Abstract
Purpose
This study aims to determine the efficacy and safety of house dust mite (HDM)-sublingual immunotherapy (SLIT) in elderly patients with AR.
Methods
A total of 45 patients aged ≥ 60 years with HDM-induced AR who had ≥ 3 A/H ratio on skin prick test and/or ≥ 0.35 IU/L to both Dermatophagoides farinae and Dermatophagoides pteronyssinus by ImmunoCAP were enrolled in 4 university hospitals. To evaluate additional effects of HDM-SLIT, they were randomized to the SLIT-treated group (n = 30) or control group (n = 15). Rhinoconjunctivitis total symptom score (RTSS), rhinoscopy score, Korean rhinoconjunctivitis quality of life questionnaire, rhinitis control assessment test, asthma control test scores, and adverse reactions, were assessed at the first visit (V1) and after 1 year of treatment (V5); for immunological evaluation, serum levels of HDM-specific immunoglobulin A/IgE/IgG1/IgG4 antibodies and basophil response to HDMs were compared between V1 and V5 in both groups.
Results
There were no significant differences in demographics, RTSS, skin reactivity to HDMs, or serum total/specific IgE levels to HDMs (P < 0.05, respectively) between the 2 groups. Nasal symptom score and RTSS decreased significantly at year 1 in the 2 groups (P < 0.05). There were no significant differences in percent decrease in nasal symptom score and RTSS at year 1 between the 2 groups (P < 0.05); however, rhinoscopic nasal symptom score decreased significantly in the SLIT-treated group (P < 0.05). Immunological studies showed that serum specific IgA levels (not specific IgE/IgG) and CD203c expression on basophils decreased significantly at V5 in the SLIT-treated group (P = 0.011 and P = 0.001, respectively), not in the control group. The control group required more medications compared to the treatment group, but there were no differences in adverse reactions.
Conclusions
It is suggested that HDM-SLIT for 1 year could induce symptom improvement and may induce immunomodulation in elderly rhinitis patients.
Keywords: Allergic rhinitis, elderly, house dust mite, sublingual immunotherapy
INTROUDCTION
Chronic disease in the elderly has become a social problem worldwide along with increasing health care expenditure. Chronic respiratory disease is the third most common burden of disease in the elderly aged ≥ 60 years.1 Although allergic rhinitis (AR) and asthma seem to decrease in the elderly, they are often underdiagnosed, undertreated and underestimated in the aspect of the current prevalence rate.2 AR and asthma are common chronic diseases that affect quality of life and further alter mood or cognitive functions.3
House dust mites (HDMs) are the most common indoor inhalant allergen that can trigger AR and asthma. Allergen immunotherapy (AIT) is a therapeutic option for AR and asthma to alleviate nasal symptoms, induce immune modulation and prevent further sensitization to other allergens.4 Guidelines regarding AIT rarely recommend and often ignore AIT in elderly rhinitis patients, since the immune system in elderly patients is down-regulated compared to younger population.3 A few studies have reported that sublingual immunotherapy (SLIT) in elderly rhinitis patients shows no severe adverse reactions with only a few local side effects and fewer interactions with other comorbid conditions compared to subcutaneous immunotherapy (SCIT).3,5,6 In a few trials, AIT for grass pollens and HDMs has been shown to be effective in elderly rhinitis patients.4,5 However, AIT in elderly patients has not been actively conducted in clinical practice. The aim of this study is to determine the efficacy and safety of SLIT in patients aged ≥60 years with HDM-induced allergic rhinoconjunctivitis in comparison with standard pharmacotherapy on demand and to evaluate immunological responses by SLIT.
MATERIALS AND METHODS
Patients and diagnostic methods
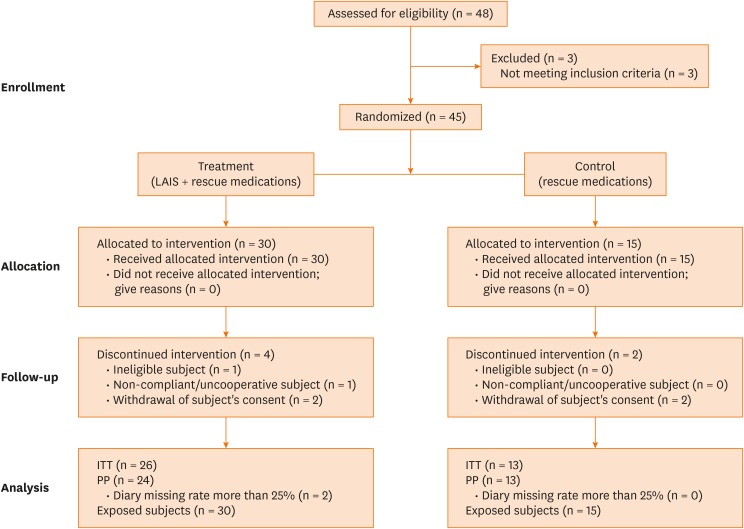
A total of 48 elderly rhinitis patients sensitized to HDMs were enrolled in 4 (Ajou, Yonsei, Korea, and Hallym University Hospitals) in South Korea, and the study flow is summarized in Fig. 1. Three patients who did not meet inclusion criteria were excluded. The patients had suffered from AR and/or bronchial asthma as defined by Allergic Rhinitis and its Impact on Asthma (ARIA) criteria and Global Initiative for Asthma (GINA), and had high serum specific IgE to Dermatophagoides pteronyssinus and/or Dermatophagoides farinae as confirmed by skin prick test and/or ImmunoCAP® (ThermoFisher Scientific, Waltham, MA, USA). At the time of enrollment, retrospective rhinoconjunctivitis total symptom score (RTSS) related to the previous winter season was at least 8 (out of a maximum of 18). RTSS is composed of 6 rhinoconjunctivitis symptoms, sneezing, rhinorrhea, nasal pruritus, nasal congestion, ocular pruritus and watery eyes during 24 hours, each symptoms assessed by 4-point-scale (0 = no symptoms to 3 = severe).7 Patients with co-sensitization to other pollens or inhalant allergens were enrolled when: 1) typical symptoms due to co-allergens did not exist and 2) the reactions of the skin prick test for co-allergens were less than those for HDMs. We excluded the following patients who: 1) participated in other studies; 2) received immunotherapy with any allergens, SCIT with HDMs, and/or anti-IgE monoclonal antibody treatment; 3) had uncontrolled asthma, other active respiratory disease, and/or malignancy; and 4) were contraindicated to SLIT. This study was approved by the Institutional Review Board of Ajou University Hospital (Ajou IRB-MED-CT4-14-159) and each center. All the study patients gave informed consent. On the assumption that the mean change in reactivity is 0.2 in the control group and 0.3 in the optimal dose group and that the variance is 0.1 in both groups, sample size was calculated to 30 patients in the treatment group and 15 patients in the control group to satisfy α = 0.05 and a power of 90%.
Fig. 1. Number of participating patients assessed for eligibility that completed the study.
ITT, intention to treat; PP, per protocol.
Treatment and follow-up schedules
The patients were randomized at the first visit (V1), and HDM-SLIT tablets (LAIS®; Lofarma SpA, Milano, Italy) with on-demand medications were given to the treatment group, while on-demand medications alone were given to the control group. From days 1 through 4, the treatment group received 300 allergenic unit (AU) of LAIS tablet; from day 5, they took 1,000 AU of LAIS twice a week for 48 weeks. The control group received standard medications on demand according to disease control status. The standard medications were defined as unexpected use of oral antihistamine, nasal corticosteroid, eye drops and inhaled corticosteroids with long acting-β2 agonists. All patients were scheduled to visit 1 week (visit 2, V2), 3 months (84 ± 7 days, visit 3, V3), 6 months (168 ± 7 days, visit 4, V4), and 1 year (336 ± 7 days, visit 5, V5) after the randomization. All patients filled out the rhinoconjunctivitis quality of life questionnaire (RQLQ), and received rhinitis control assessment test (RCAT) and asthma control test (ACT) at V1 and V5 as previously described.7,8,9 Rhinoscopy was done by the investigators to evaluate symptoms scores. Blood samples were taken from each patient at V1 and V5 for immunological evaluation and were stored at −20°C for further immunological evaluation.
Symptom diary and efficacy variables
The diary was composed of questionnaire on nasal and eye symptom scores (sneezing, rhinorrhea, nasal obstruction, nasal itching sensation, tearing, eye itching and redness) ranging from 0 to 3 (0 = no symptom, 1 = mild symptom, 2 = moderate symptom and 3 = severe symptom) and medication scores (total number of antihistamine tablets, nasal sprays, and eye drops). The daily symptoms and medication scores were recorded daily on the distributed diary.
Each patient was asked to bring the diary at each visit to confirm records. The primary efficacy variable was RTSS, which is composed of 6 rhino-conjunctivitis symptoms (sneezing, rhinorrhea, nasal pruritus, nasal congestion, ocular pruritus and watery eyes). The secondary efficacy variables were medication requirements, rhinoscopy scores as evaluated by the investigators, which were composed of nasal symptoms, nasal congestion, nasal secretion and redness (ranging from 0 = none to 3 = severe), rhinoconjunctivitis quality of life by RQLQ and RCAT, and asthma control as assessed by ACT. RQLQ is composed of 7 domains (activity limitation, sleep, non-nose/eye symptoms, practical problems, nose symptoms, eye symptoms and emotional function) and 28 questions during the previous week. The RQLQ scale scores range from 1 = no impairment to 5=severe impairment. The RCAT of Korean version is composed of 6 questions regarding nasal and eye symptoms (nasal congestion, sneezing, watery eye, sleep disturbance and activity limitation; control status: 5 = never-controlled and 1 = well-controlled) during the previous week. ACT consists of 5 questions on a 5-point scale of 1 to 5 (higher scores reflecting better control state) to assess the effect of asthma on daily activities, asthma symptoms at daytime, asthma symptoms at night, use of rescue mediations and self-assessment of asthma control.
Immunological evaluation
Blood samples were obtained from each patient at V1 and V5, and immunological changes were compared before and after the start of treatment using the following 2 methods: 1) changes in serum specific IgA, IgE, and IgG1/IgG4 antibodies to D. farinae as measured by enzyme-linked immunosorbent assay (ELISA); and 2) changes in basophil responses to D. farinae as evaluated by basophil activation test (BAT). The levels of serum specific IgE, IgA, and IgG1/IgG4 antibodies to D. farinae were measured by ELISA as previously described.10,11
For BAT, peripheral blood samples were collected from each study patient at V1 and V5 as previously described.12,13 Within 3 hours of blood sample collection in an ethylenediaminetetraacetic acid (EDTA) tube, ammonium chloride lysis buffer (0.154 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2-7.4) was used to lyse red blood cells. Using anti-IgE antibody (1 μg/mL) and calcium inophore (1 μg/mL; Sigma-Aldrich Co., St. Louis, MO, USA), basophils were incubated as positive controls. After washing out with a phosphate-buffered saline, the resuspended cells were stained with phycoerythrin-conjugated antihuman CD203c antibody (Beckman Coulter, Marseille, France) on ice in the dark for 30 minutes. After another washing with phosphate-buffered saline, the cells were evaluated by using FACS Canto II flow cytometry (Becton Dickinson, San Jose, CA, USA) and were presented as % of cells expressing CD203c.
Statistical analysis
All statistical analyses were performed using SPSS ver. 20 (SPSS Inc., Chicago, IL, USA). The values are presented as mean ± standard deviation or median (minimal and maximal values). Student's t test was used to analyze differences between the 2 groups. Wilcoxon's signed rank test was done to compare changes in symptom/medication scores, RTSS, RQLQ, ACT, RCAT and immunologic changes between V1 and V5 in both groups. A P value of < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
Total 45 patients were enrolled and randomized to the treatment group (n = 30), who received SLIT along with medications on demand, and the control group (n = 15) who were allowed to take medications, including antihistamines and intranasal steroids, on demand by using centralized, computer-generated randomization system. The mean age of the patients was 67.2 years (range, 60–81 years), and the male to female ratio was 22:17. Four patients in the treatment group and 2 in the control group were dropped out from the study, 1 patient was ineligible subject, 1 patient was uncooperative and 2 patients from each group withdrew informed consent. Among these 39 patients, 26 in the treatment group and 13 in the control group were analyzed. The mean age was 67.0 ± 5.8 years in the treatment group and 67.2 ± 6.5 years in the control group, with no significant difference. The baseline clinical characteristics, comorbid conditions, including asthma, skin reactivity or serum specific IgE to HDMs (as measured by ImmunoCAP), was not significantly different between the 2 groups (Table 1).
Table 1. Baseline clinical characteristics of the study subjects.
| Variables | Treatment group (n = 26) | Control group (n = 13) | P value | |
|---|---|---|---|---|
| Age (yr) | 67.0 ± 5.8 | 67.2 ± 6.5 | 0.956 | |
| Height (cm) | 163.6 ± 7.3 | 161.6 ± 6.7 | 0.436 | |
| Sex (male) | 15 (57.7) | 7 (53.8) | 1.000 | |
| Weight (kg) | 64.9 ± 9.9 | 63.8 ± 9.9 | 0.746 | |
| Current smoker | 6 (23.1) | 1 (7.7) | 0.388 | |
| Atopic dermatitis | 5 (19.2) | 1 (7.7) | 0.643 | |
| Food allergy | 3 (11.5) | 0 (0.0) | 0.538 | |
| Diagnosis of rhinitis (yr) | ||||
| 20 < age > 40 | 1 (3.8) | 1 (7.7) | 1.000 | |
| > 40 | 25 (96.2) | 12 (92.3) | 1.000 | |
| Asthma | 17 (65.4) | 12 (92.3) | 0.119 | |
| Baseline FEV1 (mL) | 2,419.6 ± 664.2 | 2,243.3 ± 527.1 | 0.425 | |
| Total IgE (IU/L) | 611.82 ± 923.14 | 581.09 ± 786.78 | 0.599 | |
| Specific IgE to Dp (IU/L) | 5.07 ± 9.29 | 5.06 ± 8.22 | 0.998 | |
| Specific IgE to Df (IU/L) | 12.41 ± 21.77 | 8.07 ± 9.64 | 0.541 | |
| Wheal size of Dp (mm) | 5.03 ± 4.79 | 6.667 ± 3.77 | 0.225 | |
| Wheal size of Df (mm) | 5.27 ± 3.92 | 5.500 ± 3.31 | 0.883 | |
Data are expressed as mean ± standard deviation or number (%).
FEV1, forced expiratory volume in 1 second; IgE, immunoglobulin E; Dp, Dermatophagoides pteronyssinus; Df, Dermatophagoides farinae.
When rescue medications (including oral antihistamines, histamine eye drops, and intranasal steroids) were compared between the 2 groups, the dose of antihistamines (mg) tended to be more frequently used in the control group than in the treatment group, although there was no statistical significance (P = 0.054, Table 2).
Table 2. Changes in rhinitis/eye symptoms and clinical scores in the study period between the treatment and control groups.
| Variables | Treatment group | P value* | Control group | P value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V3 | V4 | V5 | V1 | V3 | V4 | V5 | |||
| Nasal symptoms | 8 (5–10) | 2 (0–7) | 2 (0–8) | 2.5 (0–7) | < 0.001 | 8 (6–10) | 1 (0–8) | 3 (0–6) | 2 (0–5) | < 0.001 |
| Eye symptoms | 2 (0–4) | 0.5 (0–4) | 0.5 (0–5) | 1 (0–5) | 0.036 | 1 (0–4) | 1 (0–4) | 1 (0–5) | 0 (0–5) | 0.363 |
| RTSS | 10 (8–12) | 3 (0–8) | 3 (0–12) | 4 (0–9) | < 0.001 | 9 (8–13) | 2 (0–12) | 3 (0–10) | 3 (0–10) | < 0.001 |
| RQLQ | 54.5 (32–95) | 48 (34–76) | 53.5 (30–87) | 50 (33–75) | 0.053 | 59 (31–84) | 38 (28–84) | 45 (28–78) | 42 (28–94) | 0.077 |
| RCAT | 21.5 (14–27) | 23 (16–30) | 22 (19–30) | 22.5 (16–28) | 0.105 | 22 (17–29) | 25 (16–30) | 22 (13–30) | 23 (17–30) | 0.528 |
| ACT | 21 (13–24) | 20.5 (9–24) | 19.5 (14–25) | 21 (13–24) | 0.841 | 19.5 (14–25) | 22.5 (11–25) | 21 (12–25) | 22 (14–25) | 0.469 |
All values were presented as median (minimum–maximum values).
RTSS, rhinoconjunctivitis total symptom score; RQLQ, rhinoconjunctivitis quality of life questionnaire; ACT, asthma control test; RCAT, rhinitis control assessment test, V, visit.
*Wilcoxon-signed rank test comparing scores between V1 and V5.
Changes in RTSS, RQLQ, RCAT, and ACT scores
Nasal and eye symptoms decreased significantly between V1 and V5 in the treatment group (P = 0.001 and P = 0.036, respectively), while nasal symptoms and RTSS, except for eye symptoms, decreased in the control group (P < 0.001). However, RTSS decreased significantly both in the treatment and control groups (P = 0.001 for each). The RQLQ scores tended to decrease in the treatment group without statistical significance (P < 0.05). No significant change was noted in RCAT or ACT scores between V1 and V5 in both groups (Table 2).
Rhinoscopy score
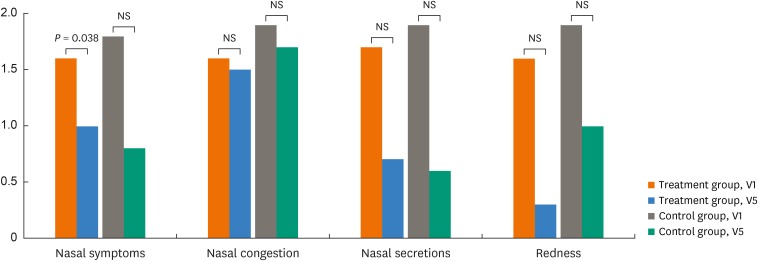
Rhinoscopy scores as measured by the investigators at each visit were compared between V1 and V5 in the treatment and control groups. A significant difference was noted only in changes in nasal symptom scores (P = 0.038), although nasal congestion, secretion, and redness scores were not significantly different in the 2 groups (P < 0.05, Fig. 2).
Fig. 2. Changes in nasal symptoms, nasal congestion, nasal secretions and redness before (visit 1, V1) and after treatment (visit 5, V5) in the treatment and control groups. All scores were analyzed by Wilcoxon's signed rank test.
NS, not significant.
Changes in immunological parameters
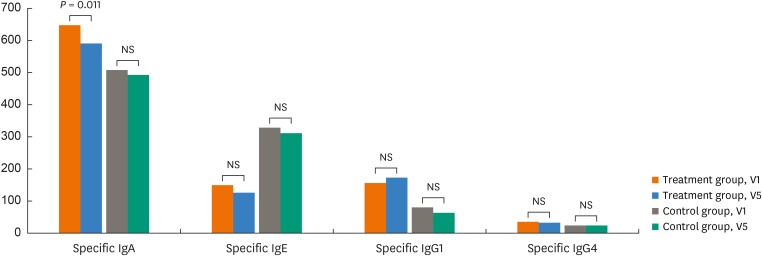
Serum specific IgE to D. farinae tended to decrease between V1 and V5 in the treatment and control groups, but no statistical significance was reached. However, the serum specific IgA levels to D. farinae decreased significantly in the treatment group (P = 0.011), while they were not in the control group. There were no significant changes in serum specific IgG1 and G4 antibodies to D. farinae between V1 and V5 in the 2 groups (Fig. 3).
Fig. 3. Changes in serum specific antibodies to Dermatophagoides farinae by ELISA before (visit 1, V1) and after the treatment (visit 5, V5) in the treatment and control groups. All values are presented as absorbance value x 1,000 and compared by using Wilcoxon's signed rank test.
ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; NS, not significant.
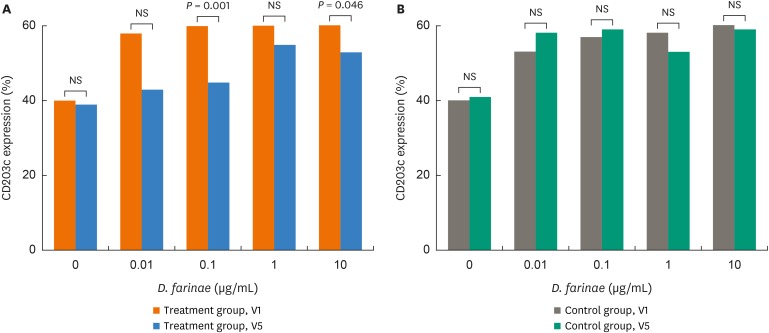
In BAT, CD203 expression levels decreased significantly after incubation with 0.1 and 10 ug/mL of D. farinae at V5 compared to V1(P = 0.001 and P = 0.046, respectively, Fig. 4), while no significant changes were noted in the control group.
Fig. 4. Changes in basophil CD203c expression with additions of Dermatophagoides farinae before (visit 1, V1) and after the treatment (visit 5, V5) in the treatment (A) and control groups (B). Wilcoxon's signed rank test was applied to compare the results between the 2 groups.
NS, not significant.
Safety assessment
There was no significant difference in adverse reactions between the 2 groups. Most of the reactions were mild, recovered completely without any reported complications. Rhinitis and conjunctivitis exacerbations were reported from the treatment and control groups. The most frequent adverse reaction was upper respiratory infection in the 2 groups (10 events from 6 patients in the treatment group, 17 events from 4 patients in the control group). No systemic or severe adverse reaction was reported.
DISCUSSION
Recent epidemiological studies have often underestimated elderly rhinitis patients and excluded elderly subjects aged ≥60 years in the analysis of rhinitis prevalence and outcomes.14 There have been few publications on the efficacy and safety of AIT in elderly patients sensitized to HDMs, and especially no published data on immunological responses during AIT in elderly rhinitis patients. In addition, elderly subjects are known to show decreased immune regulations compared to younger subjects; therefore, AIT has not been actively recommended in such subjects.15 A few studies have reported the favorable efficacy of SLIT in the elderly. SLIT with grass pollens and HDMs significantly reduced medication scores in elderly rhinitis patients. Furthermore, SLIT in elderly rhinitis patients was well tolerated without any severe adverse events.5,16 The present study is a randomized controlled trial of HDM-SLIT for 1 year in elderly rhinitis patients sensitive to HDM. During the 1-year study period, both rhinoscopy scores (an objective parameter) observed by the investigators and subjective symptom scores (RTSS) evaluated by the patients were significantly improved in the treatment group, with minimal adverse reactions. These findings can provide evidence for the favorable efficacy of HDM-SLIT in elderly rhinitis patients sensitized to HDMs, although further studies with longer study periods are needed to confirm our results.
HDMs are the most common inhalant allergen in both elderly and non-elderly patients with rhinitis in Korea.17 Moreover, the effect of SLIT with HDM can decrease medication scores and improve quality of life in adult patients with AR and in asthmatic children. Moreover, SLIT induces symptom relief and decreases medication requirements, which may lead to remission.18 Since concomitant medications can affect not only rhinitis and asthma control status, but also general conditions in elderly patients, a decrease in medication requirements is an issue in such patients.19 Antihistamines are effective in reducing rhinitis symptoms in the elderly, but cause adverse effects, such as urinary retention, dry mouth, constipation, arrhythmia and postural hypertension.20,21 In the present study, total use of antihistamines tended to be lower in the treatment group than in the control group; therefore, SLIT may be beneficial for elderly rhinitis patients to reduce total medication use.
AR results from an IgE-mediated chronic inflammation of nasal mucosa triggered by allergens and various environmental factors, provoking intermittent or persistent symptoms such as rhinorrhea, nasal congestion, nasal/ocular pruritus, sneezing and postnasal drips. These nasal symptoms are not life-threatening, but they negatively affect quality of life as it is a chronic inflammatory disease.20 Moreover, rhinitis symptoms may lead to sleep disturbance and alterations in physiological processes among geriatric patients such as endocrine function, cognitive function, glucose metabolism and appetite control.21 Rhinitis significantly affects quality of life in elderly patients compared to younger adults.22,23 In the present study, Korean versions of RQLQ and RCAT were used to assess quality of life in the study patients, and ACT was also included in the questionnaire on asthma control status in the study patients with asthma. RQLQ tended to decrease and RCAT tended to increase in the treatment group, implying that quality of life and clinical symptoms can be improved after 1-year HDM-SLIT in elderly rhinitis patients sensitized to HDMs.
Alterations in immune function due to aging, environmental factors and lifestyle cause immunosenescence, resulting in increased CD4+ T memory cells, reduced naïve T lymphocytes and decreased B-lymphocyte activity, which suppress humoral immune responses in elderly patients.24,25 Therefore, AIT has not been actively recommended for elderly rhinitis patients due to its low efficacy. To the best of our knowledge, the present study is the first trial to evaluate changes in immunological responses to HDM during 1-year HDM-SLIT in the elderly rhinitis patients. Allergen-specific IgA is known to play a critical role in the regulation of the oral mucosa immune system by repeated exposure to allergens during SLIT.26 Specific IgA in saliva and serum are increased in patients with food allergy (induced by peanuts and eggs) during allergen-specific SLIT, suggesting that specific IgA may be a useful biomarker for the efficacy and immunomodulating effect of SLIT.27,28 In the present study, serum specific IgA to D. farinae decreased; serum specific IgG4 to D. farinae tended to increase after 1-year SLIT in the treatment group, while these findings were not found in the control group. These results suggest that HDM-SLIT for 1 year may induce immune modulation as well as clinical improvement in elderly rhinitis patients, although long-term studies are needed to confirm these effects.
BAT has been widely used in the diagnosis of drug allergy as well as other allergic diseases such as chronic urticaria, food allergy and insect venom allergy.29,30 It has been applied to the assessment of AIT or anti-IgE treatment outcomes.31 The CD63 expression levels significantly decreased after 6 months of AIT in patients treated with venom immunotherapy,32 while they did not in those treated with AIT using grass pollens.33 In the present study, CD203c-expressing cells (%) decreased after 1-year SLIT, suggesting that HDM-SLIT could reduce basophil responsiveness to HDMs even in elderly rhinitis patients. Further long-term studies are needed to evaluate BAT changes as a biomarker for evaluating the efficacy of SLIT.
SLIT has several advantages over SCIT: noninvasiveness, self-administration and less frequent/severe adverse reactions.34 Common adverse events of SLIT are oral itching sensation and throat irritations.18 In the present study, no difference in the incidence or severity of adverse reactions were noted between the 2 groups, suggesting that SLIT is a safe and effective treatment modality in elderly rhinitis patients, although long-term safety studies are needed.
There are several limitations in this study. First, this study was not a placebo-controlled trial, and the patients in both groups took medications on-demand which results in symptom improvement in the control group. Drug compliance is lower in chronic diseases, such as diabetes, osteoporosis, and hypertension respiratory disease, as compared to respiratory diseases, such as chronic obstructive pulmonary disease.35 Although all patients were asked to record medications and symptoms on a daily basis, they took medications as symptoms appeared and a few patients belonging to the treatment group did not take any medication when symptoms were aggravated, because they regarded SLIT tablets as investigational products. Secondly, the patients were enrolled, irrespective of seasons. Since the questionnaires were filled out in different seasons, the patients may have reported different symptoms, affecting the questionnaire results.
In conclusion, the results of this study suggest that SLIT with HDMs can be beneficial in improving rhinitis symptoms and inducing immunomodulation in elderly rhinitis patients.
ACKNOWLDEGMENTS
This trial was supported by LoFarma (Milano, Italy) and clinical trial center of Ajou University Hospital, and by a grant of the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI6C0992).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 2.Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010;31:179–184. doi: 10.2500/aap.2010.31.3342. [DOI] [PubMed] [Google Scholar]
- 3.Scichilone N, Ventura MT, Bonini M, Braido F, Bucca C, Caminati M, et al. Choosing wisely: practical considerations on treatment efficacy and safety of asthma in the elderly. Clin Mol Allergy. 2015;13:7. doi: 10.1186/s12948-015-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96.e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 5.Bozek A, Kolodziejczyk K, Warkocka-Szoltysek B, Jarzab J. Grass pollen sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2014;28:423–427. doi: 10.2500/ajra.2014.28.4091. [DOI] [PubMed] [Google Scholar]
- 6.Hur GY, Lee JH, Park HS. Allergen immunotherapy for the treatment of respiratory allergies in the elderly. Curr Opin Allergy Clin Immunol. 2017;17:304–308. doi: 10.1097/ACI.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 7.Kim MA, Ye YM, Ban GY, Shin YS, Nahm DH, Park HS. Linguistic adaptation of the rhinitis control assessment test in Korean. Allergy Asthma Respir Dis. 2017;5:205–210. [Google Scholar]
- 8.Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, et al. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008;23:621–627. doi: 10.3346/jkms.2008.23.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report. Korean J Otolaryngol-Head Neck Surg. 2002;45:254–262. [Google Scholar]
- 10.Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016;8:329–337. doi: 10.4168/aair.2016.8.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JH, Hur GY, Ye YM, Kim JE, Park K, Park HS. Correlation between specific IgA and eosinophil numbers in the lavage fluid of patients with perennial allergic rhinitis. Allergy Asthma Proc. 2008;29:152–160. doi: 10.2500/aap.2008.29.3069. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim SH, Palikhe S, Yang EM, Ye YM, Park HS. Increased basophil activation in adult patients with anaphylaxis. Ann Allergy Asthma Immunol. 2015;115:523–5.e1. doi: 10.1016/j.anai.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The Basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res. 2016;8:541–544. doi: 10.4168/aair.2016.8.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becerril Angeles M, Vázquez Merino CL, Angeles Garay U, Alvarado Moctezuma LE, Vilchis Guízar E. Prevalence of allergic diseases in the elderly. Rev Alerg Mex. 2008;55:85–91. [PubMed] [Google Scholar]
- 16.Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43:242–248. doi: 10.1111/cea.12039. [DOI] [PubMed] [Google Scholar]
- 17.Ohn J, Paik SH, Doh EJ, Park HS, Yoon HS, Cho S. Allergen sensitization pattern by sex: a cluster analysis in Korea. Ann Dermatol. 2017;29:735–741. doi: 10.5021/ad.2017.29.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng B, Xiang H, Jin H, Gao J, Huang S, Shi Y, et al. Efficacy of sublingual immunotherapy for house dust mite-induced allergic rhinitis: a meta-analysis of randomized controlled trials. Allergy Asthma Immunol Res. 2017;9:220–228. doi: 10.4168/aair.2017.9.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardzyńska A, Kubsik B, Kowalski ML. Comorbidities in elderly patients with asthma: association with control of the disease and concomitant treatment. Geriatr Gerontol Int. 2015;15:902–909. doi: 10.1111/ggi.12367. [DOI] [PubMed] [Google Scholar]
- 20.Baptist AP, Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin North Am. 2016;36:343–357. doi: 10.1016/j.iac.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010;6:10. doi: 10.1186/1710-1492-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lue KH, Lin YH, Sun HL, Lu KH, Hsieh JC, Chou MC. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol. 2006;17:408–415. doi: 10.1111/j.1399-3038.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 23.Trebuchon F, Lhéritier-Barrand M, David M, Demoly P. Characteristics and management of sublingual allergen immunotherapy in children with allergic rhinitis and asthma induced by house dust mite allergens. Clin Transl Allergy. 2014;4:15. doi: 10.1186/2045-7022-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozek A. Pharmacological management of allergic rhinitis in the elderly. Drugs Aging. 2017;34:21–28. doi: 10.1007/s40266-016-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur SK. Allergy and asthma in the elderly. Semin Respir Crit Care Med. 2010;31:587–595. doi: 10.1055/s-0030-1265899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böttcher MF, Häggström P, Björkstén B, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy. 2002;32:1293–1298. doi: 10.1046/j.1365-2222.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 27.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA in saliva correlates with food challenge outcomes following peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1159–1162. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto M, Kamemura N, Nagao M, Irahara M, Kagami S, Fujisawa T, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016;27:276–282. doi: 10.1111/pai.12535. [DOI] [PubMed] [Google Scholar]
- 29.Gómez E, Campo P, Rondón C, Barrionuevo E, Blanca-López N, Torres MJ, et al. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J Allergy Clin Immunol. 2013;132:975–976.e1-5. doi: 10.1016/j.jaci.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Sanz ML, Sánchez G, Gamboa PM, Vila L, Uasuf C, Chazot M, et al. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne . Clin Exp Allergy. 2001;31:1007–1013. doi: 10.1046/j.1365-2222.2001.01122.x. [DOI] [PubMed] [Google Scholar]
- 31.Eberlein B, Santos AF, Mayorga C, Nopp A, Ferrer M, Rouzaire P, et al. Basophil activation testing in diagnosis and monitoring of allergic disease – an overview. Allergo J. 2016;25:26–33. doi: 10.1111/all.12698. [DOI] [PubMed] [Google Scholar]
- 32.Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007;72:196–203. doi: 10.1002/cyto.b.20142. [DOI] [PubMed] [Google Scholar]
- 33.Van Overtvelt L, Baron-Bodo V, Horiot S, Moussu H, Ricarte C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011;66:1530–1537. doi: 10.1111/j.1398-9995.2011.02696.x. [DOI] [PubMed] [Google Scholar]
- 34.Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016;8:191–197. doi: 10.4168/aair.2016.8.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagano F. Therapeutic compliance in elderly patients with COPD. J Gerontol Geriat. 2016;64:147–151. [Google Scholar]