux C, Balabaud C, Paradis V, et al. Hepatocellular nodules in vascular liver diseases. Virchows Arch 2018;473:33-44. 10.1007/s00428-018-2373-6 [DOI] [PubMed] [Google Scholar]
- 5.Valla DC, Cazals-Hatem D. Vascular liver diseases on the clinical side: definitions and diagnosis, new concepts. Virchows Arch 2018;473:3-13. 10.1007/s00428-018-2331-3 [DOI] [PubMed] [Google Scholar]
- 6.Thampy R, Elsayes KM, Menias CO, et al. Imaging features of rare mesenychmal liver tumours: beyond haemangiomas. Br J Radiol 2017;90:20170373. 10.1259/bjr.20170373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semelka RC, Nimojan N, Chandana S, et al. MRI features of primary rare malignancies of the liver: A report from four university centres. Eur Radiol 2018;28:1529-39. 10.1007/s00330-017-5102-7 [DOI] [PubMed] [Google Scholar]
- 8.Ehman EC, Torbenson MS, Wells ML, et al. Hepatic tumors of vascular origin: imaging appearances. Abdom Radiol (NY) 2018;43:1978-90. 10.1007/s00261-017-1401-3 [DOI] [PubMed] [Google Scholar]
- 9.Studer LL, Selby DM. Hepatic Epithelioid Hemangioendothelioma. Arch Pathol Lab Med 2018;142:263-7. 10.5858/arpa.2016-0171-RS [DOI] [PubMed] [Google Scholar]
- 10.Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg 2007;246:949-57. 10.1097/SLA.0b013e31815c2a70 [DOI] [PubMed] [Google Scholar]
- 11.Orlando G, Adam R, Mirza D, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 2013;95:872-7. 10.1097/TP.0b013e318281b902 [DOI] [PubMed] [Google Scholar]
- 12.Lerut JP, Weber M, Orlando G, et al. Vascular and rare liver tumors: a good indication for liver transplantation? J Hepatol 2007;47:466-75. 10.1016/j.jhep.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Lai Q, Feys E, Karam V, et al. Hepatic epithelioid hemangioendothelioma and adult liver transplantation: proposal for a prognostic score based on the analysis of the ELTR-ELITA registry. Transplantation 2017;101:555-64. 10.1097/TP.0000000000001603 [DOI] [PubMed] [Google Scholar]
- 14.Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol 2014;9:131. 10.1186/1746-1596-9-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013;52:775-84. 10.1002/gcc.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SS, Charny CK, Fong Y, et al. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J Am Coll Surg 2003;197:392-402. 10.1016/S1072-7515(03)00420-4 [DOI] [PubMed] [Google Scholar]
- 17.Brouwers MA, Peeters PM, de Jong KP, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg 1997;84:314-6. 10.1002/bjs.1800840310 [DOI] [PubMed] [Google Scholar]
- 18.Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013;131:128-40. 10.1542/peds.2012-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christison-Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg 2007;42:62-7. 10.1016/j.jpedsurg.2006.09.041 [DOI] [PubMed] [Google Scholar]
- 20.Sarıalioğlu F, Yazici N, Erbay A, et al. A new perspective for infantile hepatic hemangioma in the age of propranolol: experience at Baskent University. Exp Clin Transplant 2017;15:74-8. [DOI] [PubMed] [Google Scholar]
- 21.Roque Ramos L, Coelho ML. Hepatic haemangiomatosis: multinodular liver in an asymptomatic elderly man. BMJ Case Rep 2014;2014. doi: . 10.1136/bcr-2013-202505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keegan MT, Kamath GS, Vasdev GM, et al. Liver transplantation for massive hepatic haemangiomatosis causing restrictive lung disease. Br J Anaesth 2001;86:431-4. 10.1093/bja/86.3.431 [DOI] [PubMed] [Google Scholar]
- 23.Ishak KG, Sesterhenn IA, Goodman ZD, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol 1984;15:839-52. 10.1016/S0046-8177(84)80145-8 [DOI] [PubMed] [Google Scholar]
- 24.Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. 10.4081/oncol.2014.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 1999;85:562-82. [DOI] [PubMed] [Google Scholar]
- 26.Lau K, Massad M, Pollak C, et al. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest 2011;140:1312-8. 10.1378/chest.11-0039 [DOI] [PubMed] [Google Scholar]
- 27.Sharif K, English M, Ramani P, et al. Management of hepatic epithelioid haemangio-endothelioma in children: what option? Br J Cancer 2004;90:1498-501. 10.1038/sj.bjc.6601720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascarelli PE, Iredell JR, Maggi RG, et al. Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma. J Clin Microbiol 2011;49:4006-12. 10.1128/JCM.05527-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Jeong WK. Magnetic resonance findings of hepatic epithelioid hemangioendothelioma: emphasis on hepatobiliary phase using Gd-EOB-DTPA. Abdom Radiol (NY) 2017;42:2261-71. 10.1007/s00261-017-1119-2 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen BD. Epithelioid hemangioendothelioma of the liver with F-18 FDG PET imaging. Clin Nucl Med 2004;29:828-30. 10.1097/00003072-200412000-00019 [DOI] [PubMed] [Google Scholar]
- 31.Bagan P, Hassan M, Le Pimpec Barthes F, et al. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann Thorac Surg 2006;82:2010-3. 10.1016/j.athoracsur.2006.06.068 [DOI] [PubMed] [Google Scholar]
- 32.Demetris AJ, Minervini M, Raikow RB, et al. Hepatic epithelioid hemangioendothelioma: biological questions based on pattern of recurrence in an allograft and tumor immunophenotype. Am J Surg Pathol 1997;21:263-70. 10.1097/00000478-199703000-00001 [DOI] [PubMed] [Google Scholar]
- 33.Campione S, Cozzolino I, Mainenti P, et al. Hepatic epithelioid hemangioendothelioma: Pitfalls in the diagnosis on fine needle cytology and "small biopsy" and review of the literature. Pathol Res Pract 2015;211:702-5. 10.1016/j.prp.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 34.Dong K, Wang XX, Feng JL, et al. Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 2015;8:11015-23. [PMC free article] [PubMed] [Google Scholar]
- 35.Kitaichi M, Nagai S, Nishimura K, et al. Pulmonary epithelioid haemangioendothelioma in 21 patients, including three with partial spontaneous regression. Eur Respir J 1998;12:89-96. 10.1183/09031936.98.12010089 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez JA, Becker NS, O'Mahony CA, et al. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg 2008;12:110-6. 10.1007/s11605-007-0247-3 [DOI] [PubMed] [Google Scholar]
- 37.Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011;35:432-41. 10.1097/PAS.0b013e318206b67b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madariaga JR, Marino IR, Karavias DD, et al. Long-term results after liver transplantation for primary hepatic epithelioid hemangioendothelioma. Ann Surg Oncol 1995;2:483-7. 10.1007/BF02307080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coulier B, Pierard F, Gielen I, et al. Hepatic angiosarcoma occurring 65 years after thorium dioxide (Thorotrast) exposure: imaging, surgical and histo- pathologic findings of a historical case. JBR-BTR 2014;97:254-8. [DOI] [PubMed] [Google Scholar]
- 40.Collins JJ, Jammer B, Sladeczek FM, et al. Surveillance for angiosarcoma of the liver among vinyl chloride workers. J Occup Environ Med 2014;56:1207-9. 10.1097/JOM.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 41.Nudo CG, Yoshida EM, Bain VG, et al. Liver transplantation for hepatic epithelioid hemangioendothelioma: the Canadian multicentre experience. Can J Gastroenterol 2008;22:821-4. 10.1155/2008/418485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108-21. 10.1002/cncr.22225 [DOI] [PubMed] [Google Scholar]
- 43.Jung DH, Hwang S, Hong SM, et al. Clinicopathological Features and Prognosis of Hepatic Epithelioid Hemangioendothelioma After Liver Resection and Transplantation. Ann Transplant 2016;21:784-90. 10.12659/AOT.901172 [DOI] [PubMed] [Google Scholar]
- 44.Grotz TE, Nagorney D, Donohue J, et al. Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB (Oxford) 2010;12:546-53. 10.1111/j.1477-2574.2010.00213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desie N, Van Raemdonck DE, Ceulemans LJ, et al. Combined or Serial Liver and Lung Transplantation for Epithelioid Hemangioendothelioma: A Case Series. Am J Transplant 2015;15:3247-54. 10.1111/ajt.13430 [DOI] [PubMed] [Google Scholar]
- 46.Pallotti MC, Nannini M, Agostinelli C, et al. Long-term durable response to lenalidomide in a patient with hepatic epithelioid hemangioendothelioma. World J Gastroenterol 2014;20:7049-54. 10.3748/wjg.v20.i22.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mascarenhas RC, Sanghvi AN, Friedlander L, et al. Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology 2004;67:471-5. 10.1159/000082932 [DOI] [PubMed] [Google Scholar]
- 48.Soape MP, Verma R, Payne JD, et al. Treatment of Hepatic Epithelioid Hemangioendothelioma: Finding Uses for Thalidomide in a New Era of Medicine. Case Rep Gastrointest Med 2015;2015:326795. 10.1155/2015/326795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semenisty V, Naroditsky I, Keidar Z, et al. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 2015;15:402. 10.1186/s12885-015-1395-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly H, O'Neil BH. Response of epithelioid haemangioendothelioma to liposomal doxorubicin. Lancet Oncol 2005;6:813-5. 10.1016/S1470-2045(05)70392-2 [DOI] [PubMed] [Google Scholar]
- 51.Lakkis Z, Kim S, Delabrousse E, et al. Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol 2013;58:1254-7. 10.1016/j.jhep.2013.01.043 [DOI] [PubMed] [Google Scholar]
- 52.Kayler LK, Merion RM, Arenas JD, et al. Epithelioid hemangioendothelioma of the liver disseminated to the peritoneum treated with liver transplantation and interferon alpha-2B. Transplantation 2002;74:128-30. 10.1097/00007890-200207150-00022 [DOI] [PubMed] [Google Scholar]
- 53.Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One 2013;8:e60021. 10.1371/journal.pone.0060021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer 2013;119:2639-44. 10.1002/cncr.28109 [DOI] [PubMed] [Google Scholar]
- 55.Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257-63. 10.1093/annonc/mds237 [DOI] [PubMed] [Google Scholar]
- 56.Theurillat JP, Vavricka SR, Went P, et al. Morphologic changes and altered gene expression in an epithelioid hemangioendothelioma during a ten-year course of disease. Pathol Res Pract 2003;199:165-70. 10.1078/0344-0338-00370 [DOI] [PubMed] [Google Scholar]
- 57.Miller MA, Sandler AD. Elevated plasma vascular endothelial growth factor levels in 2 patients with hemangioendothelioma. J Pediatr Surg 2005;40:e17-9. 10.1016/j.jpedsurg.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 58.Errani C, Sung YS, Zhang L, et al. Monoclonality of multifocal epithelioid hemangioendothelioma of the liver by analysis of WWTR1-CAMTA1 breakpoints. Cancer Genet 2012;205:12-7. 10.1016/j.cancergen.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daller JA, Bueno J, Gutierrez J, et al. Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 1999;34:98-105; discussion 105-6. 10.1016/S0022-3468(99)90237-3 [DOI] [PubMed] [Google Scholar]
- 60.Selby DM, Stocker JT, Waclawiw MA, et al. Infantile hemangioendothelioma of the liver. Hepatology 1994;20:39-45. [DOI] [PubMed] [Google Scholar]
- 61.Li DB, Si XY, Wan T, et al. A pooled analysis of treatment and prognosis of hepatic angiosarcoma in adults. Hepatobiliary Pancreat Dis Int 2018;17:198-203. 10.1016/j.hbpd.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 62.Maluf D, Cotterell A, Clark B, et al. Hepatic angiosarcoma and liver transplantation: case report and literature review. Transplant Proc 2005;37:2195-9. 10.1016/j.transproceed.2005.03.060 [DOI] [PubMed] [Google Scholar]
- 63.Grassia KL, Peterman CM. Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 2017;64:11. 10.1002/pbc.26627 [DOI] [PubMed] [Google Scholar]
- 64.Saleh HA, Tao LC. Hepatic angiosarcoma: aspiration biopsy cytology and immunocytochemical contribution. Diagn Cytopathol 1998;18:208-11. [DOI] [PubMed] [Google Scholar]
- 65.Kim B, Byun JH, Lee JH, et al. Imaging findings of primary hepatic angiosarcoma on gadoxetate disodium-enhanced liver MRI: comparison with hepatic haemangiomas of similar size. Clin Radiol 2018;73:244-53. 10.1016/j.crad.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 66.Pickhardt PJ, Kitchin D, Lubner MG, et al. Primary hepatic angiosarcoma: multi-institutional comprehensive cancer centre review of multiphasic CT and MR imaging in 35 patients. Eur Radiol 2015;25:315-22. 10.1007/s00330-014-3442-0 [DOI] [PubMed] [Google Scholar]
- 67.Groeschl RT, Miura JT, Oshima K, et al. Does histology predict outcome for malignant vascular tumors of the liver? J Surg Oncol 2014;109:483-6. 10.1002/jso.23517 [DOI] [PubMed] [Google Scholar]
- 68.Tran Minh M, Mazzola A, Perdigao F, et al. Primary hepatic angiosarcoma and liver transplantation: Radiological, surgical, histological findings and clinical outcome. Clin Res Hepatol Gastroenterol 2018;42:17-23. 10.1016/j.clinre.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 69.Lin YH, Lin CC, Concejero AM, et al. Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol 2015;13:87. 10.1186/s12957-015-0489-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang NC, Kuo YC, Chiang JC, et al. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore) 2015;94:e816. 10.1097/MD.0000000000000816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hur CJ, Min BR, Lee YJ, et al. Clinical courses of primary hepatic angiosarcoma: retrospective analysis of eight cases. Korean J Gastroenterol 2015;65:229-35. 10.4166/kjg.2015.65.4.229 [DOI] [PubMed] [Google Scholar]
- 72.Husted TL, Neff G, Thomas MJ, et al. Liver transplantation for primary or metastatic sarcoma to the liver. Am J Transplant 2006;6:392-7. 10.1111/j.1600-6143.2005.01179.x [DOI] [PubMed] [Google Scholar]
- 73.Zhu YP, Chen YM, Matro E, et al. Primary hepatic angiosarcoma: A report of two cases and literature review. World J Gastroenterol 2015;21:6088-96. 10.3748/wjg.v21.i19.6088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Forbes A, Portmann B, Johnson P, et al. Hepatic sarcomas in adults: a review of 25 cases. Gut 1987;28:668-74. 10.1136/gut.28.6.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierce DB, Johnson GE, Monroe E, et al. Safety and Efficacy Outcomes of Embolization in Hepatic Sarcomas. AJR Am J Roentgenol 2018;210:175-82. 10.2214/AJR.16.17573 [DOI] [PubMed] [Google Scholar]
- 76.Young RJ, Woll PJ. Anti-angiogenic therapies for the treatment of angiosarcoma: a clinical update. Memo 2017;10:190-3. 10.1007/s12254-017-0365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sindhu S, Gimber LH, Cranmer L, et al. Angiosarcoma treated successfully with anti-PD-1 therapy - a case report. J Immunother Cancer 2017;5:58. 10.1186/s40425-017-0263-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMaster MJ, Soule EH, Ivins JC. Hemangiopericytoma. A clinicopathologic study and long-term followup of 60 patients. Cancer 1975;36:2232-44. 10.1002/cncr.2820360942 [DOI] [PubMed] [Google Scholar]
- 79.Enzinger FM, Smith BH. Hemangiopericytoma. An analysis of 106 cases. Hum Pathol 1976;7: 61-82. 10.1016/S0046-8177(76)80006-8 [DOI] [PubMed] [Google Scholar]
- 80.Flores-Stadler EM, Chou P, Walterhouse D, et al. Hemangiopericytoma of the liver: immunohistochemical observations, expression of angiogenic factors, and review of the literature. J Pediatr Hematol Oncol 1997;19:449-54. 10.1097/00043426-199709000-00008 [DOI] [PubMed] [Google Scholar]
- 81.Adams J, Lodge JP, Parker D. Liver transplantation for metastatic hemangiopericytoma associated with hypoglycemia. Transplantation 1999;67:488-9. 10.1097/00007890-199902150-00027 [DOI] [PubMed] [Google Scholar]
- 82.Meijer B, Simsek M, Blokzijl H, et al. Nodular regenerative hyperplasia rarely leads to liver transplantation: A 20-year cohort study in all Dutch liver transplant units. United European Gastroenterol J 2017;5:658-67. 10.1177/2050640616680550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 1990;11:787-97. 10.1002/hep.1840110512 [DOI] [PubMed] [Google Scholar]
- 84.Guido M, Sarcognato S, Sonzogni A, et al. Obliterative portal venopathy without portal hypertension: an underestimated condition. Liver Int 2016;36:454-60. 10.1111/liv.12936 [DOI] [PubMed] [Google Scholar]
- 85.Khanna R, Sarin SK. Non-cirrhotic portal hypertension - diagnosis and management. J Hepatol 2014;60:421-41. 10.1016/j.jhep.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 86.Breen DP, Marinaki AM, Arenas M, et al. Pharmacogenetic association with adverse drug reactions to azathioprine immunosuppressive therapy following liver transplantation. Liver Transpl 2005;11:826-33. 10.1002/lt.20377 [DOI] [PubMed] [Google Scholar]
- 87.Gane E, Portmann B, Saxena R, et al. Nodular regenerative hyperplasia of the liver graft after liver transplantation. Hepatology 1994;20:88-94. 10.1002/hep.1840200114 [DOI] [PubMed] [Google Scholar]
- 88.Dumortier J, Boillot O, Chevallier M, et al. Familial occurrence of nodular regenerative hyperplasia of the liver: a report on three families. Gut 1999;45:289-94. 10.1136/gut.45.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caturelli E, Ghittoni G, Ranalli TV, et al. Nodular regenerative hyperplasia of the liver: coral atoll-like lesions on ultrasound are characteristic in predisposed patients. Br J Radiol 2011;84:e129-34. 10.1259/bjr/17975057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krasinskas AM, Eghtesad B, Kamath PS, et al. Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transpl 2005;11:627-634; discussion 610-21. 10.1002/lt.20431 [DOI] [PubMed] [Google Scholar]
- 91.Manzia TM, Gravante G, Di Paolo D, et al. Liver transplantation for the treatment of nodular regenerative hyperplasia. Dig Liver Dis 2011;43:929-34. 10.1016/j.dld.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 92.Demetris AJ, Kelly DM, Eghtesad B, et al. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol 2006;30:986-93. 10.1097/00000478-200608000-00009 [DOI] [PubMed] [Google Scholar]
- 93.Gill RM, Buelow B, Mather C, et al. Hepatic small vessel neoplasm, a rare infiltrative vascular neoplasm of uncertain malignant potential. Hum Pathol 2016;54:143-51. 10.1016/j.humpath.2016.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joseph NM, Brunt EM, Marginean C, et al. Frequent GNAQ and GNA14 Mutations in Hepatic Small Vessel Neoplasm. Am J Surg Pathol 2018;42:1201-7. 10.1097/PAS.0000000000001110 [DOI] [PubMed] [Google Scholar]
- 95.Walcott-Sapp S, Tang E, Kakar S, et al. Resection of the largest reported hepatic small vessel neoplasm. Hum Pathol 2018;78:159-62. 10.1016/j.humpath.2018.01.013 [DOI] [PubMed] [Google Scholar]