Abstract
Endoscopic bariatric therapies (EBTs) are promising alternatives to the conventional surgeries used to treat obesity and related metabolic conditions, targeting gastrointestinal anatomical and physiological processes. Many EBTs are at various stages of development and are aimed at promoting an early sense of satiety via anatomical and physiological mechanisms. In the present study, we focused on relevant clinical issues and future perspectives with regard to gastric non-balloon methods treating obesity.
Keywords: Obesity, Stomach, Endoscopic bariatric therapy, Therapeutic endoscopy, Device
INTRODUCTION
Obesity is a pandemic disorder and has become one of the world’s greatest public health problems [1]. In the United States, obesity affects up to 36% of the total population, and the incidence thereof has increased over the past decades [1-3]. A total of US $147–210 billion is spent every year in the United States to treat obesity-related comorbidities, including cardiovascular disease (especially stroke and acute myocardial infarction, the primary causes of death in 2012), several types of cancers, and bone and joint disorders; obesity accounts for 21% of the healthcare expenditure in the United States [1,2]. Viewing obesity as a chronic medical condition helps to frame stepwise care intensification [1,3]. First, all patients should be advised to embrace evidence-based lifestyle modifications (dietary, physical activity, and behavioral changes) [1]. Second, pharmacological agents are prescribed as adjuncts [4]. These two steps are essentially noninvasive but are largely ineffective and associated with low rates of sustained weight loss [4]. The third step features endoscopy-assisted intervention or bariatric surgery; the latter afforded the most effective long-term outcomes in patients with moderate and severe obesity complicated by comorbidities rendering them unresponsive to nonsurgical treatments [4]. However, the mean bariatric mortality rate has been reported to be up to 2.5% over 8–14 years of follow-up [5]. Moreover, surgical access is limited; less than 2% of patients with obesity are eligible for surgery [4]. Therefore, endoscopic bariatric therapies (EBTs) are appropriate for high-risk patients and those who refuse surgery [4]. EBTs afford at least 10% total body weight reduction in most cases and are safer and cheaper than surgery [4]. Recently, several EBTs have directly targeted obesity. Here, we review the available EBTs, focusing on non-balloon devices, with consideration of the technical aspects and evidence of usefulness in clinical practice [2,4]. EBTs seek to reduce gastric volume via anatomical manipulations of the stomach [2,4]. This reduces the available area for caloric intake and induces early satiety by modulating gastric emptying and accommodation [2,4]. EBTs that target the stomach include the following: (1) non-balloon space-occupying devices; (2) devices/techniques influencing gastric emptying; and (3) endoscopic plication.
NON-BALLOON SPACE-OCCUPYING DEVICES
Gelesis 100 hydrogel capsules
The Gelesis 100 (Gelesis, Boston, MA, USA) is an oral capsule containing thousands of tiny hydrogel particles designed to replace food [3]. The particles (modified cellulose strands) expand rapidly after absorbing water, forming a hydrophilic lattice [3]. A capsule is ingested prior to a meal (together with water), the particles expand [3] and enter the duodenum, the luminal contents of which are particularly viscous [3]. The particles create a pseudo-barrier that blocks glucose absorption, thus improving glycemic control [3]. In a double-blinded, randomized placebo-controlled study, 128 nondiabetic overweight patients were randomized as follows: (Group 1) Gelesis 2.25 g twice daily; (Group 2) Gelesis 3.75 g twice daily; or (Group 3) placebo for 12 weeks. Group 1 exhibited a statistically significant weight loss as compared with the placebo group (6.1% vs. 4.1% weight reduction, p=0.026) [3]. Gelesis 3.75 g twice daily was associated with tolerability and compliance issues. No adverse event was recorded [3].
Full-Sense device
The Full-Sense bariatric device (Baker, Foote, Kemmeter, Walburn Lit LC, Grand Rapids, MI, USA; Fig. 1) is endoscopically placed and later removed [2]. It is a reversible, weightloss stent-type device featuring a gastric disk, a strut, and an esophageal stent. The disk is positioned on the cardia to induce satiety [1,2,4]. To the best of our knowledge, only one brief report has been published, in which the %EWL after 46 days was 28%. Thus, the device requires further review [2].
Fig. 1.
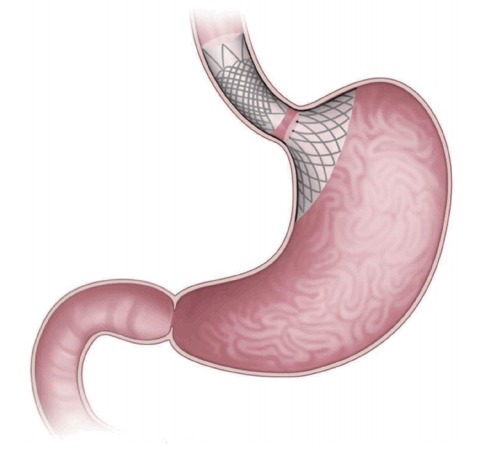
The Full-Sense bariatric device (Baker, Foote, Kemmeter, Walburn Lit LC, Grand Rapids, MI, USA). The device puts pressure on the top of the stomach to trigger signals and hormones that cause weight loss.
DEVICES/TECHNIQUES THAT INFLUENCE GASTRIC EMPTYING
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, CA, USA; Fig. 2) is delivered via an overtube and self-assembles in the stomach [4,6,7]. The shuttle features a large spherical silicon orb, a smaller cylindrical silicon bulb, and a flexible cord connecting the orb and the bulb [7]. The smaller bulb is positioned on the duodenum, and the greater orb remains behind the pylorus, engaging in natural peristalsis with the smaller bulb [2,6,7]. The greater orb prevents migration from the stomach, whereas the smaller bulb passes through the duodenum, positioning the TPS across the pylorus. Such intermittent pyloric obstruction delays gastric emptying [2,6,7]. A preliminary study featured 20 patients with obesity randomized to TPS treatment for 3 or 6 months [2,6]. The former patients exhibited a mean % excess weight loss (%EWL), excess body mass index loss (%EBMIL), and % weight loss of 25.1%, 33.1%, and 8.9%, respectively. The figures for the latter patients were 41.0%, 50.0%, and 14.5%, respectively. The device was removed in two patients because of symptomatic gastric ulceration, which resolved after removal [2]. The multicenter, randomized sham-controlled clinical study END Obesity II is currently underway [3]. The target study population (n=270, 2:1 ratio of device randomization) includes patients who underwent the conventional treatment whose body mass indexes ranged from 30.0 to 40.0 kg/m2. Subject enrolment is complete, and the early results are most encouraging [3].
Fig. 2.
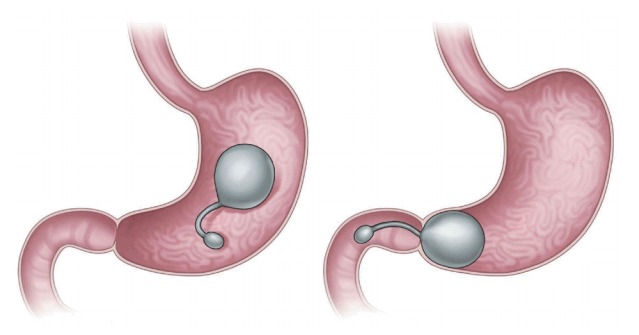
Transpyloric shuttle (BAROnova, Goleta, CA, USA). The device consists of large and small bulbs.
GASTRIC VOLUME-RESTRICTION DEVICES
Articulating circular endoscopic stapling
The articulating circular endoscopic stapler (Boston Scientific Co., Natick, MA, USA; Fig. 3) is a full-thickness stapling device that can operate up to 360º. Serial plications of the stomach restrict gastric volume [8,9]. In a human pilot study in the Netherlands, 17 patients exhibited a mean %EWL of 34.9% at the 12-month follow-up [9]. At the end of the study, all the patients underwent upper endoscopy and still showed gastric volume reduction [9]. A 24-month follow-up study is underway [9].
Fig. 3.
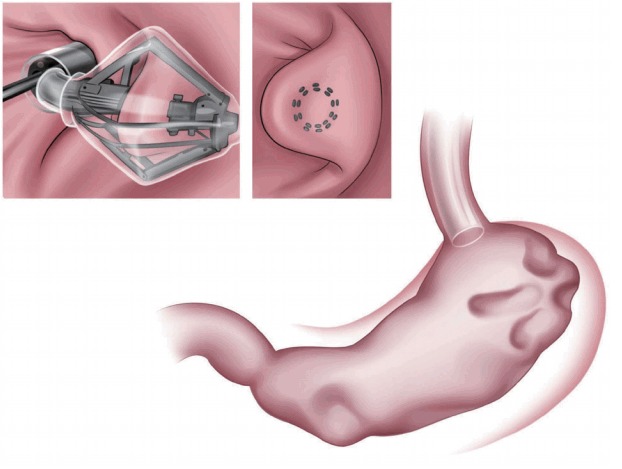
Articulating circular endoscopic stapling (Boston Scientific Co., Natick, MA, USA). The articulating circular endoscopic stapler is a modified version of the laparoscopic circular stapler for endoscopic use.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty (ESG) [8] features placement of a series of triangular full-thickness sutures though the gastric wall along the greater curvature of the stomach from the pre-pyloric antrum to the gastroesophageal junction, sparing a small pouch in the fundus. ESG creates a restrictive endoscopic sleeve that delays gastric emptying, in turn decreasing caloric intake [4,10]. In an earlier multinational report, 245 patients had a mean total body weight loss (TBWL) of 18.6% over 24 months. Compared with space-occupying devices, ESG was more effective and durable [10,11]. Five adverse events were reported as follows: two cases of perigastric inflammatory fluid collection that resolved after placement of percutaneous drainage and antibiotic treatment; one self-limiting extra-gastric hemorrhage that required blood transfusion; one pulmonary embolism developing within 3 days of suturing; and one pneumoperitoneum/pneumothorax requiring chest tube insertion [10,11].
Transoral gastroplasty
Transoral gastroplasty (TOGA; Satiety, Inc., Palo Alto, CA, USA; Fig. 4) [12,13] features placement of a series of full-thickness sutures along the lesser curvature of the stomach from the Z-line for 8 cm. TOGA mimics surgical gastric banding. It creates a restrictive endoscopic sleeve that delays gastric emptying, in turn decreasing caloric intake [4,10,12] in the same manner as ESG [12,13]. The TOGA system consists of a sleeve stapler and restrictor. More detailed descriptions of the procedures are as follows: [12,13] (1) The TOGA sleeve stapler is introduced with a guidewire into the proximal portion of the stomach, followed by an endoscope over the hole of the sleeve stapler [12,13]. The head of the sleeve stapler sucks the full thickness of the gastric wall anteroposteriorly, and then a triple line of staples is fired to form a sleeve around the stapler [12,13]. Two repetitions of the aforementioned procedures resulted in a continuous staple line extending from 8 cm of the Z line, in line with the lesser curvature [12,13]. (2) The TOGA restrictor is inserted over a guidewire after endoscopic insertion through the hole of the restrictor. The TOGA restrictor creates some folds at the distal end of the gastric sleeve, resulting in a pouch outlet [12,13].
Fig. 4.
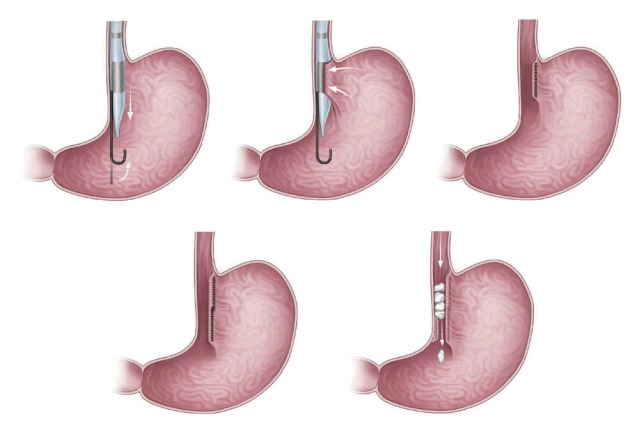
Transoral gastroplasty (TOGA; Satiety, Inc., Palo Alto, CA, USA). In TOGA, natural orifices such as the mouth are used in the surgery as an alternative to making cuts or incisions in the patient’s skin.
In an earlier multicenter trial conducted in Italy [12,13], 67 patients had a mean %EBMIL of 44.8% (p<0.05), decrease in HbA1C level of 1.3% (p=0.01), and improvement in lipid profile (p=0.001) over 12 months. Two adverse events, namely respiratory insufficiency and asymptomatic pneumoperitoneum, were reported [12,13].
Transoral endoscopic restrictive implant system
The transoral endoscopic restrictive implant system (Fig. 5) [8,14] features an implantable restrictive diaphragm over the full-thickness transmural plication in the proximal portion of the stomach from the cardia to 3 cm below the gastroesophageal junction [8,14]. For placing the implantable diaphragm on the cardia, five full-thickness plications with anchors are made using a stapling device. Then, an implantable diaphragm is attached over the five anchors [8,14].
Fig. 5.
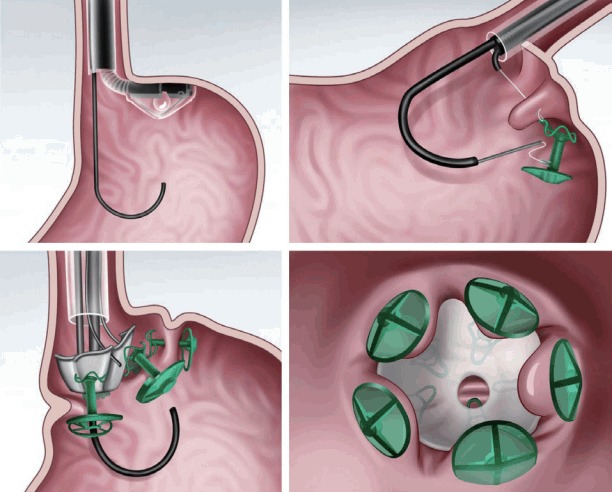
A transoral endoscopic restrictive implant system.
In a preliminary report from the Nethelands [8], 18 patients were enrolled and showed a mean TBWL of 15.1% over 6 months [8]. Three serious adverse events were reported as follows: two cases of pneumoperitoneum and one case of perforation [8].
Primary obesity surgery (endoluminal)
In primary obesity surgery (endoluminal; POSE; Fig. 6), a peroral, incisionless operating platform (USGI Medical, San Clemente, CA, USA) is used [15]. POSE reduces gastric accommodation in the fundus by placing transmural tissue-anchoring plications and delays gastric emptying by placing additional plications on the distal gastric body [15]. This increases the sense of satiation and the gut neurohormonal response [15]. In a multicenter, randomized blinded controlled trial that included 221 patients who underwent POSE combined with lifestyle interventions for 12 months and 111 patients who were receiving lifestyle interventions only [16,17], the %TBWL was 4.94%±7% in the POSE group and 1.38%±5.6% in the control group, with a statistically significant difference (p<0.05) [16,17]. Adverse events that prolonged hospital stay included vomiting (1.9%), nausea (1.6%), pain (0.4%), extragastric bleeding that required open surgical exploration (0.4%), and a hepatic abscess that required percutaneous drainage (0.4%) [16,17].
Fig. 6.
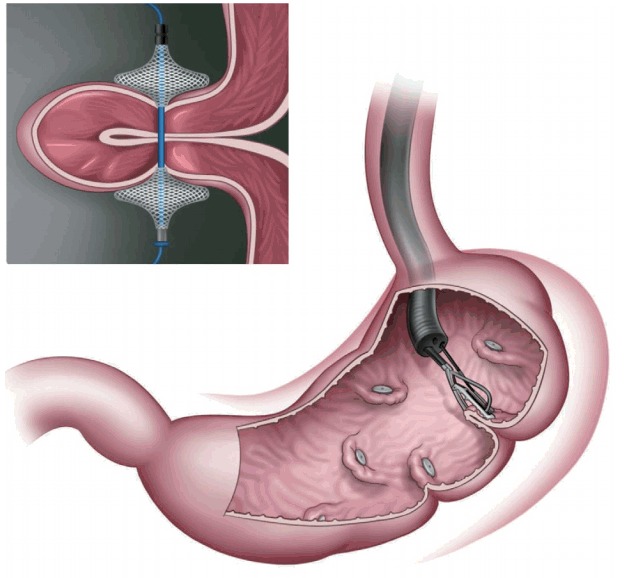
Primary obesity surgery endoluminal (USGI Medical, San Clemente, CA, USA). A peroral incisionless operating platform is used to place transmural plications in the gastric fundus and distal body with a specialized tissue anchor.
CONCLUSIONS
EBTs are effective and safe when used to treat obesity. EBTs range from promising preliminary modalities to procedures backed by strong preclinical data and are associated with varying extents of weight loss. Combinations of EBTs and lifestyle/pharmacotherapeutic interventions require further investigation. Randomized blinded controlled trials are essential to define further the effects of the various devices. Continued preclinical device development combined with well-designed clinical trials that feature standardized outcome metrics will soon personalize EBT use by physiological and clinical phenotypes.
Footnotes
Conflicts of Interest:The authors have no financial conflicts of interest.
REFERENCES
- 1.Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 2.Marinos G, Eliades C, Raman Muthusamy V, Greenway F. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis. 2014;10:929–934. doi: 10.1016/j.soard.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Sampath K, Rothstein RI. Selected endoscopic gastric devices for obesity. Gastrointest Endosc Clin N Am. 2017;27:267–275. doi: 10.1016/j.giec.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56:465–472. doi: 10.1016/j.pcad.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Telem DA, Talamini M, Shroyer AL, et al. Long-term mortality rates (>8-year) improve as compared to the general and obese population following bariatric surgery. Surg Endosc. 2015;29:529–536. doi: 10.1007/s00464-014-3714-4. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MC, Badillo R, Sullivan S. Endoscopic management. Gastroenterol Clin North Am. 2016;45:673–688. doi: 10.1016/j.gtc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017;152:1791–1801. doi: 10.1053/j.gastro.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Verlaan T, de Jong K, de la Mar-Ploem ED, Veldhuyzen EA, Mathus-Vliegen EM, Fockens P. Trans-oral endoscopic restrictive implant system: endoscopic treatment of obesity? Surg Obes Relat Dis. 2016;12:1711–1718. doi: 10.1016/j.soard.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Verlaan T, Paulus GF, Mathus-Vliegen EM, et al. Endoscopic gastric volume reduction with a novel articulating plication device is safe and effective in the treatment of obesity (with video) Gastrointest Endosc. 2015;81:312–320. doi: 10.1016/j.gie.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Sartoretto A, Sui Z, Hill C, et al. Endoscopic sleeve gastroplasty (ESG) is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: a large, international multicenter study. Obes Surg. 2018;28:1812–1821. doi: 10.1007/s11695-018-3135-x. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. 2017;27:2649–2655. doi: 10.1007/s11695-017-2693-7. [DOI] [PubMed] [Google Scholar]
- 12.Familiari P, Costamagna G, Bléro D, et al. Transoral gastroplasty for morbid obesity: a multicenter trial with a 1-year outcome. Gastrointest Endosc. 2011;74:1248–1258. doi: 10.1016/j.gie.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Moreno C, Closset J, Dugardeyn S, et al. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: results of the second human pilot study. Endoscopy. 2008;40:406–413. doi: 10.1055/s-2007-995748. [DOI] [PubMed] [Google Scholar]
- 14.Kumar N. Endoscopic therapy for weight loss: gastroplasty, duodenal sleeves, intragastric balloons, and aspiration. World J Gastrointest Endosc. 2015;7:847–859. doi: 10.4253/wjge.v7.i9.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill C, Khashab MA, Kalloo AN, Kumbhari V. Endoluminal weight loss and metabolic therapies: current and future techniques. Ann N Y Acad Sci. 2018;1411:36–52. doi: 10.1111/nyas.13441. [DOI] [PubMed] [Google Scholar]
- 16.Miller K, Turró R, Greve JW, Bakker CM, Buchwald JN, Espinós JC. MILEPOST multicenter randomized controlled trial: 12-month weight loss and satiety outcomes after pose (SM) vs. medical therapy. Obes Surg. 2017;27:310–322. doi: 10.1007/s11695-016-2295-9. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The primary obesity surgery endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 2015;11:861–865. doi: 10.1016/j.soard.2014.09.026. [DOI] [PubMed] [Google Scholar]


