Abstract
Purpose
Several markers for eosinophilic inflammation have been proposed to predict response to asthma treatment. However, definitive criteria for treatment decisions have not yet been established. We investigate a potentially useful relatively non-invasive biomarker, eosinophil-derived neurotoxin (EDN), to predict favorable responses to budesonide or montelukast, common treatment for children with asthma.
Methods
Young children (1 to 6 years old) were enrolled in this randomized, parallel, 2-group, open-label trial. Criteria for eligibility included: 1) being symptomatic during the run-in period; and 2) having a serum EDN (sEDN) level ≥ 53 ng/mL, with positive specific immunoglobulin E to house dust mite. Eligible patients were randomly placed into 2 groups: the BIS group received budesonide inhalation suspension (BIS) 0.5 mg once daily; the MONT group received montelukast 4 mg once daily. Ineligible patients were invited to receive montelukast 4 mg once daily (OBS group). Treatment period was 12 weeks.
Results
Asthma control days increased significantly in the BIS and MONT groups (P < 0.000) over the 12-week study period. There was no significant change in sEDN in the BIS group but there was a significant decrease in the MONT group (P < 0.000). Patients in the OBS group with high EDN levels (< 53 ng/mL) showed a significant decrease due to MONT treatment (P = 0.023). Rescue medication usage significantly decreased in the BIS and MONT groups (P < 0.000).
Conclusions
EDN is a useful relatively non-invasive biomarker for predicting responses to montelukast and budesonide treatment of preschool children with beta2-agonist responsive recurrent wheeze and multiple-trigger wheeze (Trial registry at UMIN Clinical Trials Registry, UMIN000008335).
Keywords: Biomarkers, children, eosinophil-derived neurotoxin, asthma
INTRODUCTION
Chronic airway inflammation is a hallmark of asthma1; consequently, inhaled corticosteroids (ICSs) are the main treatment.2 Although leukotriene receptor antagonists (LTRAs) also have anti-inflammatory properties3 and are often administered, especially to young children, guidelines clearly state ICSs are the preferred option for long-term control of asthma.2 This preference is based mainly on evidence from clinical trials comparing mean responses between treatment and control groups. However, other trials have demonstrated that there is considerable inter-individual variability in treatment response to ICSs versus LTRAs.4,5,6 It is important for a clinician to collect information that better guides them in selecting medication most likely to achieve a favorable response in each patient (i.e., tailor-made treatment regimens).
Several markers for eosinophilic inflammation or atopy, such as elevated levels of serum immunoglobulin E (IgE) due to house dust mite (HDM), Dermatophagoides pteronyssinus (Dp), sensitivity,7 sputum eosinophil counts,8 exhaled nitric oxide9 and serum eosinophil cationic protein (ECP),10 have been suggested to predict response to ICSs. However, definitive criteria for treatment decisions are still to be established. In addition, characteristics of airway pathology in preschool children may be different from those in older children and adults,11 which may lead to differential treatment responses.
In addition to ECP, elevated levels of another eosinophil degranulation product, eosinophil-derived neurotoxin (EDN), have been noted in asthmatics, with higher levels also found during asthma exacerbation when compared to healthy patients and those with stable asthma.12 Therefore, EDN has been suggested as a key marker for disease activity of asthma and other eosinophil-related disease.13
To date, there are no published studies comparing differential responses to the 2 types of anti-inflammatory therapies, namely ICSs and LTRAs, for preschool children with asthma. Furthermore, clinical markers for these possible differential responses have not been defined. Here, we propose a study to seek a useful and relatively non-invasive marker (i.e., serum EDN [sEDN]) to predict favorable responses to nebulized budesonide or montelukast, common treatment options for young children with asthma.
MATERIALS AND METHODS
Subjects
Children aged 1 to 6 years were enrolled in this study. Because asthma is a heterogeneous disease and especially difficult to diagnose in this age group, the definition of asthma used was based on the Global Initiative for Asthma (GINA)2 and Japanese pediatric guidelines.14 Major inclusion criteria were as follows: 1) 3 or more recurrent wheezing episodes within a year, with at least one confirmed by a physician, and multiple-trigger wheeze (not just common cold but other triggers such as exercise, crying or laughing); and 2) improvement of wheezing after administration of inhaled or oral short-acting beta2-agonists. Minor inclusion criteria include at least one of the following: 1) parental asthma, 2) physician-diagnosed atopic dermatitis (AD), 3) physician-diagnosed allergic rhinitis (AR) or 4) physician-diagnosed food allergy. To be included in the study, a patient must have had both major criteria and at least one of the minor inclusion criteria. Criteria for randomization included patient was: 1) symptomatic during the run-in period; and 2) had a sEDN level ≥ 53 ng/mL,13 with positive specific IgE to HDM (Immulite 2000 3gAllergy < 0.1 IUA/mL).14 Exclusion criteria included one or more of the following: 1) patients who received asthma controller medication, such as ICSs or LTRAs, in the previous 6 months; 2) chronic lung disease, heart or kidney disease or any other congenital disease; and 3) a patient whom the physician regarded as inappropriate for the study (e.g., did not attend the clinic when instructed).
Study Design
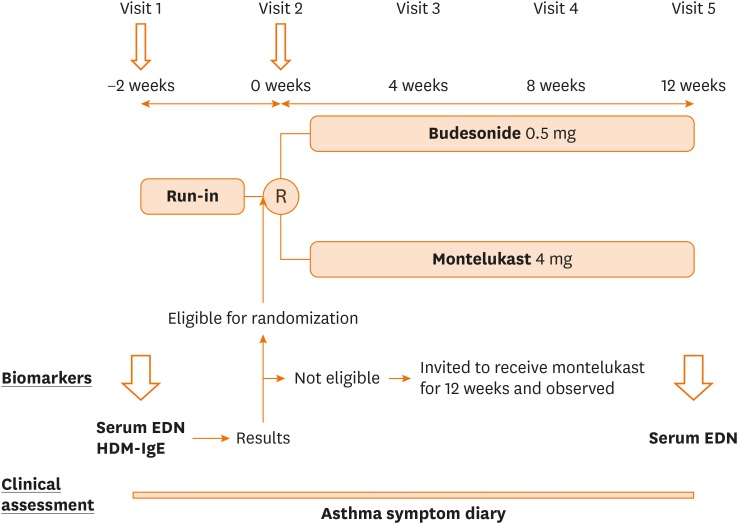
This was a randomized, parallel, 3-group, open-label trial. Patients who met the enrollment criteria entered a 2-week run-in period, with short-acting beta2-agonists as needed. Participants were then randomly selected to join 1 of the 2 groups: the BIS group received budesonide inhalation suspension (BIS) 0.5 mg once daily for 12 weeks and the MONT group received montelukast 4 mg once daily (orally) for 12 weeks. BIS was administered via a SkyNeb (Medel, Milan, Italy) compressor and nebulizer with a mouthpiece (for older children) or face mask (for children 1-3 years old). Patients that were ineligible for the trial because randomization criteria 2) was not met, EDN < 53 ng/mL and/or negative specific IgE to HDM, yet had symptoms in the run-in period were invited to receive montelukast 4 mg once daily based on recommendation by the Japanese guideline.14,15 They were observed for 12 weeks as exploratory purpose (OBS group). Parents/caregivers of all participants were instructed to record all symptoms and treatment times in an asthma diary. Patients were evaluated at 4-week intervals (Fig. 1) in either a hospital or clinic to have their asthma diary checked and to determine whether a step-up in therapy was needed as per GINA and Japanese guidelines.
Fig. 1. Study protocol.
At visit 1, patients who met inclusion criteria were tested for sEDN and HDM-IgE, then entered to run-in period. After 2 weeks of run-in period, the results of sEDN and HDM-IgE were reported to the study physician and the patients who met randomization criteria were randomized to receive budesonide or Montelukast for 12 weeks. Those who did not meet randomization criteria, yet had symptoms during run-in period were invited to receive montelukast. Clinical assessment was made at each visit and with asthma symptom diary recorded by the caregivers. At visit 5, sEDN was measured.
sEDN, serum eosinophil-derived neurotoxin; HDM-IgE, house dust mite-immunoglobulin E; R, randomization.
Computer randomization of an eligible patient into either the BIS or MONT group was performed. However, adjustments were made so that there was a statistically similar proportion of patients in each group based on age (1-2 years old versus 3-5 years old), sex and specific IgE to HDM (< 10 IUA/mL versus ≥ 10 IUA/mL).
Caregivers provided written informed consent on behalf of patients. This clinical trial was registered at UMIN Clinical Trials Registry: http://www.umin.ac.jp/ctr/index.htm (UMIN000008335).
EDN measurement
EDN levels in each patient were measured twice: at −2 weeks (during the run-in period) and at the 12-week time point. EDN was measured using an enzyme-linked immunosorbent assay (ELISA) following the manufacturer's protocol (MBL, Woburn, MA, USA), and the results were expressed in nanograms per milliliter (ng/mL). This ELISA detects human EDN with a minimum detection limit of < 0.62 ng/mL, a maximum detection limit < 300 ng/mL, and no cross-reaction with ECP. All assays were run in duplicate, and the mean value was used for statistical analyses.
Virus detection
At initial enrollment and when exhibiting asthma or upper respiratory infection symptoms at regular visits, nasopharyngeal swabs were collected and total RNA from common respiratory viruses was extracted and identified using a multiplex PCR system (Seeplex RV12 Ace Detection; Seegene, Seoul, Korea) that identifies adenovirus (AdV) A-E, coronavirus (CoV) 229E/NL63/OC43, parainfluenza virus (PIV) 1-4, rhinovirus (RV) A-C, influenza virus (IFV) A and B, respiratory syncytial virus (RSV) A and B, bocavirus (BV) 1-4, metapneumovirus (MPV), and enterovirus.
Assessment of outcome
Primary outcome was the number of asthma control days (ACD). An ACD was defined as a day with no beta-agonist rescue use; use of oral corticosteroids for asthma; daytime symptoms; nighttime awakenings; unscheduled health care visits, emergency department visits, or hospitalizations for asthma; or school absenteeism for asthma. ACD in both groups (BIS and MONT) were compared in 2 ways: 1) within group analyses comparing ACD at the beginning of the treatment period (0 week) to ACD at the other 3-time points (4, 8 and 12 weeks); and 2) between group analyses comparing ACD in each group at each time point (e.g., BIS ACD at 0 week versus MONT ACD at 0 week). An ACD value was calculated for each group during each time period. For example, ACD at 0 week was calculated during the 2-week run-in period (i.e., −2 to 0 week), ACD at 4 weeks was calculated during the time period from 0 to 4 weeks, etc. The first comparison used 2-way analysis of variance (ANOVA), followed by Sidak's multiple comparisons test. The second comparison used the Wilcoxon matched-pairs signed rank test.
A secondary outcome of this study was to compare changes in sEDN in all patients with sEDN ≥ 53 ng/mL and HDM sensitivity (BIS and MONT groups) and in those patients whose sEDN was < 53 ng/mL, were not sensitive to HDM or both (OBS group). Serum was taken from each patient at −2 weeks, sEDN was measured, and then this value was used at 0 week (termed “Pre”) to randomize each patient into either the BIS or the MONT group. Also, sEDN was measured at 12 weeks (termed “Post”). “Pre” and “Post” sEDN levels were then compared in each patient using the Wilcoxon matched-pairs signed rank test.
Another secondary outcome of this study was changes in rescue medication usage with treatment. Comparisons were done in 3 ways: 1) within each group (BIS and MONT), usage at run-in (−2 weeks) was compared to usage at the end of the treatment period (12 weeks); 2) each subject in each group was analyzed over the study period (−2 to 12 weeks) to determine any change in usage; 3) usage in BIS group was compared to usage in MONT group at each time point (−2, 0, 4, 8 and 12 weeks). For each endpoint, stratified analysis was performed based on the virus type detected.
Statistical analysis
Within group analyses of ACD at multiple time points during the entire study period (−2 to 12 weeks) were compared using 2-way ANOVA, followed by Sidak's multiple comparisons test. This was only done for the BIS and MONT groups. Between-group analyses of ACD (BIS versus MONT groups) were done using the Wilcoxon matched-pairs signed rank test. Within group analyses of treatment effect comparing “Pre” (measured during run-in period −2 to 0 week) and “Post” (measured at 12 weeks) measurements of changes in sEDN for all 3 groups (BIS, MONT and OBS) were done using the Wilcoxon matched-pairs signed rank test. Each group was then subgrouped according to presence or absence of any virus and change in sEDN with treatment from “Pre” to “Post” was analyzed using the same statistical test. Rescue medication usage within each group (BIS and MONT) and between each group were also compared. Data that were normally distributed were presented as mean values, unless otherwise noted. P values < 0.05 were considered statistically significant.
RESULTS
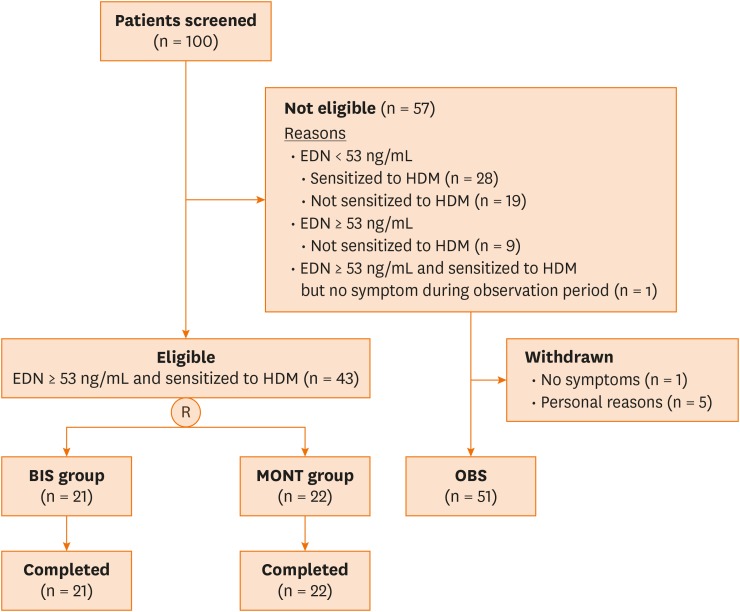
Flow chart for screening and randomization into study groups (BIS and MONT) is in Fig. 2.
Fig. 2. Patient flow.
R, randomization; BIS, budesonide inhalation suspension; MONT, eligible and receiving montelukast; OBS, not eligible and receiving montelukast; EDN, eosinophil-derived neurotoxin; HDM, house dust mite.
Demographics
ANOVA followed by the Bonn Ferroni multiple comparison test were used to determine any statistical differences in demographic data among the 3 groups (i.e., BIS, MONT, and OBS; Table 1). Age (P < 0.05) and the proportion of those with AD (P < 0.05) in the OBS group were significantly lower compared to the other groups. The age difference between the OBS groups and the other 2 groups was expected as subjects in the BIS and MONT groups were age-matched. All other demographic variables were statistically similar among the 3 groups.
Table 1. Demographics.
| Characteristics | BIS group (n = 21) | MONT group (n = 22) | OBS group (n = 51) | Total (n = 94) |
|---|---|---|---|---|
| Male/female (% male) | 6/15 (28.6) | 9/13 (40.9) | 18/33 (35.29) | 33/61 (35.1) |
| Age (mon) | 39.33 (20–63) | 40.77 (16–69) | 31.35* (9–69) | 35.34 (9–69) |
| Food allergy | 8 (38.1) | 7 (31.8) | 13 (25.5) | 28 (29.8) |
| Atopic dermatitis | 9 (42.9) | 7 (31.8) | 8 (15.7)† | 24 (25.5) |
| Body weight (grams) | 3,128 (2,505–3,827) | 3,041 (2,386–4,172) | 3,028 (1,658–3,690) | 3,053 (1,658–4,172) |
| Sibling, mean (number with at least 1 sibling, %) | 1.11 (15, 75.0) | 1.38 (18, 90) | 1.16 (36, 80.0) | 1.20 (81.9) |
| Nursery school | 16 (76.19) | 3 (13.64) | 8 (15.68) | 17 (18.09) |
| Pet | 6 (28.57) | 3 (13.64) | 8 (15.68) | 17 (18.09) |
| Family smoker | 11 (57.89) | 8 (40.0) | 27 (60.0) | 46 (48.94) |
| Parental asthma | 10 (52.63) | 9 (42.86) | 26 (57.78) | 45 (47.87) |
Data are shown as number (%) or number (range).
BIS, budesonide inhalation suspension; MONT, eligible and receiving montelukast; OBS, not eligible and receiving montelukast.
*Age in OBS group significantly lower (P < 0.05); †% Atopic dermatitis significantly less (P < 0.05).
Laboratory data
Laboratory data at entry can be found in Table 2. HDM-IgE and send levels in the OBS group were significantly lower when compared to the other 2 groups. This was expected because to be included in the OBS group a patient had to have a sEDN level < 53 ng/mL, no sensitivity to HDM or both. The proportion of the MONT group (77.3%) with a detected virus was significantly greater (P = 0.005) than that found in the other 2 groups (BIS [38.1%], OBS [37.3%]).
Table 2. Laboratory data.
| Characteristics | BIS group (n = 21) | MONT group (n = 22) | OBS group (n = 51) | Total (n = 94) |
|---|---|---|---|---|
| sEDN (ng/mL) | 106.01 (53.5–201) | 119.45 (54–201) | 52.68 (14.7–201) | 80.22 (14.7–201) |
| HDM-IgE | 112.62 (0.27–302) | 130.16 (0.1–501) | 21.66 (< 0.1–249) | 66.89 (0.09–501) |
| Detection of any virus | 8 (38.1) | 17 (77.3)* | 19 (37.3) | 44 (46.8) |
Data are shown as number (%) or number (range).
BIS, budesonide inhalation suspension; MONT, eligible and receiving montelukast; OBS, not eligible and receiving Montelukast; sEDN, serum eosinophil-derived neurotoxin; HDM-IgE, house dust mite-immunoglobulin E.
*% of OBS group with virus was significantly greater (P = 0.005).
Virus detection
PCR detection of virus was as follows: RV (A, B, C) = 29; RSV A = 3, RSV B = 6, total = 9; CoV 229E/NL63 = 7; CoV OC43 = 2, total = 9; PIV 1 = 0, PIV 2 = 3, PIV 3 = 4, PIV 4 = 1, total = 8; IFV A = 6, IFV B = 0; AdV = 2; enterovirus = 1; MPV = 0; BV = 0.
ACD
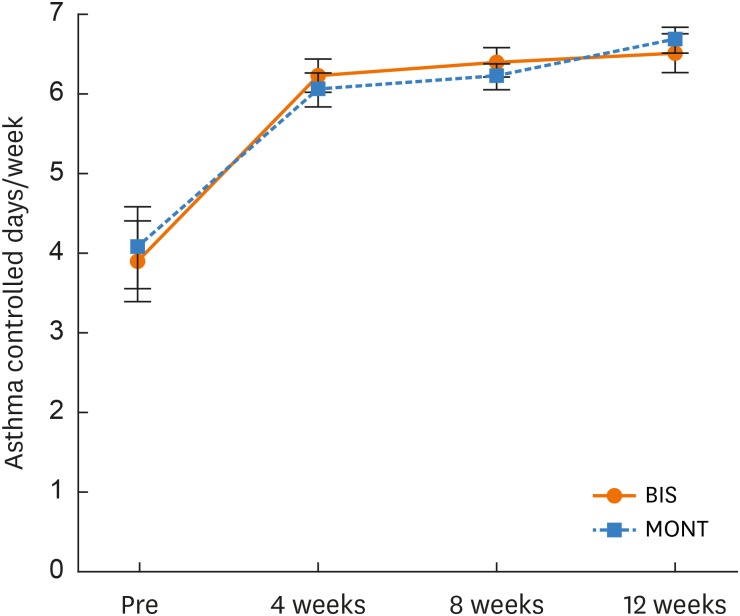
ACD increased significantly in each group (P < 0.000) from the beginning of the study (0 week) to the end of the study (12 weeks). However, when comparing ACD of each group at each time point, there were no significant differences (Fig. 3). Both treatment options had a statistically similar effect on ACD over the 12-week treatment period. The following are ACD values for each group at each time point: 1) BIS group (0 week = 14.5, 4 weeks = 23.7, 8 weeks = 23.5, 12 weeks = 23.8); 2) MONT group (0 week = 16.3, 4 weeks = 23.8, 8 weeks = 24.7, 12 weeks = 26.6).
Fig. 3. Changes in ACD per week.
ACD, asthma control days; BIS, budesonide inhalation suspension; MONT, eligible and receiving montelukast.
Changes in sEDN with treatment
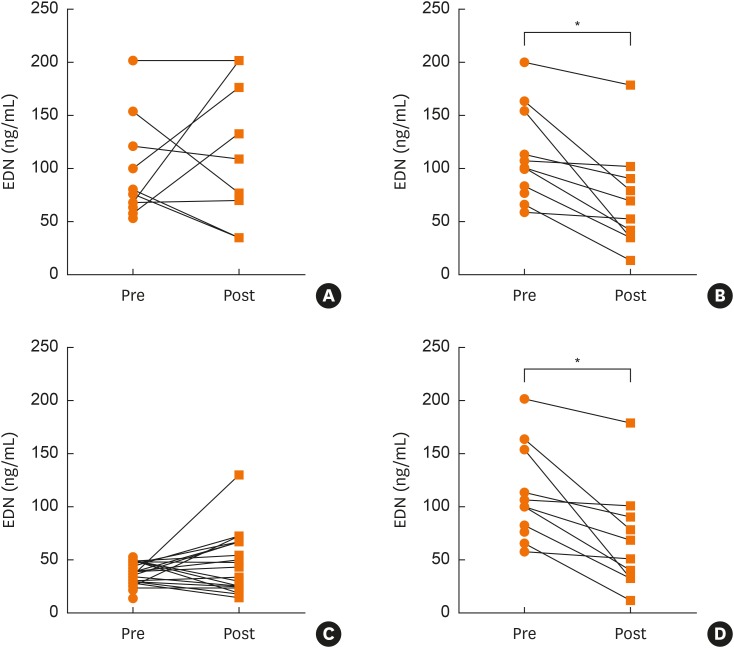
There was no significant change in sEDN in the BIS group (Fig. 4A), but there was a significant decrease found in the MONT group (P < 0.000) (Fig. 4B). When the OBS group was divided into those with low EDN (< 53 ng/mL) (Fig. 4C) and those with high EDN (< 53 ng/mL) (Fig. 4D), only patients with high EDN exhibited a significant decrease due to MONT treatment (P = 0.023), regardless of HDM sensitization.
Fig. 4. Change in sEDN with treatment. BIS group showed no significant change in sEDN level over 12-week treatment period (A). MONT group showed significant change in sEDN level over 12-week treatment period (B) (P < 0.000).
OBS group with low sEDN (< 53 ng/mL) showed no significant change in sEDN over 12-week treatment period with montelukast (C). OBS group with elevated sEDN (< 53 ng/mL) showed significant decrease (P = 0.023) in sEDN over 12-week treatment period with montelukast (D).
*P < 0.01, paired t-test.
sEDN, serum eosinophil-derived neurotoxin; Pre, sEDN level taken at −2 weeks but used at 0 weeks to determine eligibility of study subject; Post, sEDN level at 12 weeks; BIS, budesonide inhalation suspension; MONT, eligible and receiving montelukast; OBS, not eligible and receiving montelukast; EDN, eosinophil-derived neurotoxin.
Virus detection
Forty-four (46.8%) study subjects were positive for any virus tested (Table 2). When each treatment group (BIS, MONT was subgrouped according to virus-positivity, measured for sEDN “Pre” and “Post”), then changes in sEDN (ΔEDN) were compared between virus-positive and -negative subjects in the BIS and MONT groups, and ΔEDN was significantly larger in the virus-negative MONT subgroup (Supplementary Fig. S1B) and mean ΔEDN was also larger in the virus-nagative BIS subgroup, but not statistically significant (Supplementary Fig. S1B). Changes in ACD were not different between virus-positive and -negative subgroups in the BIS (Supplementary Fig. S2A) and MONT (Supplementary Fig. S2B) groups.
Rescue medication usage
Another secondary outcome of this study was change in rescue medication usage with treatment. Comparisons were done 3 ways: 1) within each group (BIS and MONT), usage at run-in (−2 weeks) was compared to usage at the end of the treatment period (12 weeks); 2) each subject in each group was analyzed over the study period (−2 weeks to 12 weeks) to determine any change in usage; 3) usage in the BIS group was compared to usage in the MONT group at each time point (−2, 0, 4, 8 and 12 weeks). There was a significant decrease (P < 0.000) in rescue medication usage within each group (BIS and MONT) and in each subject in each group when analyzed over the study period (−2 to 12 weeks) (P = 0.002). However, there were no significant differences found when comparing usage in the BIS group to that in the MONT group at each time point (−2, 0, 4, 8 and 12 weeks).
DISCUSSION
This study clearly demonstrated that preschool children with beta2-agonist responsive recurrent wheeze and multiple-trigger wheeze, a high EDN level, and HDM sensitivity responded well to both montelukast and budesonide. As expected, montelukast treatment significantly reduced sEDN levels. Unexpectedly, BIS treatment did not. Both treatments also resulted in significant decreases in rescue medication usage over the treatment period. In terms of clinical outcomes, montelukast was as effective as budesonide in the biomarker-defined (i.e., mite sensitized, eosinophilic asthma) subpopulation of preschool asthmatics.
Asthma is a heterogeneous disease with many phenotypes. The recognition of a high EDN/HDM-sensitive phenotype that exhibits a favorable response to standard asthma treatments is important. Of the 100 patients screened for eligibility, nearly half (43%) were found to be of this phenotype, which is considerable. The elevated levels of EDN and other eosinophil degranulation products found in symptomatic asthma would suggest airway inflammation is characterized not only by an increase in the number of eosinophils, but also an increase in airway eosinophil degranulation.16 Therefore, it has been suggested that the secretory activity of eosinophils — a combination of concentration of eosinophils and their propensity to release degranulation products — may be a key marker for disease activity and is more accurately assessed by eosinophil degranulation products such as EDN.17 EDN is released almost exclusively by eosinophils18; therefore, any change in EDN levels would be a direct reflection of changes in eosinophilic inflammation. Significant changes in EDN levels between disease phases have been noted in previous studies.12,19 EDN may therefore be useful in predicting therapeutic outcomes and is a welcome addition to a clinician's ability to effectively treat and monitor asthma. This was further demonstrated in our study as montelukast treatment significantly reduced elevated EDN levels in the MONT group, which has also been shown in other asthma studies12 and even in patients with post-RSV bronchiolitis recurrent wheeze.20 Among the subjects excluded from the main study group (OBS group), the subgroup with no HDM sensitivity but elevated EDN experienced a reduction in their EDN levels as well. Montelukast was the chosen treatment for the OBS group because it has been reported that ICS is less effective in non-atopic populations.21 Furthermore, from our clinical experience montelukast is preferred over ICS by both physicians and patients in Korea and Japan.
Treatment-related reduction in EDN levels may yield actual patient benefits. In our study, subjects receiving montelukast treatment over 12 weeks had not only a significant increase in the number of ACD compared to the beginning of the study, but also had a statistically similar number of ACD at each time point (4, 8 and 12 weeks) when compared to those receiving BIS treatment (BIS group). This is an interesting finding considering a recent Cochrane Review of 65 studies directly comparing the safety and efficacy of anti-leukotrienes and ICSs as monotherapy found ICS treatment to be superior in a number of respects including symptom-free days.22 However, it should be noted that no sample size calculation was done for our study. This point will be discussed further in the study limitations section. The BIS group also showed a significant increase in ACD over the 12-week treatment period.
In previous asthma12 and AD23 studies, sEDN levels have correlated well with symptom scores. It is known that eosinophils release EDN and other degranulation products when activated; in particular, peripheral blood eosinophils were found to release approximately 17%-28% of their total intracellular EDN.24 Furthermore, Sedgwick et al.24 demonstrated that intracellular EDN is also increased in asthmatic patients, while ECP is not. Montelukast is an LTRA, capable of binding with cysteine leukotriene (cysLT) receptors expressed on eosinophils and disrupting their autocrine release. CysLTs can induce eosinophil degranulation,25 which then leads to release of a number of mediators including EDN, ECP and cysLTs.26 This may explain the reduction in EDN levels in the MONT group and in patients in the OBS group with high EDN levels. Corticosteroid treatment may or may not eliminate cytokine-primed, EDN-increased and longer-surviving eosinophils resulting in decreased EDN levels.12 We observed no significant decrease in EDN levels after budesonide treatment in our study, which was contrary to our initial hypothesis that budesonide would also reduce EDN levels. This may be because budesonide, an ICS, acts locally, whereas montelukast acts systemically. Eosinophils develop principally in the bone marrow and are recruited from the circulation, where they migrate to inflammatory sites, such as the airways, and then participate in immunologic processes.26 Once activated, the eosinophil releases EDN and other granular proteins. Changes in granular protein levels signal changes in the turnover and activity of the eosinophil. Taken together, eosinophil production, recruitment and activation are a systemic process.
All patients in this study were also tested for virus infection as it is well known that respiratory viral infection is the major cause of asthma exacerbation in children.27 We detected virus in about half of the treated subjects and reduction in sEDN levels was significantly larger in the virus-negative subgroup of the MONT group, indicating that virus infection may have hindered the effect of montelukast (Supplementary Fig. S1). However, EDN levels still significantly decreased in virus-positive MONT subgroups (data not shown), which may have resulted in the same levels of improvement in ACD in the virus-positive and -negative subgroups (Supplementary Fig. S2).
Study limitations included: 1) the open-label design, which could have affected results through the placebo effect; 2) measurements for both secondary outcomes (i.e., sEDN level and rescue medication usage) were done only 2 times, once at the beginning of the study for baseline and a second time at the end of the study, so it may have been more beneficial to measure serially; 3) patient recruitment was through a few different healthcare centers, which may have introduced spectrum bias and affected study results; 4) no sample size calculation was done, so that the number of eligible patients for the study (n = 43) may have been too small to accurately assess the association between predictor (i.e., elevated sEDN and positive IgE for HDM) and outcome (significant improvement in ACD, sEDN and rescue medication following treatment).
Recent meta-analyses28 of Green et al.'s study8 and 2 others29,30 bolster the hypothesis that treatment-based eosinophil indices is more efficacious (i.e., reduces the frequency and severity of exacerbations) than treatment based on clinical symptoms and other traditional objective measures of lung function such as peak expiratory flow and spirometry. Tailoring patient treatment based on the direct and objective measurement of airway inflammation resulted in significantly less frequent and severe asthma exacerbations and, consequently a trend towards fewer asthma-related hospital admissions.28 Knowing both the inflammatory profile and the clinical categorization of the patient develops a clearer phenotype of the asthma patient and appears to contribute to improved asthma care.27,31 Using a more accurate biomarker for eosinophilic inflammation in the management of asthma may produce even better results.32,33 It has been suggested by many studies that EDN is a more accurate biomarker than eosinophil numbers or percentages.11,12,22,32 Future studies of EDN as a biomarker for the diagnosis, treatment and monitoring of conditions associated with airway inflammation — such as recurrent wheeze, multiple-trigger wheeze and asthma — are needed.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following people for their contributions to this study: Atsuko Matsuyama, Kasumi Goto, Naomi Kamioka, Fumiaki Motoyoshi, Ryosuke Inue, Reiko Tokuda, Akihiko Terada, Eiko Teranishi, Shinji Shinoda, Hidehiko Fujii, Hideo Kaneko.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Decrease in EDN levels (ΔEDN) from visit 1 (enrollment) to visit 4 (after 12 weeks of treatment) in BIS group (A) and MONT group (B).
Changes in ACD per week in virus-positive (virus+) and virus-negative (virus−) subgroups in BIS group (A) and MONT group (B).
References
- 1.Liu AH, Covar RA, Spahn JD, Leung DY. Childhood asthma. In: Kliegman RM, Behrman RE, Jenson HB, Stanton B, editors. Nelson textbook of pediatrics. 18th ed. Philadelphia (PA): Saunders; 2007. pp. 953–970. [Google Scholar]
- 2.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: Global Initiative for Asthma; 2017. [2017 Aug 18]. Available from: http://www.ginasthma.org. [Google Scholar]
- 3.Obase Y, Shimoda T, Tomari SY, Mitsuta K, Kawano T, Matsuse H, et al. Effects of pranlukast on chemical mediators in induced sputum on provocation tests in atopic and aspirin-intolerant asthmatic patients. Chest. 2002;121:143–150. doi: 10.1378/chest.121.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Jr, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 8.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 9.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh YY, Kang H, Kim CK. Ratio of serum eosinophil cationic protein/blood eosinophil counts in children with asthma: comparison between acute exacerbation and clinical remission. Allergy Asthma Proc. 2003;24:269–274. [PubMed] [Google Scholar]
- 11.Yoshihara S, Yamada Y, Abe T, Lindén A, Arisaka O. Association of epithelial damage and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol. 2006;144:212–216. doi: 10.1111/j.1365-2249.2006.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CK, Callaway Z, Fletcher R, Koh YY. Eosinophil-derived neurotoxin in childhood asthma: correlation with disease severity. J Asthma. 2010;47:568–573. doi: 10.3109/02770901003792833. [DOI] [PubMed] [Google Scholar]
- 13.Kim CK, Seo JK, Ban SH, Fujisawa T, Kim DW, Callaway Z. Eosinophil-derived neurotoxin levels at 3 months post-respiratory syncytial virus bronchiolitis are a predictive biomarker of recurrent wheezing. Biomarkers. 2013;18:230–235. doi: 10.3109/1354750X.2013.773078. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki Y, Kohno Y, Ebisawa M, Kondo N, Nishima S, Nishimuta T, et al. Japanese guideline for childhood asthma 2014. Allergol Int. 2014;63:335–356. doi: 10.2332/allergolint.14-RAI-0767. [DOI] [PubMed] [Google Scholar]
- 15.Matsson P, Hamilton RG, Esch RE. Analytical performance characteristics and clinical utility of immunological assays for human immunoglobulin E (IgE) antibodies and defined allergen specificities: approved guidelines. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 16.Motojima S, Akutsu I, Fukuda T, Makino S, Takatsu K. Clinical significance of measuring levels of sputum and serum ECP and serum IL-5 in bronchial asthma. Allergy. 1993;48:98–106. doi: 10.1111/j.1398-9995.1993.tb04709.x. [DOI] [PubMed] [Google Scholar]
- 17.Venge P. Monitoring the allergic inflammation. Allergy. 2004;59:26–32. doi: 10.1046/j.1398-9995.2003.00386.x. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Ghazaleh RI, Dunnette SL, Loegering DA, Checked JL, Kita H, Thomas LL, et al. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52:611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- 19.Morioka J, Kurosawa M, Inamura H, Nakagami R, Mizushima Y, Omura Y, et al. Increased END/EPX in ongoing asthma. Allergy. 2000;55:1203–1204. doi: 10.1034/j.1398-9995.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim CK, Choi J, Kim HB, Callaway Z, Shin BM, Kim JT, et al. A randomized intervention of montelukast for post-bronchiolitis: effect on eosinophil degranulation. J Pediatr. 2010;156:749–754. doi: 10.1016/j.jpeds.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Hofhuis W, van der Wiel EC, Nieuwhof EM, Hop WC, Affourtit MJ, Smit FJ, et al. Efficacy of fluticasone propionate on lung function and symptoms in wheezy infants. Am J Respir Crit Care Med. 2005;171:328–333. doi: 10.1164/rccm.200402-227OC. [DOI] [PubMed] [Google Scholar]
- 22.Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314. doi: 10.1002/14651858.CD002314.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniuchi S, Chihara J, Kojima T, Yamamoto A, Sasai M, Kobayashi Y. Serum eosinophil derived neurotoxin may reflect more strongly disease severity in childhood atopic dermatitis than eosinophil cationic protein. J Dermatol Sci. 2001;26:79–82. doi: 10.1016/s0923-1811(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 24.Sedgwick JB, Vrtis RF, Jansen KJ, Kita H, Bartemes K, Busse WW. Peripheral blood eosinophils from patients with allergic asthma contain increased intracellular eosinophil-derived neurotoxin. J Allergy Clin Immunol. 2004;114:568–574. doi: 10.1016/j.jaci.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Garofalo R, Kimpen JL, Welliver RC, Ogra PL. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 26.Moqbel R, Lace P, Adamko DJ, Odemuyiwa SO. Biology of eosinophils. In: Adkinson NF Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF Jr, Simons FE, editors. Allergy: principles & practice. St. Louis (MO): Mosby; 2008. pp. 295–310. [Google Scholar]
- 27.Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res. 2018;10:12–17. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petsky HL, Kynaston JA, Turner C, Li AM, Cates CJ, Lasserson TJ, et al. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2007;2:CD005603. doi: 10.1002/14651858.CD005603.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Chlumský J, Striz I, Terl M, Vondracek J. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34:129–139. doi: 10.1177/147323000603400202. [DOI] [PubMed] [Google Scholar]
- 30.Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 31.Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. doi: 10.1111/j.1398-9995.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim KW, Lee KE, Kim ES, Song TW, Sohn MH, Kim KE. Serum eosinophil-derived neurotoxin (EDN) in diagnosis and evaluation of severity and bronchial hyperresponsiveness in childhood asthma. Lung. 2007;185:97–103. doi: 10.1007/s00408-006-0054-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim CK, Callaway Z, Park JS, Kwon E. Utility of serum eosinophil-derived neurotoxin (EDN) measurement by ELISA in young children with asthma. Allergol Int. 2017;66:70–74. doi: 10.1016/j.alit.2016.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Decrease in EDN levels (ΔEDN) from visit 1 (enrollment) to visit 4 (after 12 weeks of treatment) in BIS group (A) and MONT group (B).
Changes in ACD per week in virus-positive (virus+) and virus-negative (virus−) subgroups in BIS group (A) and MONT group (B).