Abstract
Purpose
Pollen-food allergy syndrome (PFAS) is an immunoglobulin E (IgE)-mediated allergy in pollinosis patients caused by raw fruits and vegetables and is the most common food allergy in adults. However, there has been no nationwide study on PFAS in Korea. In this study, we investigated the prevalence and clinical characteristics of PFAS in Korea.
Methods
Twenty-two investigators participated in this study, in which patients with allergic rhinoconjunctivitis and/or bronchial asthma with pollen allergy were enrolled. The questionnaires included demographic characteristics, a list of fruits and vegetables, and clinical manifestations of food allergy. Pollen allergy was diagnosed by skin prick test and/or measurement of the serum level of specific IgE.
Results
A total of 648 pollinosis patients were enrolled. The prevalence of PFAS was 41.7% (n = 270). PFAS patients exhibited cutaneous (43.0%), respiratory (20.0%), cardiovascular (3.7%) or neurologic symptoms (4.8%) in addition to oropharyngeal symptoms. Anaphylaxis was noted in 8.9% of the PFAS patients. Seventy types of foods were linked to PFAS; e.g., peach (48.5%), apple (46.7%), kiwi (30.4%), peanut (17.4%), plum (16.3%), chestnut (14.8%), pineapple (13.7%), walnut (14.1%), Korean melon (12.6%), tomato (11.9%), melon (11.5%) and apricot (10.7%). Korean foods such as taro/taro stem (8.9%), ginseong (8.2%), perilla leaf (4.4%), bellflower root (4.4%), crown daisy (3.0%), deodeok (3.3%), kudzu root (3.0%) and lotus root (2.6%) were also linked to PFAS.
Conclusions
This was the first nationwide study of PFAS in Korea. The prevalence of PFAS was 41.7%, and 8.9% of the PFAS patients had anaphylaxis. These results will provide clinically useful information to physicians.
Keywords: Pollen-food allergy syndrome, pollen, food allergy
INTRODUCTION
Pollen-food allergy syndrome (PFAS) is a class 2 food allergy caused by respiratory exposure to pollen; in contrast, class 1 food allergies are sensitized by the gastrointestinal tract. The pathogenesis of PFAS is related to cross-allergenicity between pollen and foods such as fruits and vegetables.1,2 The term oral allergy syndrome (OAS) is traditionally used for PFAS, but can be confusing. OAS indicates immunoglobulin E (IgE)-mediated immediate allergy symptoms restricted to the oral mucosa, which is the most common clinical presentation of PFAS.2 Since OAS in pollinosis patients was first reported in 1942 as labile antigen in fresh fruit extracts,3 many subsequent studies reported that OAS can involve systemic symptoms such as urticaria, nausea, vomiting and anaphylaxis.4,5,6
In 1982, the first investigation of PFAS in European pollinosis patients reported a prevalence of more than 70%. Indeed, 19% of pollinosis patients sensitized to mugwort or grass pollen have a food hypersensitivity.4 The prevalence of PFAS in pollinosis patients is around 40%–50% in Europe5,6,7 and 20% in the Mediterranean region (a birch- and ragweed-free area).8 The prevalence of PFAS in pediatric pollinosis patients is around 9%–12%; e.g., 9.6%–12.2% in Mexico and 12.1% in Australia.9,10,11 In Japan, 7%–17% of OAS occurs in cedar pollinosis patients.12,13 In another study in Japan, the overall prevalence of OAS was 4.1% in the pollen-sensitized population, comprising 4% in Japanese cedar pollen, 11.5% in alder, 5.5% in orchard grass, 7.4% in ragweed and 6.4% in mugwort, where the most common causes were apple, peach and melon.14 Thus, few studies of the epidemiology and clinical characteristics of PFAS have involved children or been performed in Asian countries.
The sole previous study of PFAS in Korea reported a PFAS prevalence of 34.6% in pollen-sensitized patients in a single general hospital (48% in tree pollen- and 13% in grass or weed pollen-sensitized patients), and apple was the most common causative food.15 However, this was a small retrospective study involving only 81 subjects. Therefore, we investigated the prevalence and clinical features of PFAS in Korean pollinosis patients by means of a nationwide survey.
MATERIALS AND METHODS
Study design
This was a nationwide cross-sectional study in 20 institutes (18 university hospitals and 2 allergy clinics). The protocol was reviewed and approved by the Institutional Review Board of each institute (Hallym University Dongtan Sacred Heart Hospital, HDT-2016-04-155-003, etc.). Written informed consent was obtained from each patient or their parents.
Patients
Pollinosis patients were enrolled from March to December 2016. The inclusion criteria were as follows: 1) diagnosis with one or more of allergic rhinitis (AR), allergic conjunctivitis (AC) and/or bronchial asthma (BA); 2) sensitization to the pollen of 1 or more trees, grasses and/or weeds; and 3) aggravated symptoms of rhinitis, conjunctivitis and/or asthma when exposed to sensitized pollens. Patients who fulfilled at least one of the following criteria were regarded as being sensitized to pollen: 1) allergen wheal size greater or equal to that of histamine (A/H ratio ≥3+) or mean allergen wheal diameter of at least 3 mm by skin prick test (SPT);16,17 2) a multiple allergen simultaneous test (MAST) result of at least 2+; and 3) a serum specific IgE level of at least 0.35 kU/L measured by the ImmunoCAP® system (ThermoFisher Scientific, Uppsala, Sweden). The pollens investigated comprised tree pollens (birch, alder, hazel, beech, oak, willow, poplar, pine and tree mix), grass pollens (Bermuda, meadow, orchard, rye, timothy and grass mix) and weed pollens (mugwort, ragweed and Hop Japanese).
Clinical data collection
Clinical data were collected by questionnaire and chart review, and included demographic characteristics, underlying allergic diseases, and results of allergic tests. The demographic data included sex, birth date, birthplace, present residence area, height, body weight, family history of allergic diseases, smoking status (current, former, or never-smoker), and history of second-hand smoking. AR severity and symptom duration were classified using the Allergic Rhinitis and its Impact on Asthma (ARIA) system.18 Briefly, severity was classified as mild or moderate-severe, and symptom duration as intermittent or persistent. Moderate-severe rhinitis symptoms were defined as 1 or more bothersome symptoms such as sleep disturbance, impairment of daily activity, impairment of school or work, and troublesome symptoms. Persistent rhinitis was defined as symptoms on more than 4 days per week for at least 4 consecutive weeks. The results of allergy tests for house dust mite, cat, dog and fungus allergens were also obtained.
Questionnaires about PFAS
All subjects completed questionnaires about PFAS. The questionnaires included the following questions: 1) Have you experienced any symptoms after ingesting any of the foods listed below? and 2) If any, which symptoms have you had? The food list contained common fruits and vegetables in South Korea — apple, pear, peach, apricot, plum, cherry, watermelon, melon, Korean melon, banana, kiwi, orange, mandarin, pineapple, strawberry, mango, avocado, grape, carrot, potato, sweet potato, celery, crown daisy, perilla leaf, lettuce, kale, chicory, taro/taro stem, ginseng, deodeok (Codonopsis lanceolata), bellflower root, kudzu, lotus root, Chinese yam, eggplant, zucchini, cucumber, tomato, jujube, chestnut, peanut, walnut, pine nut and soy. The patients were also asked to record any other fruits or vegetables that caused allergy symptoms. The cooking status of the fruits and vegetables (raw and cooked) was also checked.
Symptoms of food allergy were evaluated according to the affected system — oral allergy symptoms (tingling/itching sense or edema of the lips, oral cavity, and/or throat), dermatologic symptoms (itching, urticaria or angioedema), respiratory symptoms (rhinorrhea, cough, dyspnea, wheezing, cyanosis or hypoxia), cardiovascular symptoms (chest pain, hypotension, pale, sweating or cardiac arrest), gastrointestinal symptoms (nausea or vomiting, diarrhea or abdominal pain), neurologic and systemic symptoms (dizziness, unconsciousness, anxiety, change of sense or death) and anaphylaxis.
Statistical analysis
Statistical analyses were performed using R version 3.4.0 (R Foundation, Vienna, Austria). The Wilcoxon rank-sum test and χ2 test were used to compare continuous and categorical clinical variables, respectively, according to PFAS. The CORRPLOT package was used to conduct Yules' Q correlation analysis to identify associations among ordinal variables, and P values were calculated by the χ2 test. The variables were ordered by the complete-linkage hierarchical clustering method.
RESULTS
Clinical characteristics
A total of 648 pollinosis patients were enrolled. Four hundred four patients (62.3%) were males, and 300 (42.7%) were less than or equal to 18 years of age. All of the patients had AR (90.1%), AC (45.7%) and/or BA (39.2%), the symptoms of which showed seasonal aggravation; 65.6% in spring, 28.7% in summer, 49.2% in autumn, 32.4% in winter and 17.3% perennially. Of the patients, 68.8% had a family history of allergic diseases.
Prevalence and clinical characteristics of PFAS patients
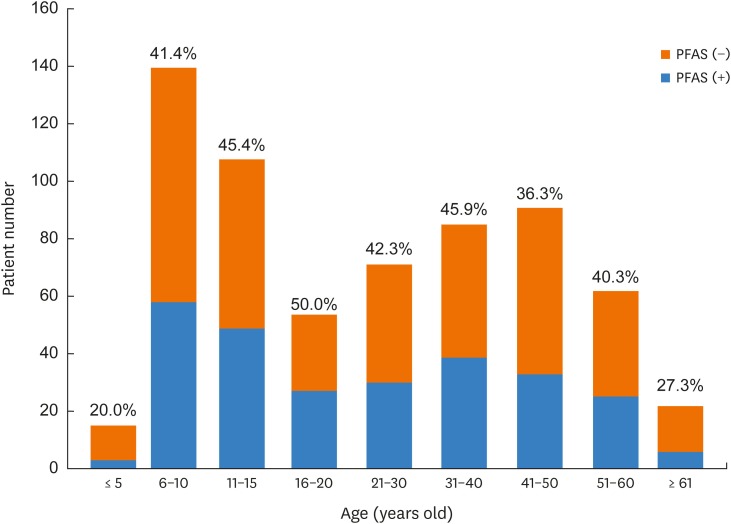
Of the 648 pollinosis patients, 270 (41.7%) had PFAS. The prevalence of PFAS did not differ significantly according to age (range, 36.3%–50%), except in those ≤5 (20%) and <60 (27.3%) years of age (Fig. 1). The prevalence of PFAS in subjects ≤18 and <18 years of age was 42.7% (128/300) and 40.8% (142/348), respectively. The prevalence of PFAS differed significantly according to current residence area (P < 0.001), and birthplace (P = 0.012), and was highest in Gyeonggi, Seoul and Jeolla Provinces (Supplementary Fig. S1). The prevalence of PFAS was not different according to birth month (P < 0.05).
Fig. 1. Prevalence of PFAS in various age groups. The prevalence of PFAS did not differ according to age, with the exception of patients less than 5 or more than 60 years of age.
PFAS, pollen-food allergy syndrome.
The clinical characteristics of the patients with PFAS are listed in Table 1. Pollinosis patients with PFAS showed higher proportions of female, allergic diseases (such as AR, AC, atopic dermatitis, drug allergy and food allergy), and family history of allergic diseases than those without PFAS. Moderate to severe AR was more prevalent in patients with than in those without PFAS (57.5% vs. 48.4%, P = 0.028), while there was no significant difference in the AR symptom duration according to PFAS (P < 0.05).
Table 1. Clinical characteristics of pollinosis patients according to PFAS.
| Variables | PFAS (n = 270) | No PFAS (n = 378) | P value | |
|---|---|---|---|---|
| Age | 26.1 ± 16.9 | 27.2 ± 18.4 | 0.672 | |
| Sex (male) | 146 (54.1) | 258 (68.3) | < 0.001 | |
| Allergic diseases | ||||
| BA | 117 (43.3) | 137 (36.2) | 0.082 | |
| AR | 259 (95.9) | 325 (86.0) | < 0.001 | |
| AC | 150 (55.6) | 146 (38.6) | < 0.001 | |
| Atopic dermatitis | 80 (29.6) | 67 (17.7) | < 0.001 | |
| Chronic urticaria | 18 (6.7) | 23 (6.1) | 0.892 | |
| Drug allergy | 28 (10.4) | 18 (4.8) | 0.010 | |
| Food allergy | 209 (76.7) | 53 (14.0) | < 0.001 | |
| Family history of allergic disease | 201 (74.4) | 245 (64.8) | 0.012 | |
| Severity of AR | 0.028 | |||
| Mild | 113/266 (42.5) | 192/372 (51.6) | ||
| Moderate/severe | 153/266 (57.5) | 180/372 (48.4) | ||
| Duration of AR | 0.698 | |||
| Intermittent | 118/266 (44.4) | 172/372 (46.2) | ||
| Persistent | 148/266 (55.6) | 200/372 (53.8) | ||
Data are means ± standard deviation or number (%).
PFAS, pollen-food allergy syndrome; BA, bronchial asthma; AR, allergic rhinitis; AC, allergic conjunctivitis.
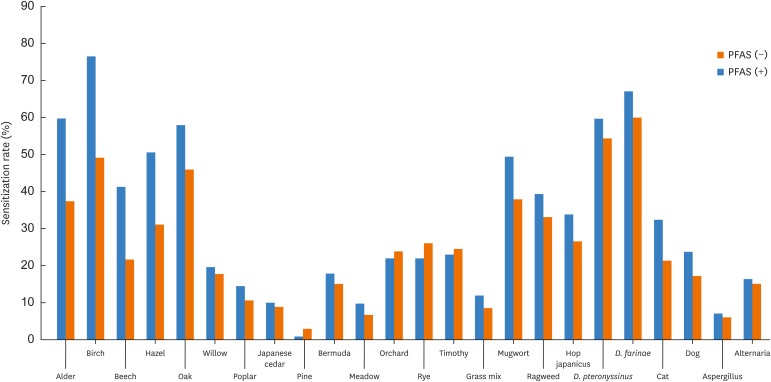
Patients with PFAS had a significantly higher rate of sensitization to alder, birch, beech, hazel, oak, mugwort and cat dander than those without (P<0.01, Fig. 2). The patients were classified according to the type of pollen to which they were sensitized: only tree pollens (T), only grass pollens (G), only weed pollens (W), tree + grass pollens (TG), tree + weed pollens (TW), grass + weed pollens (GW), or tree, grass, + weed pollens (TGW). Among the 648 pollinosis patients, 174 (26.9%) were classified as T, 36 (5.6%) as G, 52 (8.0%) as W, 51 (7.9%) as TG, 136 (21.0%) as TW, 28 (4.3%) as GW and 171 (26.4%) as TGW. The most frequent patterns were T, TGW and TW. The prevalence of PFAS was 39.7%, 25%, 21.2%, 33.3%, 55.1%, 28.6% and 47.4% in patients classified as T, G, W, TG, TW, GW and TGW, respectively. Approximately half of the patients classified as TW or TGW had PFAS.
Fig. 2. Sensitization rate to inhalant allergens according to PFAS. Patients with PFAS had a higher rate of sensitization to alder, birch, beech, hazel, oak, mugwort and cat dander than those without.
PFAS, pollen-food allergy syndrome.
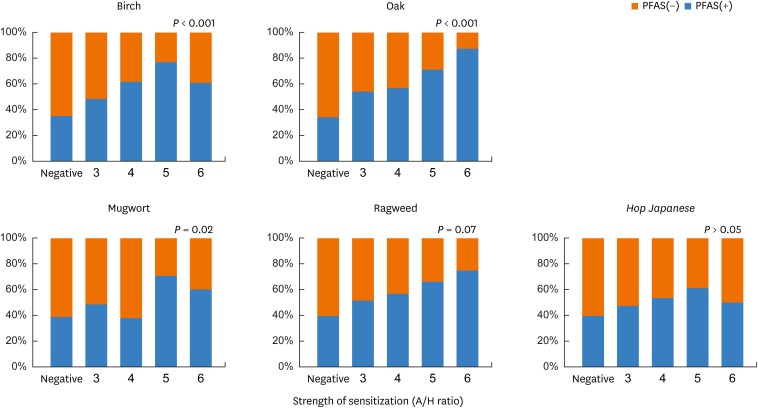
In a subanalysis according to the strength of sensitization to birch, oak, mugwort, ragweed and Hop Japanese, the prevalence of PFAS increased significantly with increasing strength of sensitization to birch, oak and mugwort (P < 0.05; Fig. 3).
Fig. 3. Prevalence of PFAS according to strength of sensitization in each pollen. The prevalence of PFAS increased with increasing strength of sensitization. X-axis, strength of sensitization (A/H ratio); Y-axis, prevalence of PFAS (%).
PFAS, pollen-food allergy syndrome.
Clinical manifestations of PFAS
All of the patients had oropharyngeal symptoms, and some had other clinical manifestations of PFAS (Table 2). Cutaneous manifestations such as pruritus, urticaria and angioedema were most frequent (43.0%), followed by respiratory (20.0%), gastrointestinal (10.7%), neurologic (4.8%) and cardiovascular (3.7%) symptoms. Interestingly, 8.9% of the PFAS patients experienced anaphylaxis.
Table 2. Clinical manifestations of PFAS.
| Clinical manifestations | No. (%) | |
|---|---|---|
| Cutaneous manifestations | 116 (43.0) | |
| Pruritus | 79 (68.1) | |
| Urticaria | 59 (50.9) | |
| Angioedema | 21 (18.1) | |
| Respiratory manifestations | 54 (20.0) | |
| Rhinorrhea | 12 (22.2) | |
| Cough | 23 (42.6) | |
| Dyspnea | 27 (50.0) | |
| Wheezing | 5 (9.3) | |
| Cyanosis | 2 (3.7) | |
| Gastrointestinal manifestations | 29 (10.7) | |
| Nausea/vomiting | 20 (69.0) | |
| Diarrhea | 8 (27.6) | |
| Abdominal pain | 9 (31.0) | |
| Cardiovascular manifestations | 10 (3.7) | |
| Chest pain | 2 (20.0) | |
| Hypotension | 6 (60.0) | |
| Pallor | 1 (10.0) | |
| Sweating | 5 (50.0) | |
| Neurologic manifestations | 13 (4.8) | |
| Dizziness | 5 (38.5) | |
| Loss of consciousness | 4 (30.8) | |
| Anxiety | 3 (23.1) | |
| Paresthesia | 4 (30.8) | |
| Anaphylaxis | 24 (8.9) | |
PFAS, pollen-food allergy syndrome.
Causative foods
The causative foods of PFAS are shown in Table 3. Peach (48.5%) was the food most frequently related to PFAS, followed by apple (46.7%), kiwi (30.4%), peanut (17.4%), plum (16.3%), chestnut (14.8%), walnut (14.1%), pineapple (13.7%), Korean melon (12.6%), tomato (11.9%), melon (11.5%) and apricot (10.7%). The following Korean foods were related to PFAS: taro/taro stems (8.9%), jujube (8.9%), ginseong (8.2%), Chinese yam (7.0%), perilla leaf (4.4%), bellflower root (4.4%), deodeok (Codonopsis lanceolate, 3.3%), crown daisy (3.0%), kudzu (3.0%) and lotus root (2.6%).
Table 3. Causative foods of PFAS.
| Foods | No. (%) |
|---|---|
| Peach | 131 (48.5) |
| Apple | 126 (46.7) |
| Kiwi | 82 (30.4) |
| Peanut | 47 (17.4) |
| Plum | 44 (16.3) |
| Chestnut | 40 (14.8) |
| Walnut | 38 (14.1) |
| Pineapple | 37 (13.7) |
| Korean melon | 34 (12.6) |
| Tomato | 32 (11.9) |
| Melon | 31 (11.5) |
| Apricot | 29 (10.7) |
| Watermelon | 26 (9.6) |
| Cherry | 24 (8.9) |
| Taro/taro stem | 24 (8.9) |
| Jujube | 24 (8.9) |
| Pear | 22 (8.2) |
| Ginseong | 22 (8.2) |
| Pine nut | 22 (8.2) |
| Soy | 20 (7.4) |
| Chinese yam | 19 (7.0) |
| Cucumber | 19 (7.0) |
| Carrot | 17 (6.3) |
| Banana | 15 (5.6) |
| Mango | 15 (5.6) |
| Grapes | 14 (5.2) |
| Perilla leaf | 12 (4.4) |
| Bellflower root | 12 (4.4) |
| Strawberry | 11 (4.1) |
| Potato | 10 (3.7) |
| Celery | 9 (3.3) |
| Deodeok (Codonopsis lanceolata) | 9 (3.3) |
| Crown daisy | 8 (3.0) |
| Kudzu | 8 (3.0) |
| Lotus root | 7 (2.6) |
| Eggplant | 7 (2.6) |
| Kale | 6 (2.2) |
| Orange | 5 (1.9) |
| Mandarin | 5 (1.9) |
| Zucchini | 5 (1.9) |
| Sweet potato | 4 (1.5) |
| Lettuce | 3 (1.1) |
| Chicory | 3 (1.1) |
| Persimmon | 3 (1.1) |
| Chili | 3 (1.1) |
| Wheat | 3 (1.1) |
| Almond | 3 (1.1) |
| Yacon | 3 (1.1) |
| Avocado | 2 (0.7) |
| Mung bean | 2 (0.7) |
| Perilla | 2 (0.7) |
| Galic | 2 (0.7) |
| Grape fruit | 2 (0.7) |
| Litchi | 1 (0.4) |
| Buckwheat | 1 (0.4) |
| Fig | 1 (0.4) |
| Ginger | 1 (0.4) |
| Mulberry | 1 (0.4) |
| Corn | 1 (0.4) |
| Burdock | 1 (0.4) |
| Cacao | 1 (0.4) |
| Cashew nut | 1 (0.4) |
| Parsley | 1 (0.4) |
| Pistachio | 1 (0.4) |
| Sunflower seed | 1 (0.4) |
PFAS, pollen-food allergy syndrome.
Ninety patients (13.9%) showed PFAS to only 1 type of fruit or vegetable, followed by 55 patients (8.5%) to 2, 37 patients (5.7%) to 3, 33 patients (5.1%) to 4, and 82 patients (12.7%) to 5 or more types of foods.
Associations of causative foods with pollen types
The causative foods according to pollen type are shown in Table 4 and Supplementary Table S1. In patients classified as T (n = 174), the causative foods were apple (60.9%), peach (46.4%), kiwi (36.2%), plum (21.7%), walnut (20.3%), peanut (18.8%), chestnut (17.4%), jujube (15.9%), taro/taro stem (14.5%), pear (13%), cherry (13%), Korean melon (13%), pine nut (13%), apricot (11.6%), melon (11.6%), pineapple (11.6%) and tomato (11.6%). Apple and peach were the foods most frequently associated with PFAS in patients classified as T, TG, TW or TGW. Kiwi was the food most frequently associated with PFAS in patients classified as W or GW.
Table 4. Causative foods of PFAS in pollen-sensitized patients.
| Tree only | Grass only | Weed only | |||
|---|---|---|---|---|---|
| Foods | No. (%) | Foods | No. (%) | Foods | No. (%) |
| Apple | 42 (60.9) | Pineapple | 3 (33.3) | Kiwi | 5 (45.5) |
| Peach | 32 (46.4) | Peach | 2 (22.2) | Apple | 3 (27.3) |
| Kiwi | 25 (36.2) | Kiwi | 1 (11.1) | Pineapple | 3 (27.3) |
| Plum | 15 (21.7) | Grapes | 1 (11.1) | Peach | 2 (18.2) |
| Walnut | 14 (20.3) | Taro/tarostem | 1 (11.1) | Watermelon | 2 (18.2) |
| Peanut | 13 (18.8) | Ginseong | 1 (11.1) | Grapes | 2 (18.2) |
| Chestnut | 12 (17.4) | Lotus root | 1 (11.1) | Tomato | 2 (18.2) |
| Jujube | 11 (15.9) | Chestnut | 1 (11.1) | Apricot | 1 (9.1) |
| Taro/tarostem | 10 (14.5) | Peanut | 1 (11.1) | Plum | 1 (9.1) |
| Pear | 9 (13.0) | Watnut | 1 (11.1) | Melon | 1 (9.1) |
| Cherry | 9 (13.0) | Pinenut | 1 (11.1) | Korean melon | 1 (9.1) |
| Korean melon | 9 (13.0) | Chilli | 1 (11.1) | Banana | 1 (9.1) |
| Pine nut | 9 (13.0) | Orange | 1 (9.1) | ||
| Apricot | 8 (11.6) | Mandalin | 1 (9.1) | ||
| Melon | 8 (11.6) | Mango | 1 (9.1) | ||
| Pineapple | 8 (11.6) | Potato | 1 (9.1) | ||
| Tomato | 8 (11.6) | Crown daisy | 1 (9.1) | ||
| Strawberry | 6 (8.7) | Pine nut | 1 (9.1) | ||
| Ginseong | 5 (7.3) | Cacao | 1 (9.1) | ||
| Soy | 5 (7.3) | ||||
PFAS, pollen-food allergy syndrome.
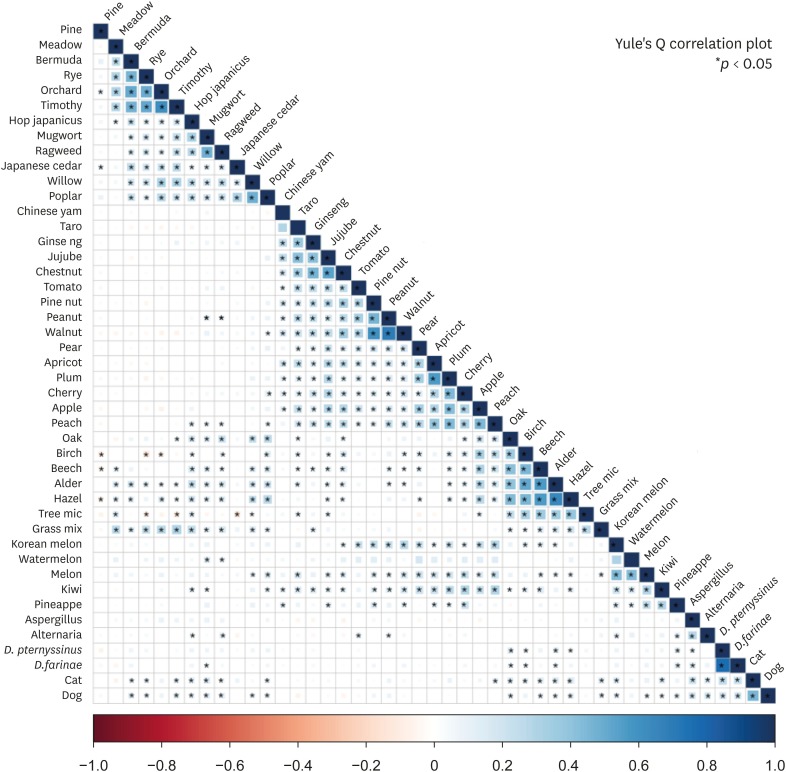
The correlations among inhalant allergens and causative foods are shown in Fig. 4. There were significant correlations among Chinese yam, taro, ginseong, jujube and chestnut; pine nut, peanut and walnut; pear, apricot, plum, cherry, apple and peach; and Korean melon, watermelon, melon, kiwi and pineapple (P < 0.05). Tree pollens were significantly associated with the Rosaceae family and nuts (P < 0.05) as well as chestnut, taro/tarostem, ginseong, jujube, Korean melon, melon and kiwi (P < 0.05). Weed pollens were associated with peanut, peach, watermelon and kiwi (P < 0.05). These data suggest that cross-allergenicity between pollens and foods is involved in the pathogenesis of PFAS in addition to cross-allergenicity among food types.
Fig. 4. Correlations among inhalant allergens and causative foods of PFAS. Significant correlations were found among Chinese yam, taro, ginseong, jujube and chest nut; pine nut, peanut and walnut; pear, apricot, plum, cherry, apple and peach; and Korean melon, watermelon, melon, kiwi and pineapple.
PFAS, pollen-food allergy syndrome.
DISCUSSION
This is the first nationwide study of the prevalence and clinical characteristics of PFAS in Korean pollinosis patients. Pollinosis was defined as 1) the presence of AR, AC or BA; and 2) a positive response to a pollen allergen by allergy skin test (AST) or serum specific IgE measurement. Previous studies enrolled subjects based on only one of these criteria. Also, we employed questionnaires comprising 44 common foods, and identified several unique and region-specific causative foods not previously implicated in PFAS. Moreover, we compared the clinical characteristics of pollinosis patients with and without PFAS to identify risk factors for PFAS. Finally, we assessed systemic symptoms of PFAS; of the PFAS patients, 8.9% had anaphylaxis.
Among the pollinosis patients, 41.7% had PFAS (42.7% in patients ≤18 years vs. 40.8% in those <18 years of age). This is comparable to the prevalence in Western countries,5,6,7 but higher than that in Japan.12,13 Interestingly, the prevalence of PFAS in pediatric pollinosis patients (≤18 years of age) was higher than previously reported in Western countries and Japan.9,10,11,12,13,14 Three of the PFAS patients were less than 6 years of age, which is unusual.19 However, 1 study in the United States reported that allergy specialists estimated the prevalence of PFAS to be 5% in children and 8% in adults, indicating that the prevalence of PFAS is underestimated in clinical practice.9
Pollinosis patients with PFAS had higher proportions of females, allergic diseases, and family history of allergic diseases. Patients with PFAS had more severe AR, but the duration of AR was not different. The prevalence of PFAS was significantly positively correlated with the strength of sensitization to birch, oak and mugwort pollen similar to a previous study of birch pollinosis.4 Therefore, the presence of allergic diseases, family history of allergic diseases, severity of AR and the strength of pollen sensitization are implicated in the development of PFAS in pollinosis patients.
The symptoms of PFAS are not limited to the oropharynx. Indeed, 8.7% of PFAS patients reportedly have systemic symptoms outside the gastrointestinal tract, 3% have systemic symptoms outside the oropharynx, and 1.7% have anaphylactic shock.9,20 In the present study, the patients exhibited cutaneous (43%), respiratory (20%), gastrointestinal (10.7%), neurologic (4.8%) and cardiovascular (3.7%) symptoms of PFAS. Of the PFAS patients, 8.9% had anaphylaxis. Our results suggest that physicians and patients should be aware of the clinical significance of PFAS and that patients may wish to consider carrying an epinephrine auto-injector.
The most frequent causative foods of PFAS were peach, apple and kiwi (Table 3). These were common causative foods in patients classified as T, while kiwi predominated in patients classified as W or GW (Table 4). Notably, PFAS to Korean melon was noted in 12.6%. The following local fruits and vegetables were also found to cause PFAS: taro/taro stem (8.9%), ginseong (8.2%), Chinese yam (7%), perilla leaf (4.4%), bellflower root (4.4%), deodeok (3.3%), crown daisy (3.0%), kudzu (3.3%), lotus root (2.6%) and lettuce (1.1%). In Korea, perilla leaf, crown daisy and lettuce are frequently ingested raw, wrapped around rice or pork. Such foods are known as “Ssam” and are popular in Korea. Therefore, these frequent causative foods of PFAS represent Korean eating habits. Moreover, various Korean foods are reportedly implicated in diverse clinical manifestations of PFAS; e.g., bronchospasm and urticaria caused by shiso leaf (Perilla frutescens),21 anaphylaxis by Chinese bellflower root,22 crown daisy-dependent exercise-induced anaphylaxis,23 and PFAS caused by celery, lettuce, chicory, radish sprouts, ginseng, mango, kiwi, tomato, crown daisy and perilla leaf.24,25,26,27
Cross-allergenicity between foods and pollen allergens is linked to the pathogenesis of PFAS.28 The major allergen of birch pollen, Bet v 1, exhibits cross-reactivity with many food allergens, particularly the Rosaceae family (e.g., apple, peach, pear, cherry, plum and apricot).29 Bet v 1 is a pathogenesis-related (PR) protein and panallergen present in many plant species. In addition to Bet v 1 homologs, cross-reactive panallergens including profilin, lipid transfer protein and cross-reactive carbohydrate determinants (CCDs) are implicated in PFAS.2,29 In this study, although we did not identify cross-reactive allergens between foods and pollens, we evaluated the associations between pollen types and culprit foodstuffs. Tree pollens were significantly associated with the Rosaceae family and nuts as well as chestnut, taro/tarostem, ginseong, jujube, Korean melon, melon and kiwi. Weed pollens were associated with peanut, peach, watermelon and kiwi. However, this association could be influenced by tree pollen as 26.4% of PFAS patients were classified as TGW. Further studies are needed to identify the cross-reactive allergens.
This study has several limitations. First, we defined PFAS according to clinical history based on questionnaires and the results of pollen allergy tests. Food-specific IgE levels were not determined, and provocation tests were not performed. However, oral provocation tests or specific IgE tests may not guarantee true PFAS due to the antigenic lability of commercial antigens. Instead, a careful clinical history can be sufficient to diagnose PFAS. Anhoej et al.30 found that a good clinical history of PFAS to apple had a negative and positive predictive value of 100% and 92%, respectively, compared to the results of food challenge tests. In this study, the diagnosis of PFAS was reliable because allergy specialists took a clinical history using questionnaires and identified positive results of allergy tests by chart review. Secondly, there is a possibility of selection bias because all but two of the institutes were university hospitals; therefore, patients with severe pollinosis may have been included in the study. In addition, recall bias could be accompanied with completion of the questionnaires. Because the questionnaires were based on the self-reporting data, the allergic reactions and symptoms might be exaggerated. However, our results are similar to those of previous reports.
In conclusion, the overall prevalence of PFAS in Korea was 41.7%, and the foods most frequently associated with PFAS were peach, apple and kiwi. Moreover, Korean foods (e.g., taro/taro stem, ginseong, perilla leaf, bellflower root, crown daisy, deodeok, kudzu root and lotus root) were also associated with PFAS, as would be expected in a study performed in Korea. A substantial proportion of the PFAS patients exhibited systemic symptoms including anaphylaxis. Physicians should be aware of the risk of PFAS in pollinosis patients and advise them to avoid the most frequently implicated foods.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Academy of Asthma, Allergy, and Clinical Immunology (2016).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest There are no financial or other issues that might lead to conflict of interests.
SUPPLEMENTARY MATERIALS
Causative foods of PFAS according to type of pollen
Total number of study subjects and prevalence of PFAS according to present residence area (A) and birthplace (B).
References
- 1.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–108. doi: 10.1016/j.anai.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Kondo Y, Urisu A. Oral allergy syndrome. Allergol Int. 2009;58:485–491. doi: 10.2332/allergolint.09-RAI-0136. [DOI] [PubMed] [Google Scholar]
- 3.Tuft L, Blumstein GI. Studies in food allergy: II. Sensitization to fresh fruits: clinical and experimental observations. J Allergy. 1942;13:574–582. [Google Scholar]
- 4.Eriksson NE, Formgren H, Svenonius E. Food hypersensitivity in patients with pollen allergy. Allergy. 1982;37:437–443. doi: 10.1111/j.1398-9995.1982.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 5.Amlot PL, Kemeny DM, Zachary C, Parkes P, Lessof MH. Oral allergy syndrome (OAS): symptoms of IgE-mediated hypersensitivity to foods. Clin Allergy. 1987;17:33–42. doi: 10.1111/j.1365-2222.1987.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 6.Bircher AJ, Van Melle G, Haller E, Curty B, Frei PC. IgE to food allergens is highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994;24:367–374. doi: 10.1111/j.1365-2222.1994.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 7.Osterballe M, Hansen TK, Mortz CG, Bindslev-Jensen C. The clinical relevance of sensitization to pollen-related fruits and vegetables in unselected pollen-sensitized adults. Allergy. 2005;60:218–225. doi: 10.1111/j.1398-9995.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 8.Cuesta-Herranz J, Lázaro M, Figueredo E, Igea JM, Umpiérrez A, De-Las-Heras M. Allergy to plant-derived fresh foods in a birch- and ragweed-free area. Clin Exp Allergy. 2000;30:1411–1416. doi: 10.1046/j.1365-2222.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003;112:784–788. doi: 10.1016/s0091-6749(03)02008-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014;50:795–800. doi: 10.1111/jpc.12658. [DOI] [PubMed] [Google Scholar]
- 11.Bedolla-Barajas M, Kestler-Gramajo A, Alcalá-Padilla G, Morales-Romero J. Prevalence of oral allergy syndrome in children with allergic diseases. Allergol Immunopathol (Madr) 2017;45:127–133. doi: 10.1016/j.aller.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Arai Y, Ogawa C, Ohtomo M, Sano Y, Ito K. Food and food additives hypersensitivity in adult asthmatics. II. Oral allergy syndrome in adult asthmatic with or without Japanese cedar hay fever. Arerugi. 1998;47:715–719. [PubMed] [Google Scholar]
- 13.Namba H, Sahashi N, Yamamoto M, Yoshida T, Yanagawa K, Hiroki N, et al. Investigation of patients suffering from Japanese cedar pollinosis conducted through a questionnaire. Jpn J Palynol. 2004;50:73–82. [Google Scholar]
- 14.Maeda N, Inomata N, Morita A, Kirino M, Ikezawa Z. Correlation of oral allergy syndrome due to plant-derived foods with pollen sensitization in Japan. Ann Allergy Asthma Immunol. 2010;104:205–210. doi: 10.1016/j.anai.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Cho YS, Lim YJ, Lee JC, Kim SH, Lim MK, Yoo B, et al. Oral allergy syndrome in pollen-sensitized patients. J Asthma Allergy Clin Immunol. 1998;18:458–465. [Google Scholar]
- 16.Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 17.Korean Academy of Asthma, Allergy, and Clinical Immunology. Asthma and allergic diseases. 1st ed. Seoul: Ryo Moon Gak; 2012. [Google Scholar]
- 18.Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Kivity S, Dunner K, Marian Y. The pattern of food hypersensitivity in patients with onset after 10 years of age. Clin Exp Allergy. 1994;24:19–22. doi: 10.1111/j.1365-2222.1994.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 20.Ortolani C, Pastorello EA, Farioli L, Ispano M, Pravettoni V, Berti C, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy. 1993;71:470–476. [PubMed] [Google Scholar]
- 21.Shin YS, Choi GS, Park HJ, Ye YM, Park HS. A case of bronchospasm and urticaria caused by Shiso ingestion. Ann Allergy Asthma Immunol. 2009;102:169. doi: 10.1016/S1081-1206(10)60250-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Lee SM, Park HW, Cho SH, Min KU, Kim YY, et al. Chinese bellflower root anaphylaxis: IgE-binding components and cross-reactivity with mugwort and birch. Korean J Intern Med. 2009;24:279–282. doi: 10.3904/kjim.2009.24.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon TY, Choi KH, Lee KM, Ahn JY, Kim MK. Crown daisy-dependent exercise-induced anaphylaxis in a patient with mugwort-sensitized pollinosis. Korean J Asthma Allergy Clin Immunol. 2011;31:63–66. [Google Scholar]
- 24.Lee SC, Son YW, Sim DW, Park KH, Lee JH, Park JW. Oral allergy syndrome associated with weed pollinosis. Allergy Asthma Respir Dis. 2016;4:458–461. [Google Scholar]
- 25.Hong GN, Kim M, Yoon MK, Lee SH, Park HS. Oral allergy syndrome caused by crown daisy and sesame leaf. Allergy Asthma Respir Dis. 2014;2:306–309. [Google Scholar]
- 26.Kim JH, Yang YS, Cho SI, Park CW, Lee CH, Kim HO. Oral allergy syndrome to watermelon and melon. Korean J Dermatol. 2013;51:730–733. [Google Scholar]
- 27.Lee SH, Ban GY, Jeong K, Shin YS, Park HS, Lee S, et al. A retrospective study of Korean adults with food allergy: differences in phenotypes and causes. Allergy Asthma Immunol Res. 2017;9:534–539. doi: 10.4168/aair.2017.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breiteneder H, Ebner C. Molecular and biochemical classification of plant-derived food allergens. J Allergy Clin Immunol. 2000;106:27–36. doi: 10.1067/mai.2000.106929. [DOI] [PubMed] [Google Scholar]
- 29.Wensing M, Akkerdaas JH, van Leeuwen WA, Stapel SO, Bruijnzeel-Koomen CA, Aalberse RC, et al. IgE to Bet v 1 and profilin: cross-reactivity patterns and clinical relevance. J Allergy Clin Immunol. 2002;110:435–442. doi: 10.1067/mai.2002.126380. [DOI] [PubMed] [Google Scholar]
- 30.Anhoej C, Backer V, Nolte H. Diagnostic evaluation of grass- and birch-allergic patients with oral allergy syndrome. Allergy. 2001;56:548–552. doi: 10.1034/j.1398-9995.2001.056006548.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Causative foods of PFAS according to type of pollen
Total number of study subjects and prevalence of PFAS according to present residence area (A) and birthplace (B).