Abstract
Corticosteroid use was associated with development of Kaposi’s sarcoma or multicentric Castleman disease in 3 patients with mycobacterial immune reconstitution inflammatory syndrome (IRIS) treated with corticosteroids. Monitoring for development of Kaposi’s sarcoma and alternative treatment may be beneficial for patients with IRIS, especially in the presence of preexisting co-infection with Kaposi’s sarcoma–associated herpesvirus.
Keywords: KSHV, TB-IRIS
Tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) is an inflammatory response in HIV-infected patients that presents as a paradoxical worsening of clinical manifestations or an unmasking of clinically silent infection upon initiation of antiretroviral therapy (ART). When attempting to diagnose TB-IRIS, other infectious diagnoses must be excluded as the treatment of TB-IRIS usually requires immunosuppression with corticosteroids.
Kaposi’s sarcoma is caused by Kaposi’s sarcoma–associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8). KSHV seroprevalence varies by patient population and geographic region, with increased prevalence noted in men who have sex with men and in Sub-Saharan Africa [1]. The risk of developing KS is increased with lower CD4 counts and higher HIV viremia; however, it may be seen in patients well controlled on ART with preserved CD4 counts [1].
Corticosteroids can reduce morbidity and symptoms in patients with severe TB-IRIS [2]; however, their use may have unintended consequences. Corticosteroid use has been associated with worsening of Kaposi’s sarcoma (KS) [3] and the development of KS [4] and KS-IRIS, an unmasking or paradoxical worsening of KS upon starting antiretroviral therapy [5]. It should be noted that not all studies have shown an increased risk of KS or KS-IRIS with steroids [2, 6], possibly due to underlying factors predicting increased risk for KS, such as ART status, study location, and exclusion of patients with preexisting KS [2–4, 6].
Here we describe 3 AIDS patients with TB-IRIS who unmasked KSHV-associated disorders during treatment with corticosteroids. The patients were participants of National Institutes of Health (NIH) institutional review board–approved protocols, Immune Reconstitution Syndrome in HIV-Infected Patients Taking Antiretroviral Therapy (IRIS; NCT00286767), or positron emission tomography (PET) imaging and lymph node biopsy in patients with AIDS starting an antiretroviral therapy protocol (PANDORA; NCT02147405). All had given informed consent.
Patient 1
A 34-year-old Liberian woman presented with weakness, weight loss, and dyspnea of several months’ duration. She was diagnosed with HIV-1 and had diffuse ground glass infiltrates on chest x-ray. Bronchoscopy revealed rifampin-resistant miliary TB, and she was started on isoniazid, levofloxacin, amikacin, ethambutol, and pyrazinamide. She was transferred to the NIH, and upon enrollment, her CD4+ T-cell count was 49 cells/µL (4%) and her plasma HIV-RNA viral load was 885 182 copies/mL.
Six weeks after ART initiation with tenofovir/emtricitabine/raltegravir, she developed fevers and altered mental status due to hypercalcemia attributed to extensive granulomas. She had a negative infectious work-up, virologic response on ART, and elevated serum inflammatory biomarkers, which led to diagnosis of paradoxical TB-IRIS (Figure 1A). She had initial improvement with intravenous hydration and corticosteroids (methylprednisolone 60 mg daily); however, 4 days after corticosteroid initiation, she developed new painful skin lesions that were biopsied and diagnosed as KS (Figure 1A). The KS lesions initially improved with tapering of corticosteroids. Persistent TB-IRIS symptoms with fevers, elevated inflammatory markers, and hypercalcemia required a prolonged course of additional prednisone. Given worsening cutaneous KS lesions while on prednisone, she received 2 doses of liposomal doxorubicin (20 mg/m2), with resolution of her KS lesions. Prednisone was eventually discontinued, and she achieved immune reconstitution (CD4 count of 671 cells/µL at week 192) and virologic suppression, without further TB-IRIS flares or recurrence of KS.
Figure 1.
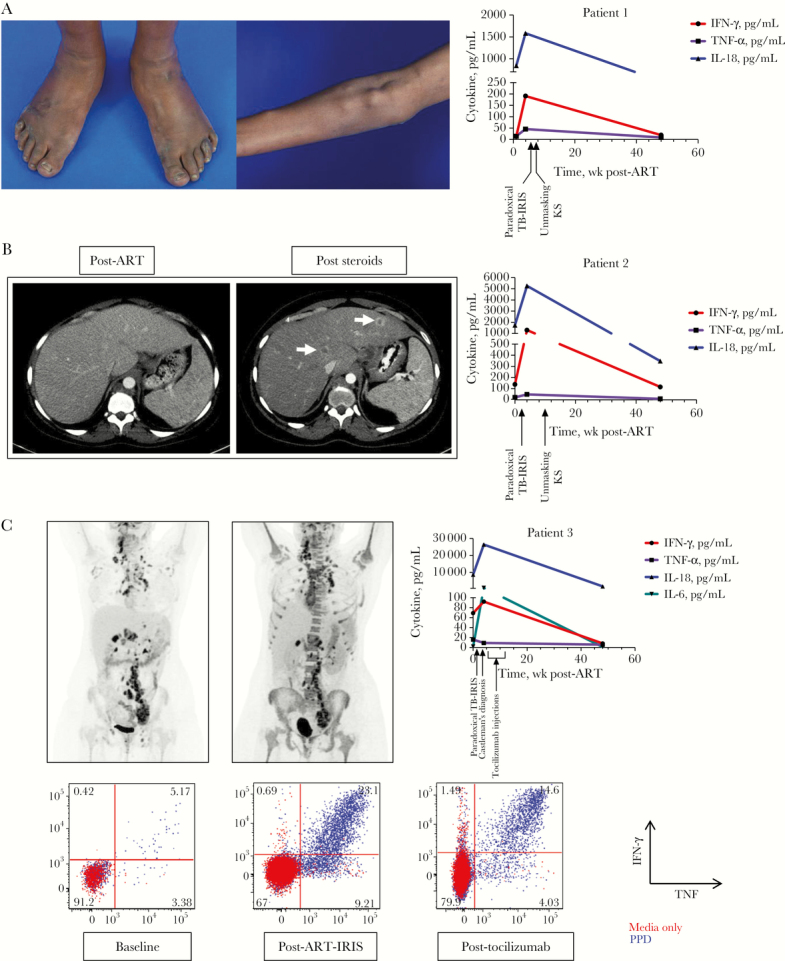
A, Kaposi’s sarcoma skin lesions and nodules and plasma levels of inflammatory cytokines of patient 1. B, Computed tomography scan of liver post–antiretroviral therapy (ART) and after initiation of prednisone and plasma levels of inflammatory cytokines of patient 2. C, Positron emission tomography scan pre- and post-ART and cytokine production of CD4 T cells after in vitro stimulations of peripheral blood mononuclear cells with tuberculin purified protein derivative (PPD) of patient 3. Plasma inflammatory markers were measured using a multi-array electrochemiluminescence assay (Meso Scale Discovery), and T-cell responses to PPD were assessed using flow cytometry. Abbreviations: IFN, interferon; IL, interleukin; KS, Kaposi’s sarcoma; TB-IRIS, tuberculosis immune reconstitution inflammatory syndrome; TNF, tumor necrosis factor.
Patient 2
A 33-year-old Cameroonian woman presented with fevers, night sweats, and cough. Her CD4 count was 93 cells/µL, with a plasma HIV-RNA viral load of >10 million copies/mL. Imaging showed retroperitoneal adenopathy and a miliary pattern in her lungs. Bronchoscopy was diagnostic for Mycobacterium tuberculosis (MTB) with rifampin and isoniazid resistance. She was treated and improved on isoniazid (subsequently discontinued), pyrazinamide, ethambutol, moxifloxacin, and amikacin.
She enrolled in an IRIS protocol and started ART 3 weeks later with tenofovir/emtricitabine/raltegravir. One week later, she developed fevers. Her clinical presentation, negative infectious work-up, and elevated serum biomarkers (and peak serum IL-6 value of 3.8 pg/mL) (Figure 1B) were consistent with TB-IRIS. She was started on prednisone 60 mg daily with subsequent clinical and radiographic improvement.
Three months later, while on prednisone taper, she developed new fevers and was found to have new ring-enhancing lesions in the liver (Figure 1B) and scattered purplish lesions over her bilateral extremities. A liver biopsy was consistent with KS. Corticosteroids were weaned quickly while ART was continued. She had resolution of cutaneous KS and liver lesions and immunologic recovery, with a CD4 count of 425 and suppression of plasma viremia.
Patient 3
A 30-year-old Cameroonian woman presented with 2 weeks of fever, neck swelling, and weight loss. She was found to have necrotizing lymphadenitis of her right cervical and occipital nodes. An excisional biopsy revealed sensitive MTB. She was also diagnosed with HIV-1 with a CD4 count of 6 cells/µL and plasma HIV-1 RNA of 980 181 copies/mL.
She was initiated on rifampin, isoniazid, pyrazinamide, ethambutol, and pyridoxine therapy with trimethoprim-sulfamethoxazole and azithromycin for prophylaxis. She enrolled in the PANDORA protocol. Efavirenz/tenofovir/emtricitabine were initiated after 4 weeks of TB therapy.
Her baseline workup was notable for hepatitis B (hepatitis B DNA viral load of 6111 c/mL) and positive KSHV by qualitative polymerase chain reaction (PCR) of the blood. On day 5 of ART, she developed high fevers, headache, and worsening right cervical lymphadenopathy. Work-up with imaging, blood, and cerebrospinal fluid cultures showed no new infectious processes. She was diagnosed with paradoxical TB-IRIS and started on prednisone 60 mg daily. In vitro testing of her blood demonstrated polyclonal cytokine responses to MTB consistent with TB-IRIS (Figure 1C). Her course was complicated by anemia requiring blood transfusions. Upon initiation of prednisone, she had immediate improvement of her symptoms.
In her fourth week of ART, she returned with recurrent fevers, abdominal discomfort, malaise, nausea, and diarrhea. Clostridium difficile colitis was diagnosed, and her diarrhea improved with treatment; however, she remained febrile and anemic (hemoglobin 7.2 g/dL) despite continuing on prednisone at 40 mg daily. She was also thrombocytopenic (platelet count 69 K/µL) with a C-reactive protein of 210.8 mg/L [5]. She underwent a PET–computed tomography scan, per protocol, which revealed increased bone marrow 18F-fluorodeoxyglucose (FDG) uptake and persistent FDG-avid lymphadenopathy (Figure 1C). An inguinal lymph node biopsy performed for research was consistent with Kaposi’s sarcoma herpesvirus–associated multicentric Castleman’s disease (KSHV-MCD). Her peripheral blood mononuclear cell (PBMC)–associated KSHV viral load was 98 555 c/million cells.
She was enrolled in a clinical trial for KSHV-MCD through the National Cancer Institute HIV/AIDS Malignancy Branch and treated with tocilizumab 8 mg/kg every 2 weeks for 6 cycles for KSHV-MCD (NCT01441063), with the aim of treating both the KSHV-MCD and the paradoxical TB-IRIS symptoms. Upon initiation of tocilizumab, she had resolution of inflammatory symptoms and became transfusion-independent. Prednisone was tapered and discontinued within 2 weeks, and she required surgical drainage by interventional radiology/otolaryngology of necrotic lymph nodes that had formed abscesses secondary to TB-IRIS. Her PBMC-associated KSHV viral load became undetectable. She completed TB therapy, her CD4 count improved to 312 cells/µL at week 96, and her KSHV-MCD remains in remission.
DISCUSSION
We have presented 3 cases of KSHV-mediated disease in TB-IRIS patients that unmasked on ART shortly after corticosteroid therapy. It is important to note that KSHV-associated disease can still present even while the patient is on ART, and we would encourage providers to be mindful of this diagnosis, particularly in high-risk populations.
There are limited studies evaluating unmasking KS-IRIS [5] or KSHV-MCD-IRIS [7], and more studies are needed to characterize this phenomenon. The development of KS-IRIS following corticosteroids has been observed [5], but the development of KSHV-MCD following initiation of prednisone has not been previously reported. The failure to recognize KSHV-MCD in this setting may be in part due to the difficulty in clinically differentiating it from TB-IRIS and the requirement of histology to make the diagnosis.
Given the prevalent use of corticosteroids for treatment of IRIS, clinicians should be vigilant for KS and other KSHV-associated disorders as these cases may be unveiled in patients at risk for KSHV infection [2, 5]. Clinicians may also consider screening with KSHV PCR as it has been shown that KSHV viremia can precede the development of KS and the viremia is elevated at the time of diagnosis of KSHV-MCD [8–10].
Corticosteroids remain the best therapeutic option for TB-IRIS as they are clinically effective [2], inexpensive, and widely available. However, we would pose that the risk of developing a KSHV-related disorder with corticosteroid therapy highlights the need for more research into targeted therapeutic options for IRIS. In addition, consideration should be given to agents that could treat both processes, specifically anti-IL-6, given the prominent role that IL-6 is thought to play in the immunopathogenesis of both IRIS and KSHV-MCD [11, 12].
This is the first case to our knowledge of tocilizumab being used for IRIS. Our patient did well following tocilizumab; anti-IL6 therapy, especially if oral alternatives become available, should be further studied for TB and KS-IRIS.
In conclusion, KSHV-related disorders can be associated with corticosteroid use for HIV-associated TB-IRIS. This underscores the need to be vigilant for such complications and, where feasible, to consider monitoring for KSHV in high-risk patients who are likely to require corticosteroid therapy. In addition, it emphasizes the continuous need for development of targeted therapies for IRIS with more specific immunosuppressive effects.
Acknowledgments
The authors thank the study participants and the staff of the outpatient clinic 8 and the inpatient ward team.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Disease and National Cancer Institute, National Institutes of Health, and by US federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhutani M, Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi sarcoma-associated herpesvirus-associated malignancies: epidemiology, pathogenesis, and advances in treatment. Semin Oncol 2015; 42:223–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill PS, Loureiro C, Bernstein-Singer M, et al. Clinical effect of glucocorticoids on Kaposi sarcoma related to the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1989; 110:937–40. [DOI] [PubMed] [Google Scholar]
- 4. Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis 2004; 190:869–78. [DOI] [PubMed] [Google Scholar]
- 5. Fernández-Sánchez M, Iglesias MC, Ablanedo-Terrazas Y, et al. Steroids are a risk factor for Kaposi’s sarcoma-immune reconstitution inflammatory syndrome and mortality in HIV infection. AIDS 2016; 30:909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gallant JE, Chaisson RE, Moore RD. The effect of adjunctive corticosteroids for the treatment of Pneumocystis carinii pneumonia on mortality and subsequent complications. Chest 1998; 114:1258–63. [DOI] [PubMed] [Google Scholar]
- 7. Siegel MO, Ghafouri S, Ajmera R, Simon GL. Immune reconstitution inflammatory syndrome, human herpesvirus 8 viremia, and HIV-associated multicentric Castleman disease. Int J Infect Dis 2016; 48:49–51. [DOI] [PubMed] [Google Scholar]
- 8. Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 2000; 96:2069–73. [PubMed] [Google Scholar]
- 9. Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet 1995; 346:799–802. [DOI] [PubMed] [Google Scholar]
- 10. Moore PS, Kingsley LA, Holmberg SD, et al. Kaposi’s sarcoma-associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS 1996; 10:175–80. [DOI] [PubMed] [Google Scholar]
- 11. Narendran G, Andrade BB, Porter BO, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One 2013; 8:e63541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013; 122:4189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]


