Abstract
Aims
Implantable cardioverter-defibrillators (ICDs) are key in the prevention of sudden cardiac death, but outcomes may vary by type of device or programming [single chamber (SC) vs. dual chamber (DC)] in patients without a bradycardia pacing indication. We sought to meta-analyse patient outcomes of randomized trials of SC vs. DC devices or programming.
Methods and results
We searched PubMed, Embase, Scopus, Web of Science, and Cochrane trials databases for relevant studies excluding those published before 2000, involving children, or not available in English. Endpoints included mortality, inappropriate ICD therapies, and implant complications. Endpoints with at least three reporting studies were meta-analysed. We identified eight studies meeting inclusion criteria representing 2087 patients with 16.1 months mean follow-up. Mean age was 62.7 years (SD 1.92); in six studies reporting sex, most patients were male (85%). Comparing patients with a SC or DC ICD or programming, we found similar rates of mortality [odds ratio (OR) 0.95, 95% confidence interval (CI) 0.54–1.68; P = 0.86] and inappropriate therapies (OR 1.46, 95% CI 0.97–2.19; P = 0.07) in five and six studies, respectively. In three studies of SC vs. DC ICDs (but not programming) rates of pneumothorax and lead dislodgement were not different (OR 2.12, 95% CI 0.18–24.72; P = 0.55 and OR 0.87, 95% CI 0.32–2.47; P = 0.83, respectively).
Conclusion
In this meta-analysis of randomized controlled trials comparing SC vs. DC ICD device or programming, there was no significant difference in inappropriate therapies, mortality, pneumothorax, or lead dislodgement. Future studies should compare these devices over longer follow-up and in specific patient populations.
Keywords: Implantable cardioverter-defibrillator , Complications , Mortality , Meta-analysis , Review
What’s new?
Multiple studies have compared single chamber and dual chamber implantable cardioverter-defibrillators (ICDs) for patients without a bradycardia pacing indication with mixed results. This meta-analysis combines the most robust of these comparisons—randomized clinical trials.
With over 2000 randomized patients, overall risks from ICD implantation is low.
When there is no bradycardia indication, there does not appear to be any significant differences in outcomes between a single chamber or dual chamber device or programming.
Introduction
The landmark clinical trials that established the benefit of the implantable cardioverter-defibrillator (ICD) did not address some decisions that are made at the time of implant. For example, some ICDs are implanted with only a lead in the right ventricle [single chamber (SC)] for the primary purpose of treating tachyarrhythmias. Other devices are implanted with a lead in the right ventricle and a lead in the right atrium [dual chamber (DC)] when the need for bradytherapy (i.e. pacing) is expected and to potentially help discriminate ventricular from supraventricular arrhythmias. In the landmark studies of the ICD, only SC devices were available.1–3 There have been major advances in implanting techniques, arrhythmia detection algorithms and treatment strategies since these studies, which may reduce risks of procedural complications and inappropriate therapy, respectively.
In patients without a pacing indication, some providers choose a SC ICD to avoid the additional time and potential complications of placement of an atrial lead including, but not limited to, infection and mechanical complications requiring reoperation.4–6 For example, lead dislodgement is a relatively common complication of device implantation typically requiring reoperation; more leads provide more opportunity for dislodgement. Others may prefer a DC ICD for the potential benefit of improved discrimination between atrial and ventricular arrhythmias to avoid inappropriate therapy, which negatively impacts quality of life and may lead to increased rates of hospitalization and death7–9 or for the relatively rare possibility of the patient developing a pacing indication during follow-up.10
Various studies of SC vs. DC ICDs, many of which were randomized6–9,11–14 or of very large size,4,5,15 have assessed whether one strategy is superior to another based on mortality, procedure-related complications, inappropriate shocks, hospitalizations, and other endpoints. However, no clear consensus has emerged regarding, which strategy is superior. A meta-analysis of randomized studies examining this question by Chen et al.16 found no benefit of DC devices over SC devices. However, since this report, there have been two large multicentre randomized trials investigating this question with more modern programming of ICDs.17,18 As such, the current ESC guidelines leave the decision to implant a SC or DC ICD in patients without a pacing indication to physician discretion.19
To address the outstanding questions regarding the risks and benefits of SC vs. DC ICD or programming in patients without a pacing indication, we conducted a systematic review of the available literature and a meta-analysis of patient outcomes. For the purposes of this analysis, ‘single chamber (SC)’ refers to a device with capability to sense and deliver back up pacing only in the right ventricle (RV) whether through hardware selection (RV lead only) or ‘programming’ [RV and right atrial (RA) leads present with RA atrial lead inactive] whereas ‘dual chamber (DC)’ refers to a device with sensing and back up pacing capability in both the right ventricle and the right atrium.
Methods
Search strategy
An expert reference librarian designed and conducted an electronic search strategy with input from the first author (E.P.Z.). The search was implemented in PubMed using a combination of medical subject headings (MeSH) and keywords (Appendix 1). After the initial search, terms were translated and implemented in each of the other databases. Duplicates were removed. The search was limited to English language. Bibliographies of selected manuscripts were reviewed manually to identify any additional relevant references not captured in our search. In addition, clinicaltrials.gov was searched for any relevant studies.
Appendix 1.
Search strategy
| Set # | Results | |
|---|---|---|
| 1 | ‘Defibrillators, Implantable’[Mesh] OR defibrillator[tiab] OR defibrillators[tiab] OR ICD[tiab] OR ICDs[tiab] | 34 887 |
| 2 | dual[tiab] | 130 292 |
| 3 | #1 AND #2 | 949 |
| 4 | #3 NOT (animals[mh] NOT humans[mh]) NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) | 761 |
| 5 | #4 Limits: English, 2000—present | 604 |
Eligibility
We included studies that systematically compared outcomes in patients with no bradycardia pacing indication receiving a SC or DC ICD or programming. This included studies in which all patients received an ICD with both an RA and RV lead, but the RA lead was ‘programmed’ inactive (e.g. VVI back up pacing mode) in the SC group and active in the DC group. We only included randomized controlled trials (RCTs) that randomized patients based on receiving a SC vs. DC device or programming; studies that randomized patients based on pacing strategies in DC devices were specifically excluded. Studies of less than 50 subjects were excluded as were studies not available in English or published before 1 January 2000. This date restriction was used since technologies studied before that time do not adequately represent those currently available and used widely.
Extraction
Two investigators (E.P.Z. and K.S.) made and tracked all screening decisions in a DistillerSR database (Evidence Partners Inc., Manotick, ON, Canada). Extracted data included patient characteristics, device programming details, procedural complications, mortality, heart failure hospitalization, inappropriate shocks, quality of life, and device-related invasive interventions and associated complications. Trial characteristics were also extracted including study design, geographic location, and whether outcomes were adjudicated centrally. Lastly, we applied recommendations of the Agency for Healthcare Research and Quality (AHRQ) to evaluate the quality of each study included in our analysis.20
Endpoints
The prespecified endpoints in this analysis were: procedural complications (including pneumothorax, bleeding/haematoma, infection, stroke/transient ischemic attack (TIA), reoperation/pocket revision, and vascular injury), mortality, heart failure hospitalization, inappropriate shocks, quality of life, generator malfunction, and device-related complications. We also collected information about device programming.
Data analysis
We first assessed the feasibility of meta-analysis for each outcome based on the number of studies with complete reported results. To perform meta-analysis, we required at least three studies with unique results that reported the number of patients experiencing the endpoint of interest. We performed meta-analysis using a random effects model in Comprehensive Meta-Analysis Version 2 (Biostat; Englewood, NJ, USA; 2005). This software uses the methods of DerSimonian and Laird.21 We report meta-analysed measures as an odds ratio (OR) with a 95% confidence interval (CI). Heterogeneity was assessed using the ratio of true heterogeneity to the overall observed variation (I2). In addition, when possible, we performed sensitivity analyses to evaluate for differences in outcomes based on randomization scheme—DC vs. SC device implants as compared with DC vs. SC programming as defined above.
Results
Search results
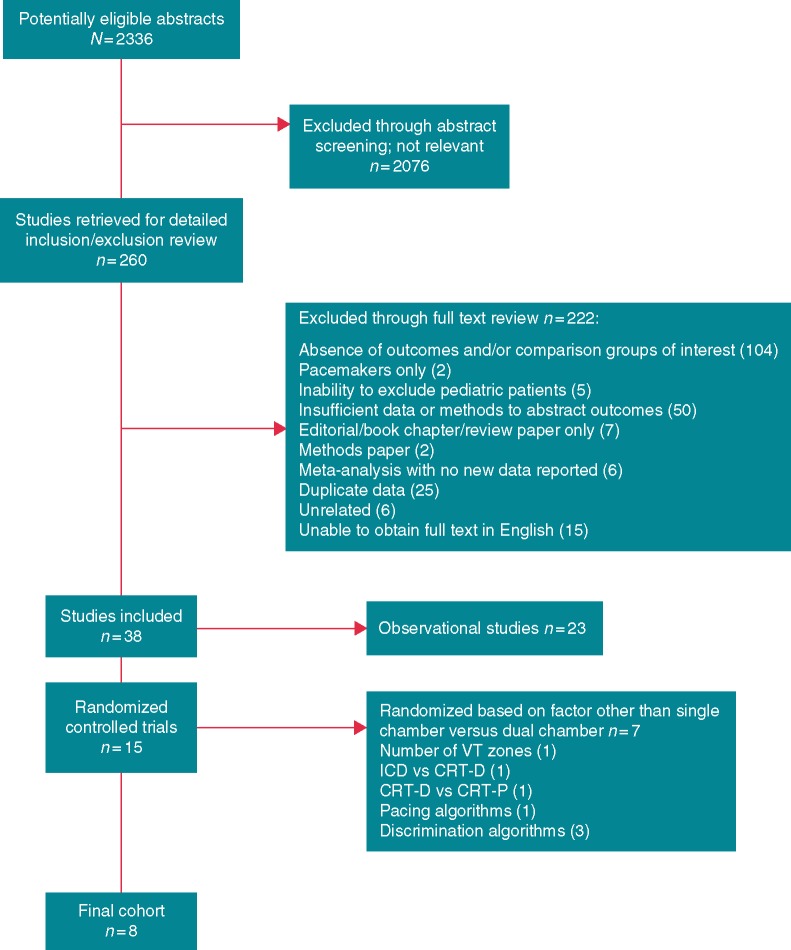
Our search identified 2336 abstracts. Of these, 2076 were excluded at the abstract level due to irrelevance or failure to meet our inclusion and exclusion criteria (Figure 1). Manuscripts of the remaining 260 studies were reviewed in full text. Of these, 222 were excluded. Many of the studies examined in full text were excluded for more than one reason. For example, many of the abstracts reviewed were excluded because they were duplicative with a full length manuscript also included in the search and because the abstract included insufficient data or methods to adjudicate endpoints. The reasons for exclusion are considered mutually exclusive, so only one reason is listed in Figure 1. Of the remaining 38 studies, 23 were observational and were, therefore, excluded. Fifteen RCTs remained. Of these, seven studies randomized patients based on a factor other than SC vs. DC devices or programming (e.g. bradycardia pacing algorithm14). Ultimately, our sample included the remaining eight studies, which compared outcomes in patients with SC vs. DC devices or programming directly. This cohort of studies represented 2087 patients. Included studies are listed in Table 1.
Figure 1.
QUORUM diagram outlining the identification and inclusion of studies in this meta-analysis. CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator.
Table 1.
Characteristics of studies included in meta-analysis
| First author, year of publication | Number of sites | Location | Overall number of subjects | Follow-upa | Endpoint adjudication | Randomization scheme |
|---|---|---|---|---|---|---|
| Deisenhofer, 200112 | Multicentre | Non-US | 92 | 7.5 months | Central | SC vs. DC devices |
| Wilkoff, 20026 | Multicentre | US | 506 | 8.4 months | Central | SC vs. DC programming |
| Bansch, 200411 | Single centre | Non-US | 102 | 6.1 months | Central | SC vs. DC programming |
| Friedman, 20068 | Multicentre | US and Non-US | 400 | 6 months | Central | SC vs. DC programming |
| Kolb, 200613 | Single centre | Non-US | 100 | 52 months | NR | SC vs. DC devices |
| Almendral, 20087 | Multicentre | Non-US | 334 | 15.7 months | Central | SC vs. DC AND SC vs. DC programming |
| Kolb, 201418 | Multicentre | Non-US | 453 | 23.4 months | Central | SC vs. DC programming |
| Friedman, 201417 | Multicentre | US and Non-US | 100 | 12 months | Central | SC vs. DC devices |
In some cases, follow-up was prespecified and in other cases, follow-up is reported as a mean.
DC, dual chamber; Non-US, outside United States; NR, not reported; SC, single chamber; US, United States.
Study characteristics
The eight RCTs included in this meta-analysis had some important differences (Table 1). First, the studies ranged considerably in size from 92 to over 500 patients. Four studies included patients with DC devices randomized to SC vs. DC programming, three studies randomized patients to SC vs. DC device implants, and one study randomized based on both programming and implants. Notably, there were minimal crossovers in the included studies representing just 5% of all participants. On average, crossovers were similar between those studies which randomized based on hardware and those that randomized based on programming. Two studies were performed at a single centre and the rest were multicentre. The endpoints in seven studies were adjudicated centrally while the adjudication methods for one study was not reported. Only one study was performed exclusively in the US while all others were performed exclusively outside the US or at a combination of US and non-US sites. Follow-up duration varied between studies with a range of 6 months to 52 months and a mean of 16 months.
Patient characteristics
The average age among all patients included in this meta-analysis was 62.7 ± 1.9 years. Seven studies reported a description of patient sex by randomized group. In all cases, patient populations were predominantly male (78–92%). No studies reported race. Rates of most important comorbidities (e.g. heart failure) were not reported in a sufficient number of studies. In six studies reporting left ventricular ejection fraction, the overall mean was 31% (range 26–38%). Only four studies reported the indication for ICD, which was primary prevention in 77, 100, 11, and 66% of patients, respectively (mean 63%).
Device programming
Device programming parameters were not reported with sufficient detail to allow for meaningful formal comparisons. In those cases in which device programming was protocolized or strongly recommended, programming reflected the best practices at the time of the study. For example, no included study prior to 2006 made mention of antitachycardia pacing (ATP) whereas the most recent two studies required the use of ATP prior to shock delivery. These latter two studies also required specific zone-based tachytherapies.
All except one study specifically reported the application of discriminating algorithms to differentiate between supraventricular tachycardia and ventricular tachycardia based on a variety of rhythm and electrographic characteristics.
Finally, most study protocols recommended some effort to reduce RV pacing through a combination of low back up pacing rates, long atrioventricular delays, and other ventricular pacing avoidance algorithms. Four of the studies in our analysis reported the rate of RV pacing by SC vs. DC group and reflected both device and programming randomization.6,7,13,18 In three studies reported from 2002 to 2008, mean RV pacing ranged from 37% to 55% in the DC group and 2–5% in the SC group. A single study from 2014 reported very low mean RV pacing rates in DC and SC groups: 3.9 vs. 2.4%, respectively18 perhaps reflecting a better appreciation for the benefits of avoiding RV pacing.
Data quality
Our analysis included only randomized controlled studies. When each of the included studies was examined according to the recommendations by the AHRQ working group, each study was found to be of good quality based on the fact that methods were clearly stated and appropriate, and bias was minimized whenever possible.
Patient outcomes
For the following outcomes as pre-defined, there was no reporting by any included study: reoperation/pocket revision, vascular injury, quality of life, device related invasive interventions, or generator malfunction. For the following endpoints, there were insufficient sources to conduct meta-analysis: heart failure hospitalization, infection, bleeding/haematoma, and stroke/TIA. Two studies reported stroke/TIA events between groups with three events in the DC group (n = 280, 1%) and one event in the SC group (n = 273, 0.3%).17,18 Two studies reported similar bleeding/haematoma events between groups with six events in the SC group (n = 334, 1.8%) and seven events in the DC group (n = 463, 1.5%).7,18 A single study reported rates of infection with 1/111 (0.9%) event and 3/223 (1.3%) events in SC and DC groups, respectively.7 Finally, in two studies, rates of heart failure hospitalization were reported: 65/497 (13%) in the SC group and 82/480 (17%) in the DC group.6,18
The following endpoints were available in at least three sources to allow for meta-analysis: mortality, inappropriate therapy, pneumothorax, and lead dislodgement.
Mortality
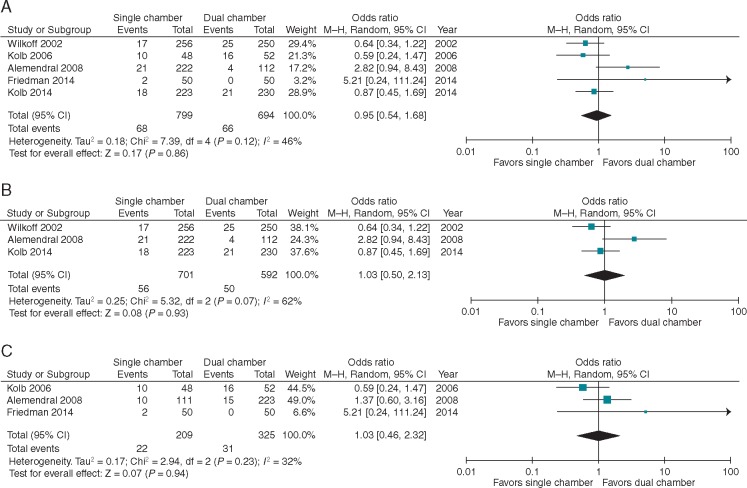
Five studies reported the mortality rate in patients who received a SC vs. a DC device or programming. Follow-up ranged from 8.4 to 52 months with an average of 23 months. The event rate ranged from 4% to 21% and 0% to 31% in the SC and DC groups, respectively, with a mean of 10% and 11%. There was no significant difference between groups (OR 0.95, 95% CI 0.54–1.68; P = 0.86) (Figure 2). Heterogeneity was moderate with I2 = 46%. When analyses were separated by randomization scheme (SC vs. DC devices or SC vs. DC programming), results were unchanged (Figure 2B and C).
Figure 2.
Forest plot for mortality in five studies reporting the outcome (A) overall, (B) randomized by programming, and (C) randomized by device type. CI, confidence interval; DC, dual chamber; SC, single chamber.
Inappropriate therapies
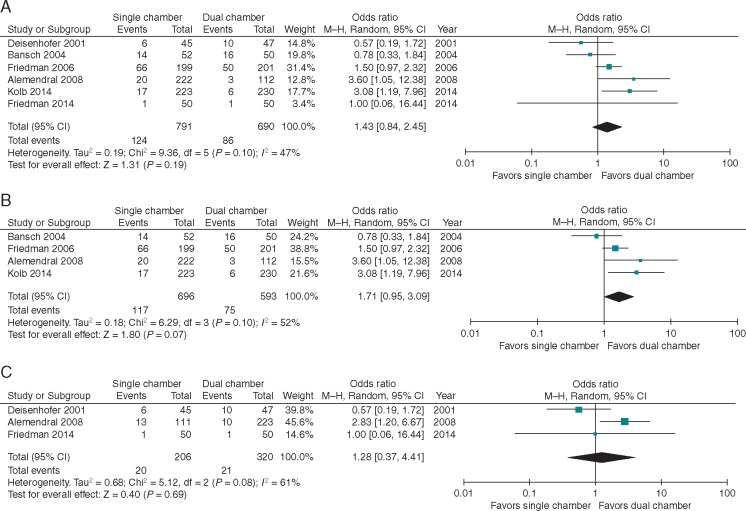
Six studies reported the rate of inappropriate therapies in patients with SC vs. DC devices or programming. Follow-up ranged from 6 to 15.6 months; average follow-up was 10 months. The event rate ranged from 2% to more than 30% in both groups with a mean event rate of 15% and 14% in the SC and DC groups, respectively. There was no difference seen between the two groups when results were pooled and meta-analysed (OR 1.43, 95% CI 0.84–2.45; P = 0.19) (Figure 3A). Heterogeneity was moderate with I2 = 47%. Similar to the mortality analysis, findings were unchanged when examined by randomization scheme (DC vs. SC devices or programming) (Figure 3B and C). We noted that earlier studies appeared to have higher overall rates of inappropriate therapy than newer studies, so we explored this formally. The OR for inappropriate therapies pre-2009 (four studies) was 1.22 favouring DC devices or programming (95% CI 0.65–2.29), while post-2009 (two studies), the difference favouring DC was greater and statistically significant (OR 2.74 95% CI 1.12–6.74).
Figure 3.
Forest plot for inappropriate therapies in six studies reporting the outcome (A) overall, (B) randomized by programming, and (C) randomized by device type. CI, confidence interval; DC, dual chamber; SC, single chamber.
Pneumothorax
Three studies reported rates of pneumothorax, and when combined, this represents seven total pneumothorax events in 379 patients. The event rate was low in both groups with a range of 0–4% in both groups with an average rate of 1.6% and 0.6% in SC and DC groups, respectively. In this meta-analysis, there was no difference in this outcome between patients with SC vs. DC devices (OR 2.12, 95% CI 0.18–24.72; P = 0.55) (Figure 4). The 95% CI is very wide reflecting the low event rate and, thus, imprecision of the point estimate despite the combination of three studies representing nearly 400 randomized patients. Heterogeneity was 50%. Notably, the pneumothorax endpoint in Almendral et al., is reported in the portion of the study, that directly compared SC vs. DC device implants (rather than DC simulated programming).
Figure 4.
Forest plot for pneumothorax in three studies reporting the outcome. CI, confidence interval.
Lead dislodgement
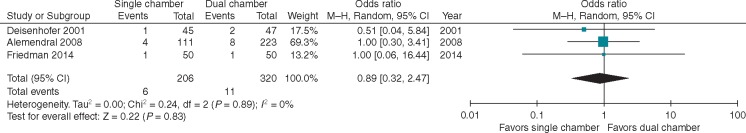
Three of the four studies comparing SC vs. DC device implants reported rates of lead dislodgement. Any lead dislodgement was reported in 6/206 (2.4%) SC devices and 11/320 (3.4%) DC devices. In the latter group, this included five atrial lead dislodgements. The time to dislodgement was not reported consistently. On meta-analysis, there was no difference in rates of lead dislodgement (OR 0.87 95% CI 0.32–2.47; P = 0.83) (Figure 5). There was no heterogeneity (I2 = 0%). Notably, in DC devices across six studies, the rate of lead dislodgement was similar between atrial [15/875 (1.7%)] and ventricular leads [17/875 (1.9%)].7,11,17,18 One additional study reported only the rate of atrial lead dislodgement of 1%.8
Figure 5.
Forest plot for lead dislodgement in three studies reporting the outcome. CI, confidence interval.
Discussion
To date, there is no clear consensus on the role of SC vs. DC ICDs in patients without a bradycardia pacing indication. Previous studies of patients similar to those studied here have demonstrated a low rate for bradycardia therapy in ICD patients over time with estimates ranging from 4% to 14% over variable follow-up.10 Importantly, these rates may be overestimates since they were established in patients with DC devices such that crossover from SC to DC pacing required only reprogramming rather than hardware revision. When there is no bradycardia pacing indication at the time of implant, reasons for implanting a DC ICD include presumed better discrimination between supraventricular and ventricular arrhythmias to reduce inappropriate therapies and to prevent another procedure to place an atrial lead if a bradycardia pacing indication develops over time. In the latter case, there are no clear epidemiological estimates to help guide the choice of SC vs. DC device, and in the former case, discriminatory functions of ICDs have not universally lived up to their promise. Alternatively, a SC device may be chosen to reduce periprocedural complications and the risk of future complications. In the context of these uncertainties, there have been multiple RCTs of SC vs. DC devices, but the results have been inconsistent. In the meta-analysis by Chen et al.,16 some relevant studies including more than 100 additional unique patients were excluded, and two additional large RCTs have been completed since its publication. In addition, The Chen et al.16 meta-analysis included the Inhibition of Unnecessary RV Pacing With AVSH in ICDs (INTRINSIC RV) study. This important study investigated the effect of one pacing algorithm designed to minimize RV pacing but allowed both SC and DC device function. Therefore, this study does not truly address differences in SC vs. DC device implantation or back up pacing programming and may have complicated the meta-analysis.
Without clear consensus in the literature or from relevant professional societies, practice patterns regarding use of SC vs. DC ICDs vary considerably. For example, a study from the National Cardiovascular Data Registry (NCDR) ICD Registry indicated that 58% and 42% of patients undergoing primary prevention ICD implantation without a bradycardia pacing indication between 2006 and 2009 received a DC and SC device, respectively.22 Another clinical cohort over the same time period reported a similar distribution.15 This nearly equal split likely reflects the effects of patient and physician preferences in the absence of clear perceived benefits of one strategy over the other. Therefore, we employed meta-analysis of the highest quality evidence—RCTs—to further evaluate the differences between SC and DC ICDs. The included studies in our analysis were similar in regard to patient characteristics but varied in size, follow-up, and other characteristics. Formal assessment suggests only moderate heterogeneity; less than 50% of the total variation across studies was due to heterogeneity across the studies rather than chance. Upon meta-analysis, we uncovered two major findings: there was no difference in regard to mortality or inappropriate therapies between SC and DC ICD groups while post-2009 (two studies), the difference favouring DC was greater and statistically significant (OR 2.74 95% CI 1.12–6.74).
When comparing mortality in patients with a SC vs. a DC ICD or programming in the absence of a pacing indication, there does not appear to be any difference. This analysis represents nearly 150 deaths in a population of 1493 patients (9%). Follow up varied between studies that were included in this analysis with a minimum of 8.4 months, a maximum of 52 months, and mean of 23 months. In this sense, the mortality rate was relatively low since studies of ICD patients in clinical practice have demonstrated much higher mortality rates as shown by an analysis of the National Cardiovascular Data Registry ICD Registry.23 This is not surprising, however, since patients included in cardiovascular clinical trials tend to be younger and healthier than those seen in clinical practice, and clinical trials include limited follow-up. Mortality may have been affected by RV pacing, but formal consideration of the impact of RV pacing on mortality was outside the scope of this analysis. Notably, there was more RV pacing in the DC groups in earlier studies which may have attenuated any beneficial effect of a DC device on mortality or other endpoints. The time of death in relation to device implant was not captured consistently across studies, so it was not possible to formally compare periprocedural mortality between groups. However, the finding of no difference in mortality when comparing SC and DC devices (Figure 2C) suggests no clear disadvantage with respect to mortality between a SC and DC ICD implant.
Secondly, there was no difference between SC and DC groups with respect to inappropriate therapies. Over an average of 10 months in six randomized studies, there was no difference in rates between SC and DC patients (OR 1.43, 95% CI 0.84–2.45; P = 0.19) demonstrating no apparent advantage or disadvantage of a DC device in respect to inappropriate therapies. In our analysis, we capture the number of patients experiencing any inappropriate therapy (rather than the total number or therapies), which does not account for the dynamic nature of this outcome. Nonetheless, the consequences of any inappropriate therapy are significant, so strategies, which reduce this outcome are reasonable to explore. We considered the impact that patient characteristics like age may have on this outcome, but without patient level data we were unable to perform formal sensitivity analyses to assess this. Furthermore, the patient populations across studies were more similar than different making meta-regression analysis less meaningful. Importantly, significant reductions in the rate of inappropriate shocks have been demonstrated with the application of less aggressive programming as well as improved discrimination algorithms including EGM morphology analysis since the early 2000s. Indeed, programming decisions may be more important that hardware selection.24 As such, studies included in our analysis demonstrate a trend towards an overall reduction in inappropriate shocks over time: the earliest studies demonstrate rates of 27% and 33% whereas the later studies demonstrate rates below 10%. We explored this finding with a sensitivity analysis of studies pre- and post-2009 and found that there was no difference between SC and DC devices or programming in the pre-2009 studies. However, post-2009, rates of inappropriate therapies were lower in the DC group. One possible explanation for this is that with improved programming, benefits of a DC device could be more fully realized. However, there was no statistically significant difference between the pre- and post-2009 ORs.
Finally, we note that there are differences in cost and resource utilization between a SC and DC ICD. While these considerations are outside the scope of this analysis, further exploration is warranted because when clinical outcomes appear to be similar, factors like these become more relevant.
Limitations
None of the studies included in our analysis reported patient or physician preferences, which may play a role in deciding to implant a SC or DC system. Furthermore, while we attempted to collect demographic and comorbidity data to put outcomes in context of other competing risks of death and morbidity, we were limited by reporting in the included studies. We did not examine outcomes in patients with cardiac resynchronization therapy (CRT), and there is evidence that CRT can impact the outcomes we examined. Surprisingly, no difference was seen in rates of pneumothorax, inappropriate therapies, or lead dislodgements between SC and DC implants. This is particularly striking in the case of lead dislodgement in which the rates of atrial and ventricular lead dislodgements were similar suggesting that the combined rate in DC devices should be twice that in SC devices. In both cases, confidence intervals were wide reflecting low event rates and resulting imprecision in the estimate with diminished opportunity to detect a difference. Finally, theoretical longer term benefits of a SC device (e.g. decreased risk of infection and ipsilateral venous occlusion) or a DC device (avoidance of adding an atrial lead if a pacing indication develops over time) could not be assessed. The absence of longer term outcomes and outcomes reported by age group may be most relevant for comparing outcomes between young and old patients.
Conclusion and clinical implications
In this systematic review and meta-analysis, we found that there have been some high quality studies of SC vs. DC ICDs or programming with no significant difference in mortality, inappropriate therapies, lead dislodgements, or pneumothorax. This reinforces a personalized approach to device selection based on specific patient characteristics and preferences, and it emphasizes the importance of discussing risks and benefits with patients prior to implantation. Indeed, such an approach is emphasized in the ‘shared decision making’ paradigm now required in some settings prior to ICD implantation.25
Acknowledgements
We wish to thank Megan Chobot MSLS for her assistance in facilitating the use of DistillerSR in conducting this review and meta-analysis. We also wish to thank Megan von Isenburg MSLS for her assistance in building and conducting the literature search strategy.
Funding
This work was was funded in part by National Institutes of Health (NIH) T-32 training [#2 T32 HL69749-11 A1 to E.P.Z.]. However, no relationships exist related to the analysis presented.
Conflict of interest: none declared.
References
- 1. Hallstrom AP, Greene HL, Wyse DG, Zipes D, Epstein AE, Domanski MJ. et al. Antiarrhythmics versus implantable defibrillators (AVID)—rationale, design, and methods. Am J Cardiol 1995;75:470–5. [PubMed] [Google Scholar]
- 2. Kuck KH, Cappato R, Siebels J, Ruppel R.. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748–54. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 4. Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD.. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol 2011;58:1007–13. [DOI] [PubMed] [Google Scholar]
- 5. Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP. et al. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA 2013;309:2025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H. et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA 2002;288:3115–23. [DOI] [PubMed] [Google Scholar]
- 7. Almendral J, Arribas F, Wolpert C, Ricci R, Adragao P, Cobo E. et al. Dual-chamber defibrillators reduce clinically significant adverse events compared with single-chamber devices: results from the DATAS (Dual chamber and Atrial Tachyarrhythmias Adverse events Study) trial. Europace 2008;10:528–35. [DOI] [PubMed] [Google Scholar]
- 8. Friedman PA, McClelland RL, Bamlet WR, Acosta H, Kessler D, Munger TM. et al. Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation 2006;113:2871–9. [DOI] [PubMed] [Google Scholar]
- 9. Theuns DA, Klootwijk AP, Goedhart DM, Jordaens LJ.. Prevention of inappropriate therapy in implantable cardioverter-defibrillators: results of a prospective, randomized study of tachyarrhythmia detection algorithms. J Am Coll Cardiol 2004;44:2362–7. [DOI] [PubMed] [Google Scholar]
- 10. Sweeney MO. The implantable cardioverter-defibrillator minimalist: an approach to patient follow-up and management of implantable defibrillators. Circulation 2012;126:369–77. [DOI] [PubMed] [Google Scholar]
- 11. Bansch D, Steffgen F, Gronefeld G, Wolpert C, Bocker D, Mletzko RU. et al. The 1 + 1 trial: a prospective trial of a dual- versus a single-chamber implantable defibrillator in patients with slow ventricular tachycardias. Circulation 2004;110:1022–9. [DOI] [PubMed] [Google Scholar]
- 12. Deisenhofer I, Kolb C, Ndrepepa G, Schreieck J, Karch M, Schmieder S. et al. Do current dual chamber cardioverter defibrillators have advantages over conventional single chamber cardioverter defibrillators in reducing inappropriate therapies? A randomized, prospective study. J Cardiovasc Electrophysiol 2001;12:134–42. [DOI] [PubMed] [Google Scholar]
- 13. Kolb C, Deisenhofer I, Schmieder S, Barthel P, Zrenner B, Karch MR. et al. Long-term follow-up of patients supplied with single-chamber or dual-chamber cardioverter defibrillators. Pacing Clin Electrophysiol 2006;29:946–52. [DOI] [PubMed] [Google Scholar]
- 14. Olshansky B, Day JD, Moore S, Gering L, Rosenbaum M, McGuire M. et al. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? Results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation 2006;115:9–16. [DOI] [PubMed] [Google Scholar]
- 15. Peterson PN, Greenlee RT, Go AS, Magid DJ, Cassidy-Bushrow A, Garcia-Montilla R. et al. Comparison of inappropriate shocks and other health outcomes between single- and dual-chamber implantable cardioverter-defibrillators for primary prevention of sudden cardiac death: results from the cardiovascular research network longitudinal study of implantable cardioverter-defibrillators. J Am Heart Assoc 2017;6:e006937.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen BW, Liu Q, Wang X, Dang AM.. Are dual-chamber implantable cardioverter-defibrillators really better than single-chamber ones? A systematic review and meta-analysis. J Interv Card Electrophysiol 2014;39:273–80. [DOI] [PubMed] [Google Scholar]
- 17. Friedman PA, Bradley D, Koestler C, Slusser J, Hodge D, Bailey K. et al. A prospective randomized trial of single- or dual-chamber implantable cardioverter-defibrillators to minimize inappropriate shock risk in primary sudden cardiac death prevention. Europace 2014;16:1460–8. [DOI] [PubMed] [Google Scholar]
- 18. Kolb C, Sturmer M, Sick P, Reif S, Davy JM, Molon G. et al. Reduced risk for inappropriate implantable cardioverter-defibrillator shocks with dual-chamber therapy compared with single-chamber therapy: results of the randomized OPTION study. JACC Heart Fail 2014;2:611–9. [DOI] [PubMed] [Google Scholar]
- 19. Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–442. [DOI] [PubMed] [Google Scholar]
- 20. Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB. et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol 2010;63:513–23. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 22. Matlock DD, Peterson PN, Wang Y, Curtis JP, Reynolds MR, Varosy PD. et al. Variation in use of dual-chamber implantable cardioverter-defibrillators: results from the national cardiovascular data registry. Arch Intern Med 2012;172:634–41; discussion 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL. et al. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes 2013;6:488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gasparini M, Proclemer A, Klersy C, Kloppe A, Lunati M, Ferrer JB. et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903–11. [DOI] [PubMed] [Google Scholar]
- 25. CMS. Decision memo for implantable cardioverter defibrillators (CAG-00157R4). 2018. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=288 (14 August 2018, date last accessed).