Abstract
Objective
Cognitive impairment is one of the nonmotor symptoms in Parkinson’s disease (PD), and olfactory dysfunction is used as a marker to detect premotor stages of PD. Serum uric acid (sUA) levels have been found to be a risk factor for PD. Our objective in this study was to examine whether sUA levels are associated with cognitive changes and olfactory dysfunction in early de novo PD patients.
Methods
The study participants included 196 de novo PD patients. We assessed cognitive function by the Korean versions of the Mini-Mental State Examination and the Montreal Cognitive Assessment and assessed olfactory function by the Korean version of the Sniffin’ Sticks test.
Results
The mean sUA level was 4.7 mg/dL and was significantly lower in women than in men. Cognitive scores were lower in women, suggesting that sUA levels were related to cognitive function. The olfactory functions were not related to sUA level but were clearly associated with cognitive scores. Olfactory threshold, odor discrimination, and odor identification were all significantly related to cognitive scores.
Conclusion
We conclude that lower sUA levels were associated with cognitive impairment, not olfactory dysfunction, in de novo PD patients. This finding suggests that UA is neuroprotective as an antioxidant in the cognitive function of PD patients.
Keywords: Cognition, Parkinson’s disease, olfaction, uric acid
Typical clinical features of Parkinson’s disease (PD), a common movement disorder, include bradykinesia, rigidity, resting tremor, and gait disturbance. In addition to these well-known motor features, nonmotor symptoms such as depression, olfactory dysfunction, psychosis, and cognitive dysfunction are often detected early in PD. Olfactory dysfunction is used as a marker to detect the premotor stages of PD, as are other symptoms, such as depression, constipation, and REM sleep behavior disorder [1,2]. Additionally, subtle cognitive changes are often detected in early PD patients [3].
Serum uric acid (sUA) levels have been found to be a risk factor for some neurological diseases. That is, high sUA levels are known to increase the risk of both cardiovascular and cerebrovascular events [4,5], whereas low sUA levels are found to be a risk factor for PD [6]. Furthermore, some studies have shown that low sUA levels are associated with cognitive dysfunction in both healthy individuals and PD patients [7,8].
To examine the hypothesis that sUA levels are associated with cognitive changes and olfactory dysfunction, we performed olfactory and comprehensive neuropsychological tests, including those for cognitive function and depression, in early de novo PD patients.
MATERIALS & METHODS
Patients
Between January 2015 and December 2017, patients presenting to the PD clinic of the Sanggye Paik Hospital for de novo PD were screened for enrollment in the study. All PD patients met the criteria of the UK Parkinson’s Disease Society Brain Bank [9]. We excluded those patients with diagnoses of secondary or atypical parkinsonism according to the available clinical criteria [10-12] and those patients diagnosed with major depression according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. For effective evaluation of olfactory function, patients who were current smokers were also excluded. All enrolled patients were admitted, and we obtained detailed information from the patients’ history and neurological examinations. On admission, we classified the progression of symptoms into stages according to the Hoehn and Yahr scale (HY) [13], and evaluated motor disability using the Unified Parkinson’s Disease Rating Scale III (UPDRS-III) [14]. Venous blood samples were drawn only at baseline in the morning after an overnight fast to evaluate UA levels. For this reason, we also excluded patients using treatments with diuretics or sUA-modifying agents and those with a body mass index > 25 or < 19 to exclude both over- and underweight patients. All patients provided signed informed consent. This study was approved by the Institutional Review Board of Sanggye Paik Hospital (IRB no. 201806008).
Neuropsychological assessment
All enrolled patients underwent a detailed neuropsychological assessment by a psychologist. Cognitive function was evaluated by means of the Korean versions of the Mini-Mental State Examination (K-MMSE) and the Montreal Cognitive Assessment (K-MoCA). The current severity of any depressive episodes experienced by patients was evaluated using the Hamilton Depression Scale.
Korean version of the Sniffin’ Sticks test
An otorhinolaryngologist examined all patients and excluded some patients with secondary anosmia, including obstruction. Olfactory function was examined by an otorhinolaryngologist using the Korean version of the Sniffin’ Sticks test (KVSS II), which is a validated test instrument for Koreans [15]. KVSS II consists of 16 different items with three components each, including olfactory threshold (i.e., detecting the lowest concentration of odor), odor discrimination (i.e., differentiating certain odors from several other odors), and odor identification (i.e., naming a certain odor). Using 16 pens, each filled with a different liquid odorant, the olfactory threshold and odor discrimination tests were carried out. Three pens (two with identical odors) were presented to each patient for odor discrimination. The odor identification test utilized all 16 odors. All patients were presented with the olfactory threshold test first, followed by the odor discrimination test and the odor identification test, with a break of 3 min between tests. The sums of the three tests are presented as threshold-discrimination-identification (TDI) scores. Total scores of 0 to 20 are defined as anosmia; 21 to 27, as hyposmia; and 28 to 48, as normosmia [15].
Statistical analysis
Data are expressed as the means (standard deviations) or numbers (percentages). Categorical data were examined by chi-square tests. Student’s t-test was used to compare normally distributed data, and the Mann-Whitney U test was used to compare nonnormally distributed data. Pearson’s and Spearman’s correlation coefficients were calculated to evaluate the relationships between sUA levels and neuropsychological assessments. Multivariate linear regression models were used to analyze the association of significant univariate variables. Two-sided null hypotheses of no difference were rejected at p < 0.05. SPSS Statistics 20 for Windows (IBM Corp, Armonk, NY, USA) was used for statistical analysis.
RESULTS
During the trial, 286 patients were assessed, and 90 were excluded. Forty-six patients met exclusion criteria (including 32 patients with below middle school education and 5 using diuretics). Forty-four patients refused to participate in the study. Finally, a total of 196 participants met the inclusion criteria and were enrolled in this study (Figure 1). Table 1 shows the baseline characteristics of the enrolled patients. The mean age of the patients was 67.4 years, and 58% of the patients were women. The mean duration of disease at recruitment was 1.4 years. The mean HY stage was 1.7, and the mean total UPDRS (I–III) score was 34. There were no significant differences in these characteristics between men and women. The mean sUA level was 4.7 mg/dL and was significantly lower in women than in men (4.3 vs. 5.2 mg/dL, p < 0.0001). The mean scores for TDI, MMSE and MoCA were 24.4, 25.7 and 21.7, respectively. These scores were lower in women than in men (TDI 22.1 vs. 26.0, p = 0.006, MMSE 24.8 vs. 27, p < 0.0001 and MoCA 20.5 vs. 23.4, p < 0.0001), suggesting that sUA was related to olfactory and cognitive function.
Figure 1.
Trial profile.
Table 1.
Baseline characteristics of enrolled patients
| Total (n=196) | Men (n=83) | Women (n=113) | p-value | |
|---|---|---|---|---|
| Age (yr) | 67.4 (9.07) | 66.2 (9.55) | 68.4 (8.63) | 0.089 |
| Duration (yr) | 1.4 (1.24) | 1.6 (1.46) | 1.3 (1.05) | 0.193 |
| HY stage | 1.7 (0.67) | 1.7 (0.66) | 1.7 (0.69) | 0.402 |
| UPDRS I–III | 34.0 (16.34) | 35.9 (17.99) | 32.5 (15.97) | 0.360 |
| UPDRS-III | 21.6 (11.52) | 21.5 (11.66) | 21.6 (11.47) | 0.979 |
| NMS | 39.6 (40.23) | 37.2 (38.77) | 41.4 (41.38) | 0.480 |
| Olfactory functions | ||||
| Threshold | 6.7 (3.41) | 6.7 (3.64) | 6.7 (3.26) | 0.994 |
| Discrimination | 7.1 (3.15) | 7.0 (3.35) | 7.3 (3.01) | 0.539 |
| Identification | 6.7 (3.41) | 6.7 (3.64) | 6.7 (3.26) | 0.994 |
| TDI score (normosmia≥28) | 24.4 (9.91) | 26.0 (9.12) | 22.1 (10.55) | 0.006* |
| MMSE score | 25.7 (3.91) | 27.0 (2.85) | 24.8 (4.30) | <0.0001* |
| MoCA score | 21.7 (5.62) | 23.4 (4.41) | 20.5 (6.09) | <0.0001* |
| HAM-D | 8.9 (6.87) | 9.0 (7.08) | 8.7 (6.74) | 0.802 |
| PDSS | 92.7 (40.84) | 98.9 (36.71) | 88.1 (43.23) | 0.088 |
| BMI (kg/m2) | 23.6 (3.04) | 23.6 (2.90) | 23.7 (3.14) | 0.911 |
| Serum uric acid (mg/dL) | 4.7 (1.16) | 5.2 (1.22) | 4.3 (0.94) | <0.0001* |
Data are presented as the mean (SD).
significant p-values.
HY: Hoehn and Yahr, UPDRS: Unified Parkinson’s Disease Rating Scale, UPDRS-III: Unified Parkinson’s Disease Rating Scale Part III, NMS: Non-Motor Symptom Scale, TDI: threshold-discrimination-identification, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive Assessment, HAM-D: Hamilton Depression Rating Scale, PDSS: Parkinson’s Disease Sleep Scale, BMI: body mass index.
Table 2 shows the correlation analysis of sUA levels with the baseline characteristics. In all participants, sUA was significantly related to being female (r = -0.367), to MMSE scores (r = 0.222), and to MoCA scores (r = 0.215). Although there was no significant difference, a trend toward lower olfactory function (TDI score) was related to decreased sUA levels (r= 0.141, p = 0.064). sUA levels were clearly associated with MMSE and MoCA scores (p < 0.008). TDI, olfactory threshold, odor discrimination, and odor identification scores were all significantly related to MMSE and MoCA scores (r > 0.150, p < 0.038). sUA was also significantly related to MMSE and MoCA scores in both male and female sexes (p < 0.047). Multiple linear regression analysis revealed that age and sUA were significantly associated with MMSE and MoCA scores in all participants, with age being the most influential factor (β coefficients of -0.369 and 0.179 for age and sUA, respectively). Figure 2 shows the age-adjusted scatterplots of the relationship between sUA levels and MMSE and MoCA scores. The sUA level was positively associated with MMSE and MoCA scores in patients with early de novo PD (r = 0.180, p = 0.017 for MMSE and r = 0.193, p = 0.010 for MoCA scores).
Table 2.
Correlation analysis of sUA level with baseline characteristics
| sUA level |
||||||
|---|---|---|---|---|---|---|
| Total (n=196) |
Men (n=83) |
Women (n=113) |
||||
| r | p | r | p | r | p | |
| Age | -0.112 | 0.139 | -0.185 | 0.094 | -0.122 | 0.254 |
| Female | -0.367 | <0.0001* | ||||
| Disease duration | -0.125 | 0.097 | -0.223 | 0.051 | -0.075 | 0.459 |
| HY stage | -0.124 | 0.137 | -0.174 | 0.134 | -0.112 | 0.272 |
| UPDRS I–III | -0.102 | 0.179 | -0.143 | 0.218 | -0.021 | 0.834 |
| UPDRS III | -0.103 | 0.178 | -0.214 | 0.074 | -0.019 | 0.854 |
| NMS | -0.143 | 0.156 | -0.112 | 0.211 | -0.088 | 0.392 |
| Olfactory functions | ||||||
| Threshold | -0.015 | 0.840 | 0.092 | 0.435 | -0.086 | 0.401 |
| Discrimination | -0.086 | 0.260 | 0.092 | 0.435 | 0.086 | 0.401 |
| Identification | 0.002 | 0.978 | -0.018 | 0.879 | -0.119 | 0.243 |
| TDI score | 0.141 | 0.064 | 0.211 | 0.079 | 0.236 | 0.054 |
| MMSE | 0.222 | 0.003* | 0.233 | 0.041* | 0.229 | 0.047* |
| MoCA | 0.215 | 0.004* | 0.237 | 0.039* | 0.231 | 0.042* |
| HAM-D | -0.124 | 0.121 | -0.198 | 0.102 | -0.137 | 0.203 |
| PDSS | 0.127 | 0.113 | 0.148 | 0.270 | 0.054 | 0.619 |
| BMI | 0.186 | 0.063 | 0.171 | 0.293 | 0.172 | 0.188 |
significant p-values.
sUA: serum uric acid, HY: Hoehn and Yahr, UPDRS: Unified Parkinson’s Disease Rating Scale, UPDRS-III: Unified Parkinson’s Disease Rating Scale part III, NMS: Non-Motor Symptom Scale, TDI: threshold-discrimination-identification, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive Assessment, HAM-D: Hamilton Depression Rating Scale, PDSS: Parkinson’s Disease Sleep Scale, BMI: body mass index.
Figure 2.
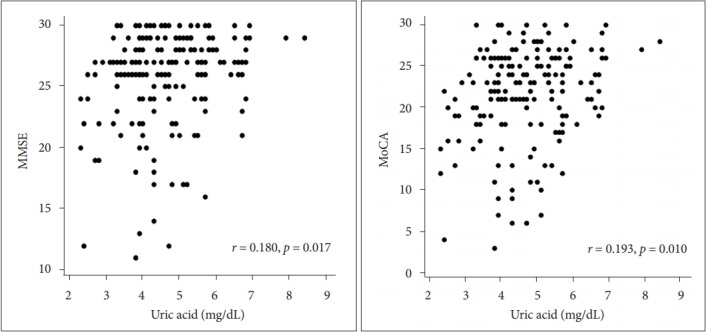
Age-adjusted scatterplots of the relationship between sUA and MMSE and MoCA scores. r: Pearson’s partial correlation coefficient. X-axes are based on calculated residuals from regressing sUA on age. Y-axes are based on calculated residuals from regressing MMSE and MoCA scores on age. sUA: serum uric acid, MMSE: MiniMental State Examination, MoCA: Montreal Cognitive Assessment.
DISCUSSION
In this study, we explored the relationships among sUA levels, cognitive function, and olfactory dysfunction. Our study clearly demonstrated that lower sUA levels were associated with more cognitive impairment in patients with de novo PD. We found that olfactory dysfunction was also related to cognitive decline. These findings support the hypothesis that UA is not neurotoxic as a pro-oxidant but is neuroprotective as an antioxidant [16], as previous study results have suggested [8,17,18].
Olfactory dysfunction and cognitive decline in early PD patients may be underestimated because there are many other causes of hyposmia and mild cognitive decline in elderly patients. Bohnen et al. [19] first suggested a correlation between cognitive function and olfactory loss in PD patients, and since then several studies have shown that olfactory dysfunction is associated with cognitive impairment [20-22]. Recently, Park et al. [22] showed that PD with minimal cognitive impairment is more likely to be associated with severe olfactory impairment than PD without cognitive dysfunction.
UA levels are considered a risk factor for cerebrovascular disease. Using MRI findings, Han et al. [23] also showed that sUA was associated with cerebral white matter hyperintensities in patients with acute lacunar infarction. In terms of cognition, sUA levels reflect opposite findings in PD patients and healthy patients [8,18], and high sUA levels are associated with cognitive deficits in a community of healthy individuals, excluding PD or Alzheimer disease (AD) patients. These results suggest that cerebrovascular disease causes cognitive decline in healthy people. However, low sUA levels have been observed in AD, vascular dementia, and PD patients with cognitive deficits. UA may play an important role in the pathophysiology of neurodegenerative disorders.
Based on this suggested mechanism, our study attempted to understand the relationship between sUA levels and olfactory dysfunction or cognitive dysfunction in PD patients. We found that sUA levels were positively related to cognitive function. A trend toward having lower olfactory function was related to decreased sUA levels in this study (p =0.064). Baba et al. [20] showed that severe anosmia can be a prognostic marker of developing dementia in PD patients. Among the subtypes of olfactory tests, odor threshold and identification can be prodromal symptoms in patients with AD [24]. Our finding that olfactory dysfunction was related to cognitive dysfunction in de novo early PD patients supports these previous results, demonstrating the correlation between olfaction and cognition in PD. In addition to olfaction and cognition, sUA can be associated with apathy, but not with depression, in de novo early PD patients [25,26]. This result supported the mechanism that sUA levels and apathy are related to the loss of presynaptic dopaminergic innervation in the striatum in early de novo PD patients [27,28]. Although we did not evaluate apathy, we found that sUA levels were unrelated to depression.
Our study has some limitations. Although we obtained a large sample size, we evaluated cognition using K-MMSE and K-MoCA rather than the detailed Neuropsychological Assessment Battery. For this reason, we could not identify the relationships between sUA levels and the subtypes of cognitive function, such as attention/executive function or visuoconstructional abilities. Additionally, as all enrolled participants were drug-naïve patients, we were unable to measure alterations in cognition and olfaction after drug therapy, including the use of levodopa or anti-UA drugs.
Despite these issues that limit our results, we discovered some unique findings through this study. We confirmed that lower sUA levels were associated with cognitive impairment in de novo PD patients. This finding suggests that UA is neuroprotective as an antioxidant for cognitive function in PD patients. However, we found no relationship between UA levels and olfactory function, despite the correlation between cognition and olfaction in PD patients. Therefore, the data from the current study support a relationship between sUA levels and cognition, but not between UA levels and olfaction or between UA levels and depression, in early de novo PD patients. Replications of our observations based on studies by independent cohorts are needed to confirm our findings.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Domellöf ME, Lundin KF, Edström M, Forsgren L. Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:41–47. doi: 10.1016/j.parkreldis.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Casjens S, Eckert A, Woitalla D, Ellrichmann G, Turewicz M, Stephan C, et al. Diagnostic value of the impairment of olfaction in Parkinson’s disease. PLoS One. 2013;8:e64735. doi: 10.1371/journal.pone.0064735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurology. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 5.Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 6.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 7.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132(Pt 2):377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 8.Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 12.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 13.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 15.Cho JH, Jeong YS, Lee YJ, Hong SC, Yoon JH, Kim JK. The Korean version of the Sniffin’ stick (KVSS) test and its validity in comparison with the cross-cultural smell identification test (CC-SIT) Auris Nasus Larynx. 2009;36:280–286. doi: 10.1016/j.anl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polidori MC, Mattioli P, Aldred S, Cecchetti R, Stahl W, Griffiths H, et al. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement Geriatr Cogn Disord. 2004;18:265–270. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- 18.Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Gordon B, Pearlson GD. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology. 2007;21:136–140. doi: 10.1037/0894-4105.21.1.136. [DOI] [PubMed] [Google Scholar]
- 19.Bohnen NI, Müller ML, Kotagal V, Koeppe RA, Kilbourn MA, Albin RL, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain. 2010;133(Pt 6):1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba T, Kikuchi A, Hirayama K, Nishio Y, Hosokai Y, Kanno S, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain. 2012;135(Pt 1):161–169. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- 21.Morley JF, Weintraub D, Mamikonyan E, Moberg PJ, Siderowf AD, Duda JE. Olfactory dysfunction is associated with neuropsychiatric manifestations in Parkinson’s disease. Mov Disord. 2011;26:2051–2057. doi: 10.1002/mds.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JW, Kwon DY, Choi JH, Park MH, Yoon HK. Olfactory dysfunctions in drug-naïve Parkinson’s disease with mild cognitive impairment. Parkinsonism Relat Disord. 2018;46:69–73. doi: 10.1016/j.parkreldis.2017.11.334. [DOI] [PubMed] [Google Scholar]
- 23.Han SW, Song TJ, Bushnell CD, Lee SS, Kim SH, Lee JH, et al. Serum uric acid is associated with cerebral white matter hyperintensities in patients with acute lacunar infarction. J Neuroimaging. 2016;26:351–354. doi: 10.1111/jon.12308. [DOI] [PubMed] [Google Scholar]
- 24.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Picillo M, Santangelo G, Moccia M, Erro R, Amboni M, Prestipino E, et al. Serum uric acid is associated with apathy in early, drug-naïve Parkinson’s disease. J Neural Transm (Vienna) 2016;123:371–377. doi: 10.1007/s00702-015-1502-5. [DOI] [PubMed] [Google Scholar]
- 26.Varanese S, Perfetti B, Ghilardi MF, Di Rocco A. Apathy, but not depression, reflects inefficient cognitive strategies in Parkinson’s disease. PLoS One. 2011;6:e17846. doi: 10.1371/journal.pone.0017846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moccia M, Pappatà S, Erro R, Picillo M, Vitale C, Amboni M, et al. Uric acid relates to dopamine transporter availability in Parkinson’s disease. Acta Neurol Scand. 2015;131:127–131. doi: 10.1111/ane.12295. [DOI] [PubMed] [Google Scholar]
- 28.Santangelo G, Vitale C, Picillo M, Cuoco S, Moccia M, Pezzella D, et al. Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat Disord. 2015;21:489–493. doi: 10.1016/j.parkreldis.2015.02.015. [DOI] [PubMed] [Google Scholar]


