Abstract
The prevalence of allergic disorders has dramatically increased over the past decade, particularly in developed countries. Apart from gastrointestinal disorders, neoplasia, genital and dermatological diseases etc., dysregulation of gut microbiota (dysbiosis) has also been found to be associated with increased risk of allergies. Probiotics are increasingly being employed to correct dysbiosis and, in turn, to modulate allergic diseases. However, several factors like strain variations and effector metabolites or component of them in a bacterial species can affect the efficacy of those as probiotics. On the other hand, host variations like geographical locations, food habits etc. could also affect the expected results from probiotic usage. Thus, there is a glaring deficiency in our approach to establish probiotics as an irrefutable treatment avenue for suitable disorders. In this review, we explicate on the reported probiotics and their effects on certain allergic diseases like atopic dermatitis, food allergy and asthma to establish their utility. We propose possible measures like elucidation of effector molecules and functional mechanisms of probiotics towards establishing probiotics for therapeutic use. Certain probiotics studies have led to very alarming outcomes which could have been precluded, had effective guidelines been in place. Thus, we also propose ways to secure the safety of probiotics. Overall, our efforts tend to propose necessary discovery and quality assurance guidelines for developing probiotics as potential immunomodulatory ‘Pharmabiotics.’
Keywords: Asthma, atopic dermatitis, food hypersensitivity, pharmabiotics, probiotics
INTRODUCTION
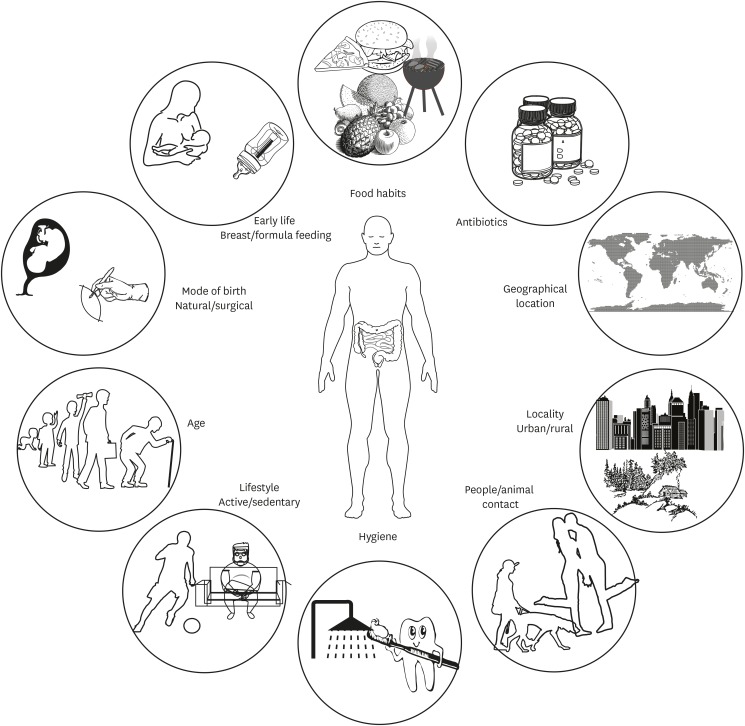
The human body harbors trillions of microbes colonized mainly in the skin and mucosal surface. The symbiotic co-evolution of commensal microbes with the host is evident from the knowledge that many of these microbial species have given up genes necessary for their survival in other microenvironments, while retaining genes which are beneficial for the host with no/little benefit to self,1 and show diversity between individuals and demographics.2 This diversity is influenced by several factors including mode of birth,3 nutritional patterns, hygiene and life-style (Fig. 1). For example, urbanization and a Westernized diet have led to the loss of ancient commensal microbes. Alterations in microbial diversity are associated with inflammatory conditions including allergies.4 Administration of probiotics has been reported to amend this dysbiotic drift by restoring the gap between the current and ancient symbiont communities.
Fig. 1. Factors affecting the diversity of intestinal microbiota. Intestinal microbial composition can vary with several factors including mode of delivery, age, dietary patterns, life style, use of antibiotics and probiotics. Microbial diversity can affect immune system maturation and has been linked with allergy incidences.4.
The Food and Agriculture Organization (FAO)/World Health Organization (WHO) defines probiotics as “live microorganisms which confer a health benefit on the host when administered in adequate amounts.”5 Researchers and regulatory authorities, however, caution against the misuse of the term ‘probiotics.’ They advise that until the beneficial properties of microorganisms are fully characterized, the term ‘probiotic’ should be used with caution. The International Scientific Association for Probiotics and Prebiotics also recently stated that the term probiotics does not apply to dead microorganisms, microbial by-products, metabolites or non-viable microbial products.5 This concept, however, should be revised as effector molecules can be derived from cellular components such as lipopolysaccharides and polysaccharides.6,7 There is increasing evidence to suggest that every probiotic microbial strain does not have a unique mechanism of action despite shared taxonomic lines.8 Advances in bioinformatics-based research and mechanistic understanding of probiotics' mode of action will perhaps allow some generalization to be made at the taxonomic level with respect to the immunological mechanism of probiotics.8
Studies in the past decade have led to a deeper understanding of the interplay between microbiome and the innate and adaptive immune system to guide immune tolerance and to alleviate allergic disease. We here attempt to review the current evidence and advancements in research for use of probiotics in allergic disorders. Also, as ‘Efficacy and Toxicity’ are interdependent characteristics of therapy, we highlight the need for improved safety regulations.
ROLE OF MICROBIOTA IN THE DEVELOPMENT OF IMMUNE TOLERANCE TO ALLERGENS
Primarily, the infant immune system presents type 2 helper T cell (Th2)-dominant phenotypes and gradually increases type 1 helper T cell (Th1) immune response to maintain immune homeostasis. Th2-skewed phenotype leads to higher immunoglobulin E (IgE) levels and mast cell activation, which in turn increases susceptibility to allergic disorders. The microflora plays a significant role in restoring Th1/Th2 immune responses.9 The Th2-dominant phenotype of an infant is reproduced in germ-free (GF) mice, which exhibits higher susceptibility to allergic reactions. Interestingly, colonization with commensal microbes can reverse this phenotype, demonstrating the key role played by microbiota. Commensals also play a role in regulating immune cell recruitment. Neonatal exposure to microbiota prevents accumulation of invariant natural killer cells in the colonic lamina propria and the lungs in a CXCL6 chemokine-dependent manner, thereby ameliorating airway allergic responses.10 Furthermore, loss of tolerance was reported in adults after extensive antibiotic treatment.9
Another perspective of these observations is reflected in ‘hygiene hypothesis.’ It suggests that reduced microbial exposure during infancy due to increased public hygiene is one of the primary causes of heightened susceptibility to allergies.9 This hypothesis is elegantly supported by a recent study in which Amish children raised in conventional farms had lower prevalence of allergic disease than Hutterite children raised in industrialized farms, despite similarities of genetic background.2 Also, house dust extracts from Amish houses inhibited ovalbumin (OVA)-induced allergic inflammation in mice in a MyD88 and TRIF-dependent mechanism.2 Of note, the bacterial composition of Amish house dust was dominated by Bartonellaceae, which had higher endotoxin levels than Hutterite house dust. Long-term exposure to low-dose endotoxin or ‘farm dust’ demonstrated protective effects in house dust mite (HDM)-induced asthma in mice.11 Protection was mediated via activation of A20 (TNFAIP3) in airway epithelial cells modifying the epithelial and dendritic cells (DCs) interactions.11 Similarly, these studies establish the role of microbiota in influencing allergic immune reactions. In the subsequent part of the review, we elaborate on probiotics-based intervention of allergic diseases and propose changes required for the advancement of the probiotics field.
PROBIOTICS IN ALLERGY MANAGEMENT
Inflammation is a fundamental defense response of body's immune system against foreign antigens; however, hypersensitivity is a host protective immune response on repeated exposure to specific antigens, but potentially deleterious to the host. Based on physiological mechanisms involved and time of response, Gell and Coombs have classified hypersensitivity into 4 types, types I to IV. However, the clinical manifestations of allergic reactions demonstrate overlap or simultaneous occurrence of humoral and cell-mediated immune responses making the differential diagnosis of 4 types difficult. Johansson et al.12 proposed a more clinically applicable classification based on involvement of IgE, excluding non-allergic non-immunological reactions. A general pathogenesis of an allergic response includes: sensitization to allergens (HDM, pollen, food, etc.), transient inflammatory response, increase in allergen specific IgE, and migration of effector T cells, mast cells and eosinophils to the site of exposure. Hyper-responsiveness is orchestrated by Th2 cells, with cytokine profiles dominated by interleukin (IL)-4, IL-5, IL-9, IL-13, and IL-31. Eosinophils play a major role in respiratory allergies by degranulation at the site of exposure causing influx of immune cells and tissue damage. Skin and food allergies can also be referred to as the second wave of allergic epidemics.
Probiotics for allergic skin disorders
Atopic dermatitis (AD) is the most common familial, chronic, recurrent and non-infectious inflammatory skin disease with a wide clinical spectrum. It is often associated with other disorders like food allergies, asthma, allergic rhinitis (AR), cardiovascular diseases and obesity. AD can be classified into extrinsic AD with high serum IgE or intrinsic/non-atopic AD with normal serum IgE levels.13 Classically, AD was considered a biphasic disease presenting acute and chronic stages, predominated by Th2 and Th1 responses, respectively. However, recent studies indicate AD is more heterogeneous — the acute phase consisting of both Th2 (IL-4, IL5, IL-13, IL-31, and CCL-18) and Th22 (IL-22 and S-100A proteins) responses, while chronic stage consisting of accentuated acute phase pathways along with Th1 signatures (interferon [IFN]-γ, CXCL-9 and CXCL-10).13 Some findings also suggest involvement of a Th17 axis, particularly in Asian patients, although cytokine patterns and clinical symptoms like parakeratosis indicate the complication of AD with psoriasis.13 Regulatory T cells (Tregs), an immunoregulatory subset of T cells, have been reported to be increased in the peripheral blood of AD patients14 as well as in skin lesions; however, contrasting findings have reported functional defects in Tregs of AD patients.15
Staphylococcal aureus is the most common pathobiont found in AD lesions and shows a close association with AD disease severity, food allergy and enhanced IgE responses.16 Furthermore, dysbiosis and the reduced microbial diversity of skin and intestine are also suggested to be associated with exacerbation of AD. Nylund et al.17 observed higher abundance of Clostridium clusters IV and XIVa and lower abundance of Bacteroidetes in 18-month-old children with eczema. It is interesting to note that microbial composition was similar between healthy and eczema patients at 6 months of age.17 In another study, Abrahamsson et al.18 discovered lower Bacteroidetes diversity at 1 month of age in a Swedish cohort of infants who subsequently developed atopic eczema at 2 years of age. Similarly, lower Bacteroidetes diversity was observed in pregnant mothers of infants who eventually developed atopic eczema, although this was not observed in the infants themselves after birth.19 However, they observed depletion of Ruminococcaceae in 1 week-old infants that developed IgE-associated eczema, suggesting a correlation between abundance of specific bacterial taxa and cytokine response to TLR ligands.19 Probiotics seem to restore the balance between Th1/Th2 immune responses and microbial composition in allergic patients. Although these studies provide compelling evidence linking microbial dysbiosis with allergic diseases, some other reports failed to reproduce these findings.
Pre-clinical models of AD have provided much immunological insights into AD pathogenesis. However, it is worth noting that existing mouse models of AD differ significantly from human AD. Comparison of human AD skin transcriptomes with mouse AD models revealed that none of them fully captured human AD.20 Interestingly, IL-23-injected mice, a conventional animal model for psoriasis, best represented the human AD profile, yet the similarity was only 37% in terms of gene expression.20
To identify specific immune-regulatory probiotic strains, we have reported an ex vivo screening system.21,22 In brief, individual probiotic strains were cultured with mesenteric lymph node cells isolated from Foxp3-reporter mice or wild type mice. End point analysis for cytokines or expression of Foxp3-transcription factors was done by enzyme-linked immunosorbent assay or flow cytometry, respectively. Based on the level of anti-inflammatory (IL-10) and pro-inflammatory (IL-12p70) cytokines, we selected the strains that induced IL-10high/IL-12low expressions. Out of several hundreds of candidate strains, we selected Bifidobacterium bifidum, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteri, and Streptococcus thermophiles; mixture of these was named IRT5.22 Administration of IRT5 suppressed the development and progression of experimental autoimmune uveitis,23 multiple sclerosis,24 myasthenia gravis,25 rheumatoid arthritis,22 age-dependent colitis26 and allergic contact hyper-sensitivity (unpublished data). IRT5 was also potent in suppressing a mouse model of experimental AD through potentiation of CD4+Foxp3+ Tregs (Table 1). 22 In general, IRT5 worked through phenotypic alteration in conventional DCs to a regulatory DCs (rDCs), which then induced de novo generation of induced Foxp3+-regulatory T cells (iTreg). Recently, we discovered another B. bifidum strain that is the most potent inducer of iTregs among all the tested probiotic strains. Monocolonization of the B. bifidum in GF mice induced Tregs in high frequencies mainly in the colon where the bacteria colonize. Furthermore, we defined cell surface polysaccharides as the key effector molecules involved in de novo generation of Tregs in a DC-dependent manner (unpublished data). Similar results were reported by Lim et al.27 using a non-conservative bacterial species of Weissella cibaria WIKIM28 in DNCB induced AD mice. Administration of probiotics reduces mast cell infiltration of lesions, suppresses Th2 responses, and increases Treg populations in peripheral lymph nodes.27 Lactobacillus plantarum CJLP133 reduced skin inflammation in HDM-induced dermatitis in Nc/NgA mice by modulating the Th1/Th2 balance and by activating Tregs.28 These findings show administration of probiotics could modulate allergic responses by targeting various immune cells (Fig. 2).
Table 1. Effect of probiotics in preclinical studies of AD in mice.
| References | Probiotics strain/s | Animal model used | Observations | Clinical/Histopa-thological scores |
|---|---|---|---|---|
| Lim et al.27 | Weissella cibaria WIKIM28 | DNCB induced AD in BALB/c mice | Reduced Th2 cytokines, generation of CD4+ Foxp3+ T cells and increased IL-10 levels in mLN | Improved |
| Choi et al.59 | Heat killed Lactobacillus brevis NS1401 | House dust mice induced AD in Nc/NgA mice | Reduced serum IgE, eosinophil & mast cell infiltration, allergen specific IgG1 and Th1/Th2 cytokines | Improved |
| Shin et al.60 | Lactobacillus acidophilus CBT LA1, Lactobacillus rhamnosus CBT LR5, Lactobacillus plantarum CBT LP3, Bifidobacterium bifidum CBT BF3, Bifidobacterium breve CBT BR3, Lactococcus lactis CBT SL6, Streptococcus thermophilus CBT ST3 | DNCB induced AD in Nc/NgA mice | Generation of CD4+ Foxp3+ T cells in mLN, low serum IgE, IL-4 and IL-5, high Th1 IFN-γ, IL12p40 | Improved |
| Kim et al.61 | Lactobacillus casei, L. plantarum, L. rhamnosus and Bifidobacterium lactis | DNCB induced AD in Nc/NgA mice | Low serum IgE, IL-4 and IL-5, high Th1 cytokines IFN-γ, IL12p40 | Improved |
| Weise et al.62 | Escherichia coli Nissle1917 | Allergen induced AD in BALB/c mice | Increased IFN-γ, IL-10, TGF-β and proportion of CD4+ Foxp3+ T cells | Improved |
| Won et al.28 | L. plantarum CJLP133 | House dust mice induced AD in Nc/NgA mice | Reduced cell infiltration and Th2 cytokines | Improved |
| Activation of Tregs | ||||
| Kwon et al.22 | IRT5 probiotic mixture: L. acidophilus, L. casei, Lactobacillus reuteri, B. bifidum, S. thermophilus | HDM/DNCB induced AD in BALB/c mice | Generation of CD4+ Foxp3+ Tregs | Prophylactic & therapeutic effect |
| Migration of Tregs to the inflamed region | Suppression of ongoing AD progression | |||
| Reduction of serum (total and allergen specific) IgE level, cellular infiltration and Th2 cytokines |
AD, atopic dermatitis; DNCB, dinitrochlorobenzene; Th2, type 2 helper T cell; IL, interleukin; mLN, mesenteric lymph node; IgE, immunoglobulin E; Th1, type 1 helper T cell; IFN, interferon.
Fig. 2. Overview of mechanisms of action of probiotics in allergic diseases. Primary mode of action of probiotics includes restoration of Th1/Th2 cytokine balance13 and induction of CD4+Foxp3+ Treg38 cells. Other specific mechanisms include reduction in allergen specific IgE38,52 and increased SCFA56,57 levels. Probiotics also aids in constant homeostasis by maintaining intestinal epithelial integrity, increased anti-microbial production and competitively inhibiting survival of pathogens.
Th2, type 2 helper T cell; Th1, type 1 helper T cell; Treg, regulatory T cell; IgE, immunoglobulin E; SCFA, short-chain fatty acid.
Probiotics treatment in AD patients, however, shows inconsistent results in clinical trials (Table 2). Administration of L. plantarum CJLP133 improves Scoring Atopic Dermatitis (SCORAD) index in pediatric patients of AD by decreasing levels of IFN-γ, IL-4 and eosinophil counts.29 Another randomized, double-blind, placebo-controlled trial (RCT) on pediatric AD investigated the effect of Lactobacillus paracasei and Lactobacillus fermentum and observed improvement in SCORAD scores up to 4 months after discontinuation of probiotics.30 Bifidobacterium lactis CECT 8145, Bifidobacterium longum CECT 7347 and L. casei CECT 9104 were able to reduce SCORAD index in a 12-week RCT on young Spanish patients with AD.31 A meta-analysis of 25 RCTs suggested that administration of probiotics improved AD from 1–18 years of age but results for less than 1 year of age were not confirmatory.32 They also observed that a mixture of Lactobacillus was better than Bifidobacteria alone.32 On the other hand, recent review of 13 RCTs on children with AD suggested no robust differences between probiotic and placebo treated groups.33 The beneficial effects of probiotic administration in allergic disorders are still questionable due to the small cohort size and heterogeneity among studies. Interestingly, differential efficacy may also depend on geographical or racial-genetic effects as Asian children (1–18 years) were responsive, while Europeans children presented no effect.33 Moreover, strain-specific effects have been observed. For example, Lactobacillus sakei and L. fermentum were effective, while L. plantarum and Lactobacillus rhamnosus GG had no effect against allergy.33
Table 2. Effects of probiotics in clinical trials on patients with AD.
| References | Probiotics strain/s | Clinical patients | Observations | Clinical outcome (SCORAD index, clinical symptoms) |
|---|---|---|---|---|
| Navarro-López et al.31 | Bifidobacterium lactis CECT 8145, Bifidobacterium longum CECT 7347, and Lactobacillus casei CECT 9104 | Pediatric | Reduced use of topical corticosteroids | Improved |
| Wang et al.30 | Lactobacillus paracasei, Lactobacillus fermentum and mixture | Pediatric | Reduced IL-4, marginally increased TGF-β, IFN-γI | Improved |
| Niccoli et al.63 | Lactobacillus salivarius LS01 | Pediatric | - | Improved |
| Yang et al.64 | Mix: L. casei, Lactobacillus rhamnosus, Lactobacillus plantarum, and B. lactis | Pediatric | No change in cytokine profile | No change from placebo |
| Inoue et al.65 | Heat killed Lactobacillus acidophilus strain L-92 | Pediatric | Reduced eosinophil count, increased TGF-β | Improved |
| Wickens et al.35 | L. rhamnosus HN001 | Pediatric | Reduced serum total IgE, | Improved |
| Yeşilova et al.66 | Mix: Bifidobacterium bifidum, L. acidophilus, L. casei, and L. salivarius | Pediatric | Reduced IL-5, IL-6, IFN-γ and total serum IgE | Improved |
| Iemoli et al.67 | Mix L. salivarius LS01 and Bifidobacterium breve BR03 | Adults | Reduced Th1/Th2 cytokines and Th17/Treg ratio | Improved |
| Han et al.29 | L. plantarum CJLP133 | Pediatric | Reduced IL-4, IFN-γ and total eosinophil count | Improved |
| Gore et al.68 | L. paracasei CNCM I-2116 or B. lactis CNCM I-3446 | Pediatric | - | No change from placebo |
| Drago et al.69 | L. salivarius LS01 | Adults | Reduced Th1 cytokines (IL-12, IFN-γ), Th1/Th2 cytokines ratio and serum IgE | Improved |
| Woo et al.70 | Lactobacillus sakei KCTC 10755BP | Pediatric | Lower serum levels of CCL17 and CCL27 | Improved |
AD, atopic dermatitis; SCORAD, Scoring Atopic Dermatitis; IL, interleukin; TGF, transforming growth factor; IFN, interferon; IgE, immunoglobulin E; Th2, type 2 helper T cell; Th1, type 1 helper T cell; Treg, regulatory T cell.
Use of probiotics for the prevention of eczema has also been explored. An RCT showed maternal supplementation with L. rhamnosus HN001 (HN001) starting from 35 weeks of gestation until 6 months of breast-feeding and infant supplementation from birth to 2 years of age significantly reduced the prevalence of eczema up to 6 years of age.34 On the other hand, Bifidobacterium animalis subsp lactis HN019 (HN019) had no significant effect on any outcome.35 A recent meta-analysis of 6 trials, comprising 1,955 patients, suggested use of probiotics early in life is likely to prevent AD later in life.36 However, it is difficult to generalize the efficacy of probiotics in AD modulation due to considerable heterogeneity between studies.
Probiotics for allergic asthma
Although asthma is conventionally considered a Th2-type inflammatory condition, it has been recognized as a clinically heterogeneous disease and etiology for many of the asthma phenotypes are yet to be understood. Several studies have described the microbiota composition of the respiratory or gastrointestinal tracts associated with asthma occurrence. However, it is still not clear how dysbiosis affects susceptibility to asthma. Neonatal susceptibility to allergic airway inflammation is reversed in adult mice post development of an airway microbiome. Gollwitzer et al.37 observed a shift in lung microbiome from Gammaproteobacteria and Firmicutes to Bacteroides in the first 2 weeks of life. These changes were associated with PD-L1 dependent emergence of CD4+Helios−Foxp3+ Treg cells in lungs.37 Immune modulating effects of commensals are not limited to local tissue alone; intestinal commensals also affect respiratory immune responses. For example, short-chain fatty acids (SCFAs) that are produced by commensals during fermentation of dietary fibers suppress allergic airway responses. Higher serum SCFA, particularly propionate, suppress induction of Th2 responses in the lungs by modulating DC progenitors in bone marrow in a G-protein coupled receptor 41-dependent manner.38 Among the SCFAs, butyrate is the most potent immunoregulatory metabolite. Butyrate producing mixture of Clostridia species types IV, XIVa and XVIII induce colonic Treg cells in mice.39 Clostridium butyricum suppressed inflammatory responses by inducing IL-10-producing macrophages.7 Butyrate and propionate have HDAC inhibitory activity, enhance histone acetylation state in the Foxp3 locus,39,40 and induce tolerogenic DCs to enhance Treg generation.40 Bacteria belonging to the clostridial family Lachnospiraceae and Ruminococcaceae are also known to ferment dietary fibers in the colon to produce SCFAs, thereby supporting epithelial integrity and homeostasis. However, whether these findings could be recapitulated in humans should be tested in clinical trials.
Therapeutic effects of probiotics in human asthmatic patients are not well established. An 8-week RCT on children with asthma and AR treated with Lactobacilllus gasseri A5 showed a significant reduction in symptoms along with improvement of pulmonary function.41 Oral administration of L. rhamnosus GG (LGG) alleviated asthma symptoms in an OVA-sensitized model of mouse asthma.42 In other studies, however, probiotic treatment early in life had no effect in preventing asthma.43 Collectively, the current evidence does not support use of probiotics in prevention or treatment of asthma.
Probiotics for food allergy and food-induced anaphylaxis
Adverse reactions to foods manifest in 2 forms, food allergy and food intolerance. Food allergy consists of immune responses against certain foods, while food intolerance is a non-immunological reaction. The best characterized food allergies are IgE-mediated immediate hypersensitivity reactions, the others being non-IgE-mediated delayed type reactions. Most cutaneous allergic reactions to food are IgE-mediated.44 Cross-linking of allergen-specific IgE antibodies to their receptor FcεRI, expressed on mast cells and basophils, results in the release of cellular mediators and inflammation. Food allergy can also be explained as failure of oral tolerance, which is usually established in infancy. Oral tolerance refers to immunological hypo-responsiveness towards dietary antigens and endogenous gut microflora. Intestinal DCs sample intestinal antigens promoting development of CD4+Foxp3+ Tregs. On the other hand, epithelial cells produce the inflammatory trio, thymic stromal lymphopoietin, IL-33 and IL-25 upon insult, activating mast cells, innate lymphoid cells (ILCs) and DCs to induce Th2 type immune response.45 IL-33 also up-regulates OX40L on DCs driving Th2 immunity.45 Oral exposure to food antigens early in life is believed to induce tolerogenic responses to dietary antigens leading to decreased occurrence of food allergy. A ‘dual exposure hypothesis’ for food allergy, proposed by Lack, suggests that development of oral tolerance occur by oral exposure to antigen, while sensitization occurs upon cutaneous exposure to low-dose antigen.46 Eczema and mutations in filaggrin (a protein necessary for cutaneous integrity) are associated with increased risk of AD as well as food allergy.
Intestinal microbiome can also influence development of oral tolerance and sensitization to food antigens. Abundance of Clostridia and Firmicutes were linked to cow's milk allergy in infants. Administration of LGG-supplemented casein formulas in infants with cow's milk allergy led to enrichment of butyrate-producing taxa accelerating tolerance acquisition. A 3-year RCT on a similar cohort revealed LGG supplementation reduced the risk of other allergic manifestations.47 In murine models of food allergy, colonization of gnotobiotic mice with Clostridia suppressed sensitization to food allergen. Mono-association of Clostridia-increased CD4+Foxp3+ Treg cell numbers and IgA production while promoting IL-22 production by RORγt+ ILCs and T cells in the lamina propria, thus restoring epithelial integrity. Certain Clostridia species were also found to suppress food allergic reactions therapeutically.48 In a different strategy, transfer of intestinal microbiota from food allergy-prone mice to wild type GF mice induced food allergy phenotype.49 Similarly, food allergy-prone IL-4 receptor alpha chain mutant mice (IL-4RαF709) showed a distinct microbial signature with differential abundance of Lachnospiraceae, Lactobacillaceae, Rikenallaceae and Porphyromonadaceae.49 Tang et al.50 evaluated the effect of probiotics as an adjuvant to oral immunotherapy (OIT) in children with peanut allergy. Co-administration of L. rhamnosus CGMCC1.3724 and peanuts led to sustained desensitization and reduced peanut-specific IgE levels.50 However, evidence for therapeutic and preventive effects of probiotics on food allergy in human subjects is still sparse. Since induction of antigen-specific oral tolerance requires a tolerogenic microenvironment, high IL-10-inducing probiotic strains might be employed for OIT to treat allergy and autoimmunity in the future.21,22
PROPHYLACTIC AND THERAPEUTIC EFFICACY OF PROBIOTICS TO TREAT ALLERGIC DISORDERS
As described above, although health benefits of taking probiotics have been reported in allergic disorders, it is still early to draw any conclusions. Prominent regulatory organizations like the American Academy of Pediatrics, National Institute of Allergy and Infectious Diseases, European Academy of Allergy and Clinical Immunology, European Society for Pediatric Gastroenterology, Hepatology and Nutrition and FAO of the United Nations/WHO, do not support the use of probiotics for primary prevention of allergic diseases.51 In 2015, the World Allergy Organization (WAO) applied Grading of Recommendations, Assessment, Development and Evaluation approach to develop evidence-based recommendations for using probiotics in the prevention of allergic diseases.52 Findings were reported in a systemic review of 29 RCTs by Cuello-Garcia et al.43 The panel observed that there is insufficient evidence to support recommendation to use probiotics in primary prevention of allergic diseases. However, they suggested the use of probiotics in pregnant/lactating women and infants with a family history of allergic disease. Although strain-specific activity of different bacterial species is recognized, no recommendations were made by the WAO regarding strain or dose of probiotics in light of insufficient evidence.52
SAFETY OF PROBIOTICS FOR HUMAN PATIENTS
Probiotics are generally considered safe for consumption. However, increasing evidence raises concern over generalizing the safety of probiotics. In 2002, a joint report released by WHO and FAO of the United Nations indicated that probiotics may be responsible for 4 types of side effects: systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals and gene transfer.53 The ‘Probiotics in Pancreatitis Trial (PROPATRIA),’ a prophylactic clinical trial for acute pancreatitis in human patients, created a furor as large number of deaths were reported in the probiotic-treated group compared to control, 16% versus 6%.54 It was also reported that treatment of patients with a multi-strain probiotic led to bowel ischemia development.54 An enquiry into the trial identified inadequate knowledge on the safety of probiotics used. This investigation grabbed the attention of the scientific community towards looking into adverse effects of probiotics. Lactobacillus and Bifidobacterium are generally considered safe, and therefore dominate the commercial formulas of probiotics. A recent investigation, however, identified Bifidobacterium adoloscentis as a potent inducer of IL-17, which plays pathogenic roles in inflammatory bowel disease (IBD) and rheumatoid arthritis.55 Although Bifidobacterium species have long been associated with Treg induction,22 including Bifidobacterium adolescentis,55 the current findings indicate that a harmless probiotic strain can be counterproductive in disease conditions. Tan et al.55 further tested 6 probiotic formulas containing Bifidobacterium species, 4 of which similarly induced Th17 cells in mice, although their effects in an autoimmune set-up were not confirmed. These observations warrant an investigation into the clinical safety of probiotics before commercialization. Some groups further argue that probiotics with medical claims must receive Food and Drug Administration (FDA) approval like any other drugs, including filing of Investigational New Drug Application (IND) and clinical trials, phases I–III. Other groups support the necessity of regulations, but disagree on receiving FDA approval as this will incur huge expenses and cause major delays in the overall research.5 We believe the safety of probiotics must not be overlooked; the IND application process can be modified for probiotics products to expedite research without adding much to the cost.
CHALLENGES AND FUTURE PERSPECTIVE IN PROBIOTIC RESEARCH
Advances in molecular and microbiological techniques have enabled us to perform in-depth research into microbial diversity found in the human commensal pool. To-date, however, most of the probiotic research has focused on the bacteria itself rather than the host-bacteria interaction. Moreover, available data omits the importance of mycobiome and virome. This has arisen from difficulty in processing fungal DNA, underdeveloped fungal genome databases, unavailability of viral 16S rRNA sequencing and inefficient bioinformatics tools. In our observations, commensal yeast, recognized mostly as an opportunistic pathogen, can act as a potent immunoregulator in inflammation and autoimmune diseases (unpublished data). The interaction and harmony between microbiome, mycobiome and virome can be important in maintaining homeostasis and offers a window for further investigation.
As more academic and clinical research groups focus on commensals, the number of probiotic species with health claims is increasing. However, there is no international consensus for the screening of these microbial species. Current screening methods focus on the cytokine induction ability of microbes using cell lines or ex vivo isolated peripheral immune cells, even though they do not represent the phenotype of intestinal cells. There is a need to devise high-throughput screening methods to ensure the specificity and efficacy of selected probiotics. Most commercially available probiotics are mixtures of bacterial strains with defined colony forming units (CFUs); therefore, it is also important that consumers be informed of the product shelf life of individual strains to ensure viability of bacteria in the administered dosage. A recent report also suggested the manufacturing process of probiotic mixtures can affect their activity. VSL#3, a probiotic mixture made in the USA, was found to be beneficial in HIV-infected subjects and also IBD by reducing the 1,3 dihydroxyacetone (DHA) levels in feces.56 On the contrary, a similar mix made in Italy enhanced DHA levels leading to adverse effects.56 These findings suggest the same bacteria can have different functions, depending on their culture conditions. Thus, using functional markers rather than CFU counts might be a better readout of probiotic quality control. Moreover, as the composition of commensals varies among populations, efficacy of probiotics may also vary among populations. Hence, clinical trials should be expanded to include different geographical locations. With this in mind, it is advisable not to perform meta-analyses on pooled data when different strains of bacteria were used, since the effects can vary dramatically among strains.55
The majority of disease-related health claims for probiotics have been rejected due to lack of supportive data. Then, how can we establish probiotics as a modulator of specific diseases? Analysis of disease-associated markers could be a simple approach. For example, Roessler et al.57 explored different effects of selected probiotics in healthy and AD patients, and found probiotic treatment increased CD57+ NK cells in healthy subjects, but not in AD patients. Kim et al.58 reported that characterization of clinical phenotype of patients and selection of patients based on the immunological parameters (i.e., high IgE level) could enhance the possibility of probiotic efficacy. However, these approaches do not cover many important issues such as the molecular and cellular identity of the probiotic strain, full genome sequences and mechanisms of action in homeostatic/disease conditions. Therefore, application of probiotic strains in patients should not be allowed unless the safety and effector molecules of the probiotics are well defined. Moreover, the term “Probiotic” should not be generically used as some exacerbate disease under different conditions.
CONCLUSION
The current understanding of probiotics suggests their beneficial effects to prevent AD. However, evidence is still lacking to support the general efficacy of probiotics in the modulation of other allergic conditions. Valid conclusions cannot be drawn based on pooling of data from different probiotic strains or combinatory administrations. Thus, there is a need to develop international standards for study designs on probiotics to ensure uniformity across clinical trials. Further, there is a glaring deficiency in quality standards of probiotics which shall be addressed by defining genetic identity of strains and uniform production guidelines. Moreover, future studies are warranted to refine the effector molecules of probiotics and to identify their mode of action in healthy and diseased conditions. We also want to emphasize the need of functional markers for screening probiotics, which could serve as a standard for quality control toward each batch of manufactured products. Annotating genomic information involved in coding the functional molecules is also necessary as bacteria could lose their genetic information during large-scale cultures. Once the safety and efficacy of the effector molecule(s) are assured, application of probiotic bacteria for treating allergic disorders might be possible. In addition, once they show beneficial effects in clinical trials, we could call them “Probiotics.” Furthermore, if we can purify the effector molecules from the probiotic bacteria in large amounts, we may use this to treat allergy symptoms in the future (Fig. 3). Although, current definition excludes these products from the category ‘Probiotic,’5 we propose that microbial derived molecules with health benefits could be included in a separate category within probiotics, termed “Pharmabiotics.”
Fig. 3. Strategy for the development of probiotics as prophylactic and therapeutic microbial agent. More stress should be laid on strain specificity and identification of effector molecules derived from probiotics in the assessment of preclinical efficacy of probiotics. Preclinical safety assessment should include studies in healthy and immunocompromised animals. Clinical community should develop standardized protocols to avoid heterogeneity in studies and allow pooling of data and generalization of results obtained. We recommend qualitative and quantitative assessments for the post-production aspects of probiotics before marketing, to ensure uniformity in marketed products. Future research should focus on these aspects to improvise the current methods to find a balance between safety and efficacy while maintaining quality of product delivered.
ACKNOWLEDGMENTS
Authors appreciate Amit Sharma (POSTECH, Korea) for his contribution to figures and Chan Johng Kim (POSTECH, Korea) for the editing. We appreciate their intellectual contribution to the manuscript. This work was supported by the Institute for Basic Science, Korea (IBS-R005 for Sin-Hyeog Im).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2011;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 2.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee E, Kim BJ, Kang MJ, Choi KY, Cho HJ, Kim Y, et al. Dynamics of gut microbiota according to the delivery mode in healthy Korean infants. Allergy Asthma Immunol Res. 2016;8:471–477. doi: 10.4168/aair.2016.8.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 5.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. 2018;49:207–216. doi: 10.1016/j.copbio.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. 2015;77:220–228. doi: 10.1038/pr.2014.160. [DOI] [PubMed] [Google Scholar]
- 10.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 12.Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 13.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roesner LM, Floess S, Witte T, Olek S, Huehn J, Werfel T. Foxp3(+) regulatory T cells are expanded in severe atopic dermatitis patients. Allergy. 2015;70:1656–1660. doi: 10.1111/all.12712. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YY, Wang AX, Xu L, Shen N, Zhu J, Tu CX. Characteristics of peripheral blood CD4+CD25+ regulatory T cells and related cytokines in severe atopic dermatitis. Eur J Dermatol. 2016;26:240–246. doi: 10.1684/ejd.2015.2709. [DOI] [PubMed] [Google Scholar]
- 16.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016;137:1272–1274.e3. doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 17.Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, et al. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013;13:12. doi: 10.1186/1471-2180-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129:434–440. 440.e1–440.e2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 19.West CE, Rydén P, Lundin D, Engstrand L, Tulic MK, Prescott SL. Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin Exp Allergy. 2015;45:1419–1429. doi: 10.1111/cea.12566. [DOI] [PubMed] [Google Scholar]
- 20.Ewald DA, Noda S, Oliva M, Litman T, Nakajima S, Li X, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol. 2017;139:562–571. doi: 10.1016/j.jaci.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Kim JE, Chae CS, Kim GC, Hwang W, Hwang JS, Hwang SM, et al. Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J Funct Foods. 2015;13:350–362. [Google Scholar]
- 22.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Choi SH, Kim YJ, Jeong HJ, Ryu JS, Lee HJ, et al. Clinical effect of IRT-5 probiotics on immune modulation of autoimmunity or alloimmunity in the eye. Nutrients. 2017;9:E1166. doi: 10.3390/nu9111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, et al. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol. 2013;146:217–227. doi: 10.1016/j.clim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Chae CS, Kwon HK, Hwang JS, Kim JE, Im SH. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PLoS One. 2012;7:e52119. doi: 10.1371/journal.pone.0052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong JJ, Woo JY, Ahn YT, Shim JH, Huh CS, Im SH, et al. The probiotic mixture IRT5 ameliorates age-dependent colitis in rats. Int Immunopharmacol. 2015;26:416–422. doi: 10.1016/j.intimp.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Lim SK, Kwon MS, Lee J, Oh YJ, Jang JY, Lee JH, et al. Weissella cibaria WIKIM28 ameliorates atopic dermatitis-like skin lesions by inducing tolerogenic dendritic cells and regulatory T cells in BALB/c mice. Sci Rep. 2017;7:40040. doi: 10.1038/srep40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won TJ, Kim B, Lee Y, Bang JS, Oh ES, Yoo JS, et al. Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice. Cell Immunol. 2012;277:49–57. doi: 10.1016/j.cellimm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2012;23:667–673. doi: 10.1111/pai.12010. [DOI] [PubMed] [Google Scholar]
- 30.Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin Exp Allergy. 2015;45:779–787. doi: 10.1111/cea.12489. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, Ruzafa-Costas B, Genovés-Martínez S, Chenoll-Cuadros E, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37–43. doi: 10.1001/jamadermatol.2017.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217–226. doi: 10.1016/j.anai.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392. doi: 10.3389/fcimb.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. 2012;42:1071–1079. doi: 10.1111/j.1365-2222.2012.03975.x. [DOI] [PubMed] [Google Scholar]
- 35.Wickens K, Stanley TV, Mitchell EA, Barthow C, Fitzharris P, Purdie G, et al. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy. 2013;43:1048–1057. doi: 10.1111/cea.12154. [DOI] [PubMed] [Google Scholar]
- 36.Cao L, Wang L, Yang L, Tao S, Xia R, Fan W. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: a meta-analysis. J Dermatolog Treat. 2015;26:537–540. doi: 10.3109/09546634.2015.1027168. [DOI] [PubMed] [Google Scholar]
- 37.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 38.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 39.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 40.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YS, Lin YL, Jan RL, Chen HH, Wang JY. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. 2010;45:1111–1120. doi: 10.1002/ppul.21296. [DOI] [PubMed] [Google Scholar]
- 42.Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J Microbiol Immunol Infect. 2016;49:625–635. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Castellazzi AM, Valsecchi C, Caimmi S, Licari A, Marseglia A, Leoni MC, et al. Probiotics and food allergy. Ital J Pediatr. 2013;39:47. doi: 10.1186/1824-7288-39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129:1187–1197. doi: 10.1016/j.jaci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 47.Berni Canani R, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139:1906–1913.e4. doi: 10.1016/j.jaci.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 48.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 49.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135:737–744.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 51.West CE, Dzidic M, Prescott SL, Jenmalm MC. Bugging allergy; role of pre-, pro- and synbiotics in allergy prevention. Allergol Int. 2017;66:529–538. doi: 10.1016/j.alit.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8:4. doi: 10.1186/s40413-015-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015;60(Suppl 2):S129–S134. doi: 10.1093/cid/civ085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 55.Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113:E8141–E8150. doi: 10.1073/pnas.1617460113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinchieri V, Laghi L, Vitali B, Parolin C, Giusti I, Capobianco D, et al. Efficacy and safety of a multistrain probiotic formulation depends from manufacturing. Front Immunol. 2017;8:1474. doi: 10.3389/fimmu.2017.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, et al. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008;38:93–102. doi: 10.1111/j.1365-2222.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 58.Kim J, Lee BS, Kim B, Na I, Lee J, Lee JY, et al. Identification of atopic dermatitis phenotypes with good responses to probiotics (Lactobacillus plantarum CJLP133) in children. Benef Microbes. 2017;8:755–761. doi: 10.3920/BM2017.0034. [DOI] [PubMed] [Google Scholar]
- 59.Choi CY, Kim YH, Oh S, Lee HJ, Kim JH, Park SH, et al. Anti-inflammatory potential of a heat-killed Lactobacillus strain isolated from Kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. J Appl Microbiol. 2017;123:535–543. doi: 10.1111/jam.13515. [DOI] [PubMed] [Google Scholar]
- 60.Shin JH, Chung MJ, Seo JG. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4+Foxp3+ T cells. Food Nutr Res. 2016;60:32550. doi: 10.3402/fnr.v60.32550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MS, Kim JE, Yoon YS, Seo JG, Chung MJ, Yum DY. A probiotic preparation alleviates atopic dermatitis-like skin lesions in murine models. Toxicol Res. 2016;32:149–158. doi: 10.5487/TR.2016.32.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weise C, Zhu Y, Ernst D, Kühl AA, Worm M. Oral administration of Escherichia coli Nissle 1917 prevents allergen-induced dermatitis in mice. Exp Dermatol. 2011;20:805–809. doi: 10.1111/j.1600-0625.2011.01326.x. [DOI] [PubMed] [Google Scholar]
- 63.Niccoli AA, Artesi AL, Candio F, Ceccarelli S, Cozzali R, Ferraro L, et al. Preliminary results on clinical effects of probiotic Lactobacillus salivarius LS01 in children affected by atopic dermatitis. J Clin Gastroenterol. 2014;48(Suppl 1):S34–S36. doi: 10.1097/MCG.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 64.Yang HJ, Min TK, Lee HW, Pyun BY. Efficacy of probiotic therapy on atopic dermatitis in children: a randomized, double-blind, placebo-controlled trial. Allergy Asthma Immunol Res. 2014;6:208–215. doi: 10.4168/aair.2014.6.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue Y, Kambara T, Murata N, Komori-Yamaguchi J, Matsukura S, Takahashi Y, et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: a double-blind, randomized, clinical trial. Int Arch Allergy Immunol. 2014;165:247–254. doi: 10.1159/000369806. [DOI] [PubMed] [Google Scholar]
- 66.Yeşilova Y, Çalka Ö, Akdeniz N, Berktaş M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol. 2012;24:189–193. doi: 10.5021/ad.2012.24.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. 2012;46(Suppl):S33–S40. doi: 10.1097/MCG.0b013e31826a8468. [DOI] [PubMed] [Google Scholar]
- 68.Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2012;42:112–122. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 69.Drago L, Iemoli E, Rodighiero V, Nicola L, De Vecchi E, Piconi S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int J Immunopathol Pharmacol. 2011;24:1037–1048. doi: 10.1177/039463201102400421. [DOI] [PubMed] [Google Scholar]
- 70.Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol. 2010;104:343–348. doi: 10.1016/j.anai.2010.01.020. [DOI] [PubMed] [Google Scholar]