Abstract
Aims
Examination of long-term results following different treatments in hypertrophic obstructive cardiomyopathy (HOCM) in a complete geographical cohort.
Methods and results
HOCM patients attending during 2002–13 in all 10 hospitals in the West Götaland Region, Sweden, were identified (n = 251), follow-up 14.4 (±8.9) years (mean ± SD), 121 managed medically, 42 treated with myectomy and 88 with short atrioventricular (AV) delay pacing as first interventional procedure. Post-intervention follow-up was 12.9 ± 8.7 years and 12.2 ± 5.0 years, respectively. Both intervention treatments improved New York Heart Association (NYHA) class and outflow gradients significantly. Patients treated with pacing were older (median age 64 vs. 43 years, P < 0.001). Freedom from disease-related death post-procedure at 5, 10, and 20 years were 93%, 80%, 56% vs. 93%, 93%, 57% in pacing and myectomy groups, respectively (log-rank P = 0.43). Survival after diagnosis was not different in patients just treated conservatively (P = 0.51 pacing/conservative; P = 0.39 myectomy/conservative). Reintervention for outflow gradients in patients ≥18 years at procedure occurred in 3.5% in pacing group and 15.6% in myectomy group (P = 0.007). Pacing therapy was equally effective in patients aged 13–64 years (n = 44), as in patients ≥65 years (n = 44): resting gradient pre-procedure and at last follow-up were median (IQR) 65 (71) and 12 (20) mmHg for <65 year-olds (P < 0.001), and 75 (64) and 14 (38) mmHg, respectively, for ≥65 year-olds (P < 0.001). New York Heart Association class improved significantly in both age ranges to 1.6 ± 0.6 and 1.8 ± 0.7, respectively (P < 0.001; P < 0.001).
Conclusion
Short AV delay pacing provided lasting satisfactory relief of symptoms and outflow obstruction in the majority of patients, with low risk of requiring reintervention. Our findings support the view that pacing therapy should be considered a valid option to treat patients with HOCM.
Keywords: Hypertrophic obstructive cardiomyopathy, Myectomy, Pacing, Survival, Reintervention rate
What’s new?
This is the largest study so far with very long-term results after short atrioventricular (AV) delay pacing therapy, with 100% complete follow-up and comparison with surgical myectomy patients in the same geographical cohort.
Long-term survival after pacing is not inferior to that after myectomy.
Gradient control is maintained long-term after pacing, and New York Heart Association class improved to the same degree as after myectomy.
Pacing therapy was equally effective in patients aged 13–64 years as in the ≥65-year-old age group.
The rate of reintervention for recurring outflow tract obstruction in our patient cohort was significantly higher after myectomy than after pacing therapy in patients ≥18 years at the procedure.
Currently, short AV delay pacing has been relegated to a last-choice option in international guidelines. We believe that this is not justified on available evidence and that the procedure should still be considered a valid first-line option even in patients <65 years of age, particularly if an implantable cardioverter defibrillator implantation is planned.
Introduction
Hypertrophic cardiomyopathy (HCM) is frequently associated with muscular left ventricular outflow obstruction (LVOTO) and then termed hypertrophic obstructive cardiomyopathy (HOCM). The severity of obstruction is a risk factor for death and symptomatic deterioration.1 In the American Heart Association (AHA) guidelines, surgical myectomy is considered the gold standard management for symptomatic HOCM resistant to medical therapy.1 Short atrioventricular (AV) delay pacing in DDD-mode also provides haemodynamic and symptomatic improvement.2–4 There are no randomized controlled studies comparing surgical myectomy with short AV delay pacing. Published long-term follow-up studies all arise from highly specialized tertiary centres and do not have comparison groups with similar age profile.5,6 We have therefore assessed survival and event-free survival stratified for treatment mode in a total geographical cohort of HOCM-patients.
Methods
Cardiac care for the 1.6 million inhabitants of the West Götaland Region in Sweden is provided by 10 hospitals (The Sahlgrenska, Östra, Mölndal, Uddevalla, Trollhättan, Skövde, Lidköping, Alingsås, Borås, and Kungälv Hospitals), supplying adult cardiological services for separate districts. Cardiac surgery was carried out at the Sahlgrenska University Hospital, Gothenburg, and short AV delay pacing was also undertaken in Uddevalla, Trollhättan, and Skövde hospitals. We searched all hospital diagnostic databases for all patients attending adult hospital services from January 2002 through December 2013 with diagnostic codes relating to HCM and to myectomy. In addition, we identified 10 patients who had myectomy after infancy but <18 years of age, but who at last follow-up were adults (n = 6) or teenagers (n = 4). All hospital records were reviewed on-site by two investigators (D.J. and I.Ö.S.) to identify those with primary HCM and some degree of LVOTO. Vital status was censored on 28 February 2015.
The diagnosis of HCM was based on two-dimensional echocardiographic demonstration of a hypertrophied (wall thickness ≥ 15 mm) and non-dilated left ventricle (LV) in the absence of another disease capable of producing the magnitude of hypertrophy present.7 Clinical features and electrocardiography (ECG) and ultrasound measurements were documented at pre-procedure, and at last follow-up visit, as well as medical therapy and interventional procedures during follow-up.
Patients were classified as HOCM if they had an LVOTO pressure gradient of ≥30 mm Hg at rest.7
During the study period, 1141 patients with primary HCM were identified. LVOTO at rest (HOCM) was present in 251 (22%) patients, 128 male and 123 female, with a mean follow-up time of 14.4 (SD ± 8.9) years from the time of original diagnosis. All patients were initially treated medically, and 121 patients remained on medical therapy only (Conservative group). Of those having insufficient control of gradients or symptoms on medical therapy, 88 patients were treated with short AV delay pacing, and 42 patients underwent a surgical myectomy (Table 1). The first choice of intervention for patients <18 years of age was myectomy. However, four patients (all now adults) received pacing therapy <18 years of age: in patients aged 13–17 years where an ICD was indicated (three primary, one secondary prophylaxis) and the ICD also was used for short AV delay pacing (two of four had unsatisfactory results from a prior myectomy). For patients aged ≥18 years, first-line procedure was determined by the responsible physician and patient preference.
Table 1.
Characterization of treatment groups at baseline,
| Groups | ||||||
|---|---|---|---|---|---|---|
| Variables | Conservative (Con) | Pacing | Myectomy (Mye) |
P-value |
||
| Con vs. pacing | Con vs. mye | Pacing vs. mye | ||||
| n (Female %) | 121 (48) | 88 (52) | 42 (45) | 0.57 | 0.22 | 0.45 |
| Age at diagnosis (years) | ||||||
| Mean ± SD | 53.9 ± 20.6 | 55.3 ± 18.3 | 32.1 ± 23.0 | |||
| Median (IQR) | 59.0 (23.0) | 59.0 (23.0) | 34.4 (40.0) | 0.872 | <0.001 | <0.001 |
| Post-diagnosis FU (years) | ||||||
| Mean ± SD | 11.3 ± 7.5 | 16.4 ± 8.4 | 19.2 ± 10.2 | |||
| Median (IQR) | 9.5 (9.1) | 14.5 (12.9) | 16.3 (16.8) | <0.001 | <0.001 | 0.131 |
| NYHA class | ||||||
| Mean ± SD | 2.1 ± 0.8 | 2.3 ± 0.6 | 2.0 ± 0.8 | |||
| Median (IQR) | 2 (1) | 2 (1) | 2 (1) | 0.350 | 0.242 | 0.050 |
| Beta-blocker therapy, n (%) | 88 (73) | 59 (67) | 32 (76) | 0.375 | 0.830 | 0.206 |
| Metoprolol dose (mg/day)a | 200 (200) | 100 (100) | 100 (125) | 0.017 | 0.006 | 0.174 |
| Verapamil/diltiazem, n (%) | 14 (12) | 14 (16) | 3 (7) | 0.363 | 0.920 | 0.153 |
| LVOT gradient (mmHg) | 61 (54) | 64 (66) | 73 (64) | 0.663 | 0.048 | 0.149 |
| LVOT gradient >50 mmHg, n (%) | 73 (58) | 60 (68) | 34 (79) | 0.128 | 0.013 | 0.194 |
| Septum (mm) , n (%) | 18 (5) | 20 (5) | 18 (7) | 0.031 | 0.429 | 0.051 |
| Posterior LV wall (mm), n (%) | 12 (3) | 13 (3) | 12 (5) | 0.147 | 0.772 | 0.527 |
| LVEDD (mm), n (%) | 43 (9) | 46 (10) | 42 (7) | 0.047 | 0.233 | 0.021 |
| LAD (mm), n (%) | 40 (9) | 44 (13) | 40 (11) | <0.001 | 0.762 | 0.013 |
| LVEF (%), n (%) | 69 (15) | 69 (16) | 68 (16) | 0.665 | 0.651 | 0.651 |
Values are represented as median (IQR) where not otherwise specified. P-values: Mann–Whitney U test (inter-group) and χ2 test (categorical variables).
Con, Conservative, medical therapy only; FU, follow-up; IQR, interquartile range; LAD, left atrium diameter; LV, left ventricle; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; n, number; NYHA, New York Heart Association Class; SD, standard deviation.
Metoprolol was the most common beta-blocker used, and thus doses of other beta-blockers have been converted to metoprolol equivalents. P-values in bold indicate significance.
Dual-chamber permanent pacemakers were implanted with fluoroscopy and echocardiography verification that the ventricular electrode tip was placed optimally in the apex of the right ventricle. Atrioventricular delay was set under ECG control to ensure abolition of spontaneous conduction and then evaluated by echocardiography to obtain maximal LVOT gradient reduction without deterioration of diastolic filling.3 Septal myectomy was performed as described by Robbins and Stinson.8
No patients were lost to follow-up, as all inhabitants in Sweden are provided with a unique personal identification number; all death certificates were obtained to establish the causes of death. The primary endpoint was disease-related death (henceforth: ‘cardiac mortality’), in which we included death due to heart failure, arrhythmia, death-certificate classified sudden deaths, bacterial endocarditis (n = 1), fatal strokes in patients with atrial fibrillation and due to embolism from a presumed cardiac source, and cardiac transplantation that was considered a death equivalent. The secondary endpoint was reintervention for residual LVOTO. The study complies with the Declaration of Helsinki and was approved by local ethics committee of the University of Gothenburg (ÖS no 1012-12).
Statistical analysis
Parameters without normal distribution are represented with median [interquartile range (IQR)] in Tables 1–4 and normally distributed by mean ± standard deviation. Statistical comparisons were performed by the Mann–Whitney U test for continuous measures, and by the Fisher’s exact test and the χ2 test for categorical measurements as appropriate. Paired data were analysed by Wilcoxon signed rank test. Survival and hazard of reintervention were analysed by the Kaplan–Meier curves and log-rank test. All tests were two sided, and P-values <0.05 were considered statistically significant. Analysis was carried on SPSS software (version 22.0; IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
There was no significant gender imbalance between treatment groups (Table 1). Myectomy patients were nearly 25 years younger than patients treated with medical therapy alone and patients receiving pacemaker treatment (P < 0.001). Thus, the myectomy group contains many patients with early onset of obstruction. The conservatively treated group on the other hand had a comparable age profile with the pacing group, although it has less severe septal hypertrophy (P = 0.031) and left atrial enlargement (P = 0.001) compared with the pacing group, so probably contains milder cases. The LVOT gradients at rest were comparable between myectomy and pacing groups but significantly lower in the conservative group when compared with myectomy group. Septal hypertrophy was slightly more pronounced in the group treated with pacing, and LV end-diastolic diameter and left atrial diameter significantly greater than in both myectomy group and conservative group. The New York Heart Association (NYHA) class at diagnosis tended to be worse in the pacing group compared with the myectomy group (P = 0.05). One difference between the patients who did not need to proceed to an intervention procedure and the two interventional groups was that the group for whom medical treatment sufficed had significantly higher initial beta-blocker dose (expressed in metoprolol equivalents), median 200 mg metoprolol daily vs. 100 mg daily (P = 0.002), when compared with interventional groups combined, and P = 0.017 compared with the pacing group. Verapamil doses did not differ between groups.
Effect of therapy
Values are shown in Table 2 and are considered group by group below. In the total cohort, there were 65 disease-related deaths (cardiac mortality), and 25 non-cardiac deaths from unrelated causes. Mean duration of post-procedure follow-up was similar in both intervention groups (Table 2).
Table 2.
Findings at last follow-up in the treatment groups, values as median (IQR) where not otherwise specified
| Groups | ||||||
|---|---|---|---|---|---|---|
| Variables | Conservative (Con) n = 121 | Pacing n = 88 | Myectomy (Mye) n = 42 |
P-value |
||
| Con vs pacing | Con vs mye | Pacing vs mye | ||||
| Age at intervention (years) | ||||||
| Median (IQR) | 64 (22) | 43 (41) | <0.001 | |||
| Post-intervention FU (years) | ||||||
| Mean ± SD | 12.2 ± 5.0 | 12.9 ± 8.7 | ||||
| Median (IQR) | 11.8 (9.1) | 10.5 (12.4) | 0.81 | |||
| Cardiac mortality. n (%) | 25/121 (21) | 25/88 (28) | 15/42 (36) | 0.51 L-R | 0.31L-R | 0.45L-R |
| Annual cardiac mortality from diagnosis (%) | 1.8 | 1.7 | 1.9 | |||
| Annual sudden death mortality from diagnosis (%) | 0.44 | 0.21 | 0.63 | |||
| Annual cardiac mortality post-procedure (%) | 2.4 | 2.8 | ||||
| NYHA class | ||||||
| Mean ± SD | 1.9 ± 0.81a | 1.7 ± 0.7b | 1.8 ± 0.6a | |||
| Median (IQR) | 2 (1) | 2 (1) | 2 (1) | 0.21 | 0.70 | 0.43 |
| Beta-blocker use (%)c | 88 | 85 | 83 | 1.0 | 0.62 | 0.79 |
| Metoprolol dose (mg/day)d | 200 (150) | 150 (100) | 100 (150)e | 0.053 | 0.037 | 0.280 |
| Verapamil/diltiazem use (%)c | 12 | 17 | 5 | 0.342 | 0.161 | 0.057 |
| Disopyramide use (%)c | 12 | 7 | 24 | 0.192 | 0.163 | 0.016 |
| ICD implantations, n (%) | 5 (4) | 5 (6) | 7 (17) | 0.601 | 0.009 | 0.048 |
| LVOT gradient (mmHg)f | 23 (42)b | 14 (32)b | 11 (16)b | 0.013 | 0.003 | 0.381 |
| LVOT gradient >50 mmHg (%) | 24 | 17 | 14 | 0.23 | 0.19 | 0.92 |
| Septum (mm) | 20 (5) | 18 (5)a | 16 (7) | 0.17 | 0.002 | 0.013 |
| Posterior LV wall (mm) | 13 (5) | 13 (5) | 11 (4)a | 0.67 | 0.002 | 0.004 |
| LVEDD (mm) | 43 (7) | 45 (9) | 45 (11)a | 0.011 | 0.019 | 0.60 |
| LAD (mm) | 47 (10)b | 51 (14)b | 49 (15)e | 0.011 | 0.954 | 0.124 |
| LVEF (%) | 65 (10) | 63 (12)e | 60 (18)b | 0.36 | 0.12 | 0.41 |
Values are represented as median (IQR) where not otherwise specified.
Con, Conservative, medical therapy only; FU, follow-up; ICD, implantable cardioverter defibrillator; IQR, interquartile range; L-R, log-rank test of Kaplan–Meier analysis; LAD, left atrium diameter; LVEF, left ventricular ejection fraction; LVEDD, left ventricle end-diastolic diameter; LVOT, left ventricular outflow tract; Mye, myectomy; NYHA, New York Heart Association Class; PM, pacemaker; SD, standard deviation. Mann Whitney U test was used for inter-group comparisons unless otherwise specified. P-values in bold indicate significance.
Significant on paired test vs. pre-treatment values: P ≤ 0.01–0.002.
Significant on paired test vs. pre-treatment values: P ≤ 0.001. Mann–Whitney U test was used for inter-group comparisons unless otherwise specified.
χ2 test.
Metoprolol was the most common beta-blocker used, and thus doses of other beta-blockers have been converted to metoprolol equivalents.
Significant on paired test vs. pre-treatment values: P < 0.05–0.02.
Final result after any re-intervention.
Conservative group
There was significant improvement in NYHA class (P = 0.006) and significant reduction in LVOT gradient at last follow-up to a median of 23 (42) mm Hg (P < 0.001). Nevertheless, some patients had suboptimal results and 29 of 121 had gradients of >50 mm Hg at last follow-up. There was no reduction in ventricular hypertrophy. Left ventricular-end-diastolic diameter remained unchanged, but left atrial size increased (P < 0.001). Proportion receiving beta-blocker therapy increased from 73% to 88%, other medication is detailed in Table 2. The conservatively treated group had freedom from cardiac death of 94%, 82%, and 73% respectively (see Figure 1) at 5, 10, and 20 years, and the survival is no different from the intervention groups on log-rank testing (P = 0.51 pacing/conservative; P = 0.39 myectomy/conservative).
Figure 1.
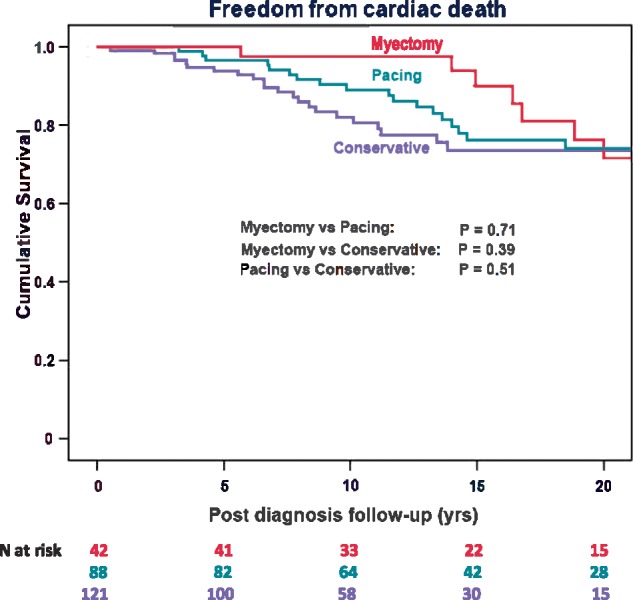
Kaplan–Meier survival curves of cardiac mortality (according to the defined primary endpoints in Methods) from diagnosis in the total cohort of HOCM patients managed. Conservative group (blue), short atrioventricular delay pacing (green), and myectomy (red). N at risk refers to number of patients left in Kaplan–Meier curve. As myectomy group is 25 years younger at the time of diagnosis, the lower attrition rate during early follow-up is to be expected but rapidly increases during later follow-up.
Short atrioventricular delay pacing
Short atrioventricular (AV) delay pacing also improved the NYHA class significantly (P < 0.001) and achieved good long-term relief of LVOTO in the majority of the patients (median 14 mm Hg). In 96.6% of patients, this was achieved at the initial procedure. Residual gradients during follow-up (range 50–120 mmHg) required a further intervention on the LVOT in 3 of 88 patients [3.4%; 1 myectomy, 2 alcohol septal ablation (ASA)]. None of those were among the four patients between 13 and 17 years of age, where final follow-up gradients were 4, 4, 10, and 16 mmHg, respectively. In the total pacing cohort, 17% had a gradient ≥50 mmHg at last follow-up, mostly very elderly [median age 82 (IQR 25) years], and 47% had atrial fibrillation rendering consistent pre-excitation of the ventricle difficult. Septal hypertrophy was significantly reduced (P = 0.012). Left ventricular end-diastolic diameter was unchanged, but left atrial size increased (P < 0.001). Beta-blocker use increased from 67% to 85% during follow-up. Post-procedural annual sudden death mortality was 0.28%.
In a previous study, it was suggested that the beneficial effect of pacing occurs only in patients >65 years of age,9 and in Table 3, we therefore compare the results of pacing in patients <65 years and ≥65 years at procedure. There are fewer females among the <65-year-olds (P = 0.0028). Beta-blocker treatment from diagnosis was more common in the younger group (P = 0.023), and a higher dose was used (P < 0.001), but at last follow-up, beta-blocker use had increased in the older pacing group and the difference was no longer significant. In both age groups, there were significant improvements after pacing in both outflow tract gradients (P < 0.001 for both) and NYHA class (P < 0.001 for both). Final results in the <65 year-old group was a median gradient of 12 (20) mmHg, and only 9.5% had residual gradient ≥50 mmHg. Thus treatment effect was in no way inferior in <65-year-olds. Unsurprisingly, cardiac mortality was significantly lower in the younger group on log-rank testing (P = 0.006), with post-procedural annual cardiac mortality of 1.3% vs. 3.6%, and annual sudden death mortality of 0.16% and 0.43%, respectively. Freedom from cardiac death post-procedure at 5, 10, and 20 years were 93%, 80%, and 56%, respectively, in the whole pacing group, but in <65-year-olds 10-year freedom was 86%. Corresponding all-cause survival were 91%, 70%, 37% in the whole group and 10-year survival in <65-year-olds 83%.
Table 3.
Comparison of results in pacing therapy according to age, values as median (IQR) where not otherwise specified
| Variables | <65 years at procedure (n = 44) | 65 years at procedure (n = 44) | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Female, n (%) | 16 (36) | 30 (68) | 0.0028 | |||||
| Age at diagnosis (years) | ||||||||
| Mean ± SD | 42 ± 16 | 68 ± 8 | ||||||
| Median (IQR) | 47 (21) | 68 (13) | <0.001 | |||||
| Age at intervention (years) | ||||||||
| Mean ± SD | 48.1 ± 13.0 | 73.3 ± 6.2 | ||||||
| Median (IQR) | 51.5 (16.5) | 72.5 (8.0) | <0.001 | |||||
| Post intervention FU (years) | ||||||||
| Mean ± SD | 13.9 ± 6.1 | 10.6 ± 5.4 | ||||||
| Median (IQR) | 13.6 (9.6) | 9.4 (9.4) | 0.008 | |||||
| Cardiac mortality n (%) | 8/44 (18) | 17/44 (39) | 0.006 (L-R) | |||||
| Annual cardiac mortality from diagnosis (%) | 1.0 | 2.7 | ||||||
| Annual cardiac mortality post-procedure (%) | 1.3 | 3.6 | ||||||
| Annual sudden death mortality post-procedure (%) | 0.16 | 0.43 | 0.34 (L-R) | |||||
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value |
P value <65 vs. ≥65 |
||
| Baseline | Follow-up | |||||||
| Beta-blocker use (%) | 80 | 91 | 0.13 | 55 | 82 | 0.006 | 0.023 | 0.21 |
| Metoprolol dose (mg/day)a | 175 (100) | 187.5 (100) | 0.46 | 100 (150) | 100 (125) | 0.32 | <0.001 | 0.010 |
| NYHA class | ||||||||
| Mean ± SD | 2.8 ± 0.7 | 1.6 ± 0.6 | 2.8 ± 0.5 | 1.8 ± 0.7 | ||||
| Median (IQR) | 3 (1)b | 2 (1) | <0.001 | 3 (1)b | 2 (1) | <0.001 | 0.79 | 0.32 |
| LVOT gradient (mmHg) | 65 (71)b | 12 (20) | <0.001 | 75 (64)b | 14 (38) | <0.001 | 0.08 | 0.42 |
| LVOT gradient >50 mmHg (proportion in %) | 28 (65)b | 4 (9.5) | <0.001 | 34 (79)b | 9 (22.0) | <0.001 | 0.15 | 0.12 |
| Septum (mm) | 20.0 (6.3) | 19.3 (6.0) | 0.14 | 20.0 (6.0) | 18.0 (4.0) | 0.08 | 0.18 | 0.029 |
| Posterior LV wall (mm) | 13.0 (3.0) | 13.0 (5.0) | 0.91 | 13.0 (3.5) | 13.0 (4.0) | 0.74 | 0.48 | 0.80 |
| LVEDD (mm) | 44 (10) | 44 (9) | 0.95 | 46 (10) | 43 (6) | 0.16 | 0.78 | 0.46 |
| LAD (mm) | 47 (11) | 52 (16) | 0.003 | 44 (12) | 48 (16) | 0.86 | 0.17 | 0.96 |
| LVEF (%) | 65 (15) | 61 (10) | 0.78 | 70 (15) | 65 (14) | 0.74 | 0.51 | 0.010 |
χ2 test for categorical variable and Mann–Whitney U test was used for inter-group-comparisons.
EF, ejection fraction; FU, follow-up; IQR, interquartile range; L-R, log-rank test of Kaplan–Meier analysis; LAD, left atrium diameter; LVEDD, left ventricle end-diastolic diameter; LVOT, left ventricular outflow tract; NYHA, New York Heart Association Class; SD, standard deviation. P-values in bold indicate significance.
Metoprolol was the most common beta-blocker used, and thus doses of other beta-blockers have been converted to metoprolol equivalents.
Pre-operation.
Myectomy group
Myectomy group also improved the NYHA class significantly (P = 0.011), and final reduction in outflow gradient (though in some cases only after reintervention) was good, median 11 (16) mmHg (P = 0.003), see Table 2. Patients treated with myectomy were much younger than the pacing group at the procedure. During follow-up, 9 of 42 patients in the myectomy group had a recurrence of outflow gradient (range 60–110 mmHg) and required a further LVOT intervention to obtain that final result (5 repeat surgery, 1 ASA, and 3 short AV delay pacing). At last follow-up, 14% had remaining gradient ≥50 mmHg. Septal thickness did not change significantly, but posterior wall thickness showed a significant decrease (P = 0.003). Left ventricular end-diastolic diameter (P = 0.002) and left atrial enlargement (P = 0.015) increased. Beta-blocker use increased from 76% to 83%. Annual cardiac mortality post-first myectomy was 2.8%, and post-procedure annual sudden death mortality was 0.92% in total myectomy group. Freedom from cardiac death post-procedure at 5, 10, and 20 years were 93%, 93%, and 57%, respectively, in the myectomy group (log-rank pacing/myectomy P = 0.43) and all-cause survival was 88%, 88%, and 51%, respectively.
Inter-group comparisons
All three groups showed some reduction in initial systolic hypercontractility as measured by ejection fraction (Table 2). The conservative group had less good final reduction of outflow gradient compared both with pacing group (P = 0.013) and myectomy group (P = 0.003) and was the only group not showing some significant reduction in hypertrophy. All three groups, however, developed increasing left atrial enlargement over time. Disopyramide therapy was used in a higher proportion in myectomy group (24%) than in pacing group (7%; P = 0.016). Survival after time of diagnosis, which for the intervention groups is longer than post-procedural survival, is illustrated in Figure 1 for all treatment groups (log-rank P = 0.39–0.71).
Inter-group comparison of patients adult at procedure
Four of those nine requiring reintervention after myectomy had the initial procedure at age <18 years. We therefore decided to focus any comparisons on outcome after pacing and surgery on patients aged ≥ 18 years at procedure (Table 4). In patients adult at myectomy repeat surgery for recurring LVOTO occurred in 4 of 32 (12.5%) during the first 5 years of follow-up with an additional one later giving median reintervention time of 1.9 years (range 0.5–9.5 years) later, a total surgical reoperation rate of 15.6%. In pacing ≥18 years group (n = 86), three reinterventions were required after 1.4–5.1 years, one myectomy, and two ASA procedures, with a total reintervention rate of 3.5%. A Kaplan–Meier analysis of hazard of reintervention in those groups confirms a significantly higher hazard in the adult myectomy group (P = 0.007).
Table 4.
Effect of therapy in the interventional groups with age ≥ 18 years at intervention, values as median (IQR) where not otherwise specified
| Groups | |||
|---|---|---|---|
| Variables | Pacing (n = 86) | Myectomy (n = 32) | P-value |
| Female n (%) | 45 (52) | 15 (47) | 0.68 |
| Age at diagnosis (years) | |||
| Mean ± SD | 56.4 ± 16.7 | 41.9 ± 18.0 | |
| Median (IQR) | 58.8 (21.9) | 42.8 (31.7) | <0.001 |
| Age at intervention (years) | |||
| Mean ± SD | 61.8 ± 14.8 | 48.8 ± 16.7 | |
| Median (IQR) | 65.0 (21.3) | 49.0 (26.0) | <0.001 |
| Total FU post diagnosis (years) | |||
| Mean ± SD | 16.2 ± 8.4 | 19.5 ± 10.4 | |
| Median (IQR) | 14.5 (13.0) | 16.3 (16.7) | 0.123 |
| Post-intervention FU (years) | |||
| Mean ± SD | 11.5 ± 5.7 | 12.9 ± 8.9 | |
| Median (IQR) | 11.3 (8.9) | 10.7 (15.5) | 0.92 |
| Cardiac mortality n (%) | 25 (29.1) | 10 (28.6) | 0.16 (L-R) |
| Annual cardiac mortality from diagnosis (%) | 1.8 | 1.5 | |
| Annual cardiac mortality post-procedure (%) | 2.5 | 2.2 | |
| Post-procedural sudden deaths on follow-up, n (%) | 2 (2.3) | 2 (6.3) | 0.78 (L-R) |
| Annual sudden death mortality post-procedure (%) | 0.20 | 0.48 | |
| Beta-blocker use (%) | 86 | 81 | 0.57 |
| Metoprolol dose mg/daya | 125 (100) | 100 (112.5) | 0.032 |
| Pre-procedural NYHA class | |||
| Mean ± SD | 2.7 ± 0.5 | 2.7 ± 0.7 | |
| Median (IQR) | 3 (0) | 3 (0) | 0.64 |
| NYHA class at last follow-up | |||
| Mean ± SD | 1.7 ± 0.69 | 1.8 ± 0.59 | |
| Median (IQR) | 2 (1) | 2 (1) | 0.26 |
| LVOT gradient pre-procedure (mmHg) | 69 (55) | 80 (54) | 0.022 |
| LVOT-gradient last follow-up (mmHg) | 14 (33) | 7 (16) | 0.12 |
| LVOT gradient >50 mmHg, n (%) | 15 (17) | 4 (13) | 0.59 |
| Septum (mm) | 19 (5) | 15 (7) | 0.006 |
| Posterior LV wall (mm) | 13 (5) | 11 (3) | 0.033 |
| LVEDD (mm) | 45 (9) | 47 (11) | 0.082 |
| LAD (mm) | 51 (14) | 51 (14) | 0.54 |
| LVEF (%) | 65 (10) | 60 (13) | 0.028 |
χ2 test for categorical variables and Mann–Whitney U test was used for inter-group-comparisons.
EF, ejection fraction; FU, follow-up; IQR, interquartile range; L-R, log-rank test of Kaplan–Meier analysis; LAD, left atrium diameter; LVEDD, left ventricle end diastolic diameter; LVOT, left ventricular outflow tract; NYHA, New York Heart Association Class; SD, standard deviation. P-values in bold indicate significance.
Metoprolol was the most common beta-blocker used, and thus doses of other beta-blockers have been converted to metoprolol equivalents.
Comparison between short atrioventricular delay pacing and myectomy therapy in patients of similar age range
It is noteworthy that the <65 years pacing group (Table 3) had a similar median age at procedure as the ≥18 years myectomy group (Table 4; 51.5 years vs. 49.0 years), and thus the most relevant mortality comparisons are between those two groups with <65 years pacing group having post-procedural cardiac annual mortality rate of 1.3% and post-procedural sudden death mortality rate of 0.28%, and the ≥18 years myectomy group having post-procedural cardiac annual mortality rate of 2.2% and sudden death mortality rate of 0.48%. Figure 2 shows the result on outflow gradients after the first procedure, prior to any reintervention, with total pacing group compared to similar age profile conservative group and ≥18 years myectomy group compared with <65 pacing group. There is no difference in relief of LVOTO between myectomy and pacing group (P = 0.89), but the conservative group has less good relief (P = 0.028).
Figure 2.

Final results after first intervention (not immediate post-intervention LVOTO gradients, but prior to any reintervention) compared in groups with most similar age profiles, namely conservative medical therapy (n = 121) vs. the total pacing group (n = 88), and pacing <65 years of age (n = 44) with myectomy ≥18 years of age (n = 32). Box-and-whisker plots, with box enclosing two middle quartiles and horizontal line showing the median, whiskers outlining upper and lower quartile values, and with outlier values as dots; conservative (blue), pacing (green), and myectomy (red).
Discussion
Surgical septal myectomy has long been considered the gold standard treatment for patients with HOCM that have severe remaining symptoms or significant LVOTO in optimal medical therapy.1,5,6 In 1992, it was shown that the outflow gradient in HOCM patients could be significantly reduced by short AV delay pacing,2 and a non-randomized study with 84 patients confirmed significant benefit on gradient and NYHA functional class.3 Subsequently, a European multicentre double-blind cross-over trial in 83 patients of active vs. inactive short AV delay pacing demonstrated that active pacing was associated with a significantly lower outflow gradient, reduction in symptoms, and improved NYHA class and quality of life with effect persisting at 1 year.4 A smaller American randomized, double-blind cross-over study with 48 patients confirmed significant benefit of pacing therapy on outflow gradient and quality of life score but without improved exercise ability.9 Another small non-randomized study compared 20 patients who, based on patient preference, were treated with myectomy with 19 patients treated by short AV delay pacing.10 This study reported significantly better reduction in gradient and greater improvement in exercise capacity in the myectomy group than in the pacing group. However, duration of follow-up in the study was longer for myectomy than pacing therapy patients, and the myectomy patients were on average 17 years younger, which may be relevant to their ability to increase exercise performance. These two studies were probably influential in short AV delay pacing being dropped from consideration as first-line intervention treatment in the AHA guidelines for HCM treatment.1 Pacing therapy continued to be fairly widely used in Europe, at least until the increasing adoption of ASA as an alternative treatment option for symptomatic LVOT obstruction.7
Long-term results after short atrioventricular delay pacing, and reason for discrepancies
Reports on pacing therapy in HOCM tend to include older patients than myectomy studies. Four of four pacing studies with greater than 2 years follow-up showed a satisfactory relief of LVOTO,4,11–13 similar to this study. Both Galve et al.13 and Lucon et al.12 documented that outflow gradients continued to fall, and exercise ability continued to improve, for more than 1 year after the pacing procedure. Lucon et al.12 reported 51 patients with an average age of 59 years, and follow-up of 11.5 years with survival rates at 5 and 10 years of 90% and 65%, respectively. Thus, our total pacing group of 88 patients is by far the largest hitherto reported in the literature with very long-term results after pacing therapy. The total survival, including non-cardiac deaths, in our pacing group was 91%, and 70% for survival at 5 and 10 years, similar to Lucon et al’s12 study. One smaller study of 12 months duration (M-PATHY) showed a less good gradient reduction in the 32 of 48 patients who completed the study.9 This study, however, had an unusual feature of the design in that after 3 months, there was an attempt to reduce or withdraw medical therapy so that at the end of the study drug therapy was reduced and three patients were drug free. Beta-blocker therapy markedly reduces exercise-induced increase in gradient and improves symptoms and exercise capacity as well as diastolic function as reviewed in Elliott et al.7. It is thus hardly surprising that if negatively inotropic drugs were withdrawn outflow gradients and symptoms may deteriorate. We believe that it is of importance that medical therapy continues after intervention and especially beta-blockers. It may be the case that our high use of beta-blocker therapy post-procedure contributes to the low reintervention rate in the pacing group. The report of the M-PATHY study also claimed that pacing therapy was effective only in >65-year-olds (n = 6)9 but that claim is clearly refuted by our comparative analysis in Table 3, which confirms equally good haemodynamic results in the <65 year olds (n = 44) as in the ≥65-year-olds (n =44). Furthermore, we found no evidence that pacing had inferior survival or haemodynamic results to myectomy in age-comparable groups in our cohort (Tables 3 and 4; Figure 2).
Long-term results after myectomy
Long-term results with respect to survival and rate of reinterventions are important in comparing different treatment modalities. The Mayo clinic reported 96% survival at 10 years after myectomy, with 6.2 years of average follow-up.6 With a longer follow-up, the Stanford clinic reported 81%, and a Swiss group 80%, survival at 10 years.5,14 In our single-centre single-region low-volume total myectomy cohort, we report freedom from cardiac deaths at 10 years follow-up of 93%, and total survival at 10 years of 88%. Thus, our survival compares well to large tertiary centres with patients of comparable age at myectomy. Those studies had 11–14% of patients lost to follow-up and did not report reintervention rates. However, in a Cleveland Clinic study, excluding patients <18 years of age, and >65 years at surgery, reintervention rate with surgery was 3.4% over an 8.7year follow-up,15 the same as in our pacing cohort. Reintervention rate is considerably higher in patients operated on as children or young adults (range 0.2–20 year), where 14.3% had required additional cardiac surgical procedures during an average follow-up of 8.6 years.16 This could be compared to our 11.9% rate of repeat LVOT surgery over 12.9 years of follow-up in the total myectomy group, where four of nine patients requiring reinterventions had had their first myectomy <18 years of age. However, even in our ≥18-year-old myectomy group, the eventual total reintervention rate was quite high (15.6%). However, all but one reintervention occurred more than 1 year after the first myectomy, most commonly caused by gradual worsening of a moderate residual gradient.
What is the current place for short atrioventricular delay pacing in the treatment of left ventricular outflow obstruction?
It has been claimed that ASA have similar outcomes as surgical myectomy and could become an alternative first-line treatment to surgical myectomy, but randomized comparisons are lacking.7 However, a recent large study suggested that ASA might be associated with a higher risk of late sudden cardiac death than myectomy, with annual post-procedural sudden death mortality rates of 0.96% and 0.75%, respectively.17 These figures are substantially higher than the 0.28% sudden death rate observed in our pacing group. Periprocedural mortality is similar for myectomy and ASA, but 7–20% of patients subjected to ASA require permanent pacing,7 when compared with around 4% after myectomy,18 although recent ASA studies report lower pacing rates of 4%.19 Short AV delay pacing is a reversible and non-destructive mode of therapy with lower periprocedural mortality and requiring fewer resources than surgery and ASA. We believe that it is not warranted to virtually discard it as a first-line treatment option as has been done in the 2011 AHA guidelines.1 The recent ESC guidelines have also relegated short AV delay pacing to a last-choice option for patients unsuitable for, or unwilling to consider, myectomy or ASA.7 The good very long-term relief of LVOTO reported by Galve et al.13 and Lucon et al.12 was confirmed in our larger study group. It was also noteworthy that our rate of reintervention for LVOTO in the ≥18-year pacing group at 3.5% was clearly lower than in our ≥18 year old myectomy group at 15.6% (P = 0.007) and clearly lower than comparable age range patients treated with ASA (reintervention rate 9.7%).17 In a large multicentre study of ASA, reintervention rate was higher still, 10.6% in the <51-year-olds and 10.4% in 51–64 year-olds,19 and LVOT-gradients was 26 and 27 mmHg respectively 1 month after the procedure.19 The 51–64-year-olds had 10 year survival of 80%,19 which compares with our <65-year-old pacing group having a total survival at 10 years of 83%. In summary, the results from short AV delay pacing in our cohort with 100% complete follow-up give no support for the notion that short AV delay pacing is an inferior treatment approach to ASA or indeed in adult patients to myectomy. It thus seems justifiable to propose that short AV delay pacing should again be considered as a reasonable first-line option for patients who are less than ideal candidates for myectomy and with significant remaining gradient/and or symptoms on medical therapy. As suggested by Qintar et al.20 commenting upon a Cochrane review, further larger randomized trials of short AV delay pacing are justified.
There is a complete absence in the literature of randomized studies comparing different interventional treatment approaches for HOCM patients not adequately controlled by medical therapy. It seems unlikely that a randomized study between surgery and either pacing or ASA will ever be carried out. As randomizing patients to long-term placebo pacing would not conform to the Helsinki Declaration, a randomized trial comparing pacing therapy with ASA would seem well justified. Such a trial might be more realistic than a randomized comparison with myectomy. However, no randomized trial is likely to provide the length of follow-up after procedure available in some cohort studies.
Limitations
The findings were generated from a post hoc analysis. Our group of myectomy patients is too small to have statistical power to detect small differences in long-term survival, reflecting the reality of specialized cardiac surgery in nations with small populations (total population in Sweden 2015: 9.85 million). Nevertheless, since our myectomy long-term survival results are comparable to high-volume centres, they appear a fair comparison group for our pacing patients.
Conclusions
Although not being a randomized study, this complete geographical cohort study showed that short AV delay pacing was not inferior to myectomy in the relief of LVOTO long-term. Furthermore, pacing therapy was associated with a low need for later reintervention. Our data support the view that short AV delay pacing should be considered a valid option to treat patients with HOCM.
Acknowledgements
We are most grateful for the helpful assistance of medical and clerical staff at the Sahlgrenska, Östra, Mölndal, Uddevalla, Trollhättan, Skövde, Lidköping, Alingsås, Borås and Kungälv Hospitals in the West Götaland Region, Sweden.
Funding
The study was supported by grants from the Swedish Heart and Lung Foundation [20080510], and by Gothenburg University ALF project grant [ALFGbg-544981].
Conflict of interest: none declared.
References
- 1. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS.. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2011;58:e212–60. [DOI] [PubMed] [Google Scholar]
- 2. Jeanrenaud X, Goy JJ, Kappenberger L.. Effects of dual-chamber pacing in hypertrophic obstructive cardiomyopathy. Lancet 1992;339:1318–23. [DOI] [PubMed] [Google Scholar]
- 3. Fananapazir L, Epstein ND, Curiel RV, Panza JA, Tripodi D, McAreavey D.. Long-term results of dual-chamber (DDD) pacing in obstructive hypertrophic cardiomyopathy: evidence for progressive symptomatic and hemodynamic improvement and reduction of left ventricular hypertrophy. Circulation 1994;90:2731–42. [DOI] [PubMed] [Google Scholar]
- 4. Kappenberger LJ, Linde C, Jeanrenaud X, Daubert C, McKenna W, Meisel E. et al. Clinical progress after randomized on/off pacemaker treatment for hypertrophic obstructive cardiomyopathy. Pacing in cardiomyopathy (PIC) study group. Europace 1999;1:77–84. [DOI] [PubMed] [Google Scholar]
- 5. Schonbeck MH, Rocca HPB, Vogt PR, Lachat M, Jenni R, Hess OM. et al. Long-term follow-up in hypertrophic obstructive cardiomyopathy after septal myectomy. Ann Thorac Surg 1998;65:1207–14. [DOI] [PubMed] [Google Scholar]
- 6. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S. et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470–6. [DOI] [PubMed] [Google Scholar]
- 7. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P. et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 8. Robbins RC, Stinson EB.. Long-term results of left ventricular myotomy and myectomy for obstructive hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 1996;111:586–93. [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ, Nishimura RA, McKenna WJ, Rakowski H, Josephson ME, Kieval RS.. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY). Circulation 1999;99:2927–33. [DOI] [PubMed] [Google Scholar]
- 10. Ommen SR, Nishimura RA, Squires RW, Schaff HV, Danielson GK, Tajik AJ.. Comparison of dual-chamber pacing versus septal myectomy for the treatment of patients with hypertrophic obstructive cardiomyopathy - a comparison of objective hemodynamic and exercise end points. J Am Coll Cardiol 1999;34:191–6. [DOI] [PubMed] [Google Scholar]
- 11. Fananapazir L, Cannon RO, Tripodi D, Panza JA.. Impact of dual-chamber permanent pacing in patients with obstructive hypertrophic cardiomyopathy with symptoms refractory to verapamil and beta-adrenergic blocker therapy. Circulation 1992;85:2149–61. [DOI] [PubMed] [Google Scholar]
- 12. Lucon A, Palud L, Pavin D, Donal E, Behar N, Leclercq C. et al. Very late effects of dual chamber pacing therapy for obstructive hypertrophic cardiomyopathy. Arch Cardiovasc Dis 2013;106:373–81. [DOI] [PubMed] [Google Scholar]
- 13. Galve E, Sambola A, Saldana G, Quispe I, Nieto E, Diaz A. et al. Late benefits of dual-chamber pacing in obstructive hypertrophic cardiomyopathy: a 10-year follow-up study. Heart 2010;96:352–6. [DOI] [PubMed] [Google Scholar]
- 14. Sedehi D, Finocchiaro G, Tibayan Y, Chi J, Pavlovic A, Kim YM. et al. Long-term outcomes of septal reduction for obstructive hypertrophic cardiomyopathy. J Cardiol 2015;66:57–62. [DOI] [PubMed] [Google Scholar]
- 15. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW. et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209–16. [DOI] [PubMed] [Google Scholar]
- 16. Minakata K, Dearani JA, O’Leary PW, Danielson GK.. Septal myectomy for obstructive hypertrophic cardiomyopathy in pediatric patients: early and late results. Ann Thorac Surg 2005;80:1424–9. discussion 1429-1430 [DOI] [PubMed] [Google Scholar]
- 17. Vriesendorp PA, Liebregts M, Steggerda RC, Schinkel AF, Willems R, Ten Cate FJ.. Long-term outcomes after medical and invasive treatment in patients with hypertrophic cardiomyopathy. JACC Heart Failure 2014;2:630–6. [DOI] [PubMed] [Google Scholar]
- 18. Liebregts M, Vriesendorp PA, Mahmoodi BK, Schinkel AF, Michels M, ten Berg JM.. A systematic review and meta-analysis of long-term outcomes after septal reduction therapy in patients with hypertrophic cardiomyopathy. JACC Heart Failure 2015;3:896–905. [DOI] [PubMed] [Google Scholar]
- 19. Liebregts M, Faber L, Jensen MK, Vriesendorp PA, Januska J, Krejci J. et al. Outcomes of alcohol septal ablation in younger patients with obstructive hypertrophic cardiomyopathy. JACC Cardiovasc Interv 2017;10:1134–43. [DOI] [PubMed] [Google Scholar]
- 20. Qintar M, Morad A, Alhawasli H, Shorbaji K, Firwana B, Essali A. et al. Pacing for drug-refractory or drug-intolerant hypertrophic cardiomyopathy. Cochrane Database Syst Rev 2012;5:CD008523. [DOI] [PMC free article] [PubMed] [Google Scholar]


